Artigo
| Optimization of solvent-free geranyl butanoate production using novozyme 435 and homemade polyurethane immobilized novozyme NZL-102-LYO-HQ as catalysts |
|
Carla R. SbardelottoI; Suelen P. PiazzaI; Nadia L. D. NyariI; Rogério M. DallagoI; Débora de OliveiraII; Vladimir de OliveiraII; Jamile ZeniI; Rogério L. CansianI,*; Natalia ParoulI
I. Departamento de Engenharia de Alimentos, Universidade Regional Integrada do Alto Uruguai e das Missões, 99700-000 Erechim - RS, Brasil Recebido em: 04/06/2018 *e-mail: cansian@uricer.edu.br This study reports the optimization of geranyl butanoate production by esterification of geraniol and butanoic acid in a solvent-free system using two immobilized lipases as catalyst. The operating conditions that optimized geranyl butanoate production were determined to be 40 °C, a geraniol to butanoic acid molar ratio of 3:1, 150 rpm, 5 wt% of enzyme, and 1 h of reaction, which resulted in a reaction conversion of about 97% for Novozyme 435. When homemade Novozyme NZL-102-LYO-HQ (Cal-B) immobilized in polyurethane foam was used as catalyst, the experimental conditions of an alcohol to acid molar ratio of 5:1, 70 °C, 150 rpm, 5 wt% of enzyme, and 1 h of reaction gave a conversion of 95%. New experimental data on enzymatic esterification of geraniol and butanoic acid for geranyl butanoate production are reported in this work, showing that the technique is promising for overcoming the well-known drawbacks of the chemical-catalyzed route. INTRODUCTION The flavor and fragrance industry is a world market worth billions of dollars and growing annually; it was valued at approximately US $26.0 billion in 2015 and is expected to exceed $37.0 billion in 2021, with an annual compound growth rate of 6.4% and predicted growth to $33.5 billion by 2019.1 This sector is not only a multi-billion dollar global market but also a source of scientific development and constant innovation. Esters of aroma are currently obtained by two major processes: chemical synthesis or extraction from natural sources. The classic process for producing esters is by acid catalysis, in which a Bronsted acid is typically used as a catalyst. The fatty acid residues and catalyst can be removed by an alkaline treatment which further increases the environmental impact by generating a certain load of non-biodegradable waste besides having a high energy production cost.2 Industrial interest in clean technologies is increasing, especially in engineering of proteins and enzymology. The use of lipases in industry allows for the development of technological processes that are very close to the efficient processes executed in nature due to the versatility of applications and ease of large-scale production. Energy savings and minimization of thermal degradation are probably the greatest advantages of replacing current chemical technologies by biological ones.3 An overview of the role of biocatalysts in the synthesis of various cosmetic esters and a discussion of the limitations of conventional biocatalytic synthesis of cosmetic esters are presented by Khan and Rathod.4 Lipases play an important role in the production of aromatic esters, with several advantages over synthetic routes. Study of the process parameters and their interaction is very important to understand the optimization of the system and achieve the maximum reaction yield for scaling up.5 Although the industrial applications of lipases are currently concentrated in the detergent and food industries, mostly based on hydrolysis reactions, new applications resulting from the synthesis reactions and transesterification according to the features of enantiomers and regioselectivity of lipases come settling in various fields: pharmaceuticals, fine chemicals, cosmetics, oleochemical, leather, pulp and paper, and the treatment of industrial waste.6-8 The natural synthesis of esters in solvent-free systems can be defined as solvolysis, a reaction in which the reactant itself acts as a solvent. Various investigations are focused on the study of enzymatic esterification in systems free of organic solvents.9-14 Esters of fatty acids of low molar mass from acyclic terpene alcohols, such as geraniol, linalool, and citronellol, are important compounds for the fragrance and flavor industries. The acetate groups occur in large amounts in essential oils, but low quantities have been verified for formates, propionates, and butyrates.15 Geranyl butanoate (3,7-dimethylocta-2,6-dien-1-yl butanoate) is one of the most frequently used terpenoid fragrance materials and has a flowery rose-like composition that does not discolor soaps. This ester is frequently used in perfumes and cosmetics such as lipsticks with or without the conventional ones. In flavor (for rose, orange oil, and lemon type) compositions, geranyl butanoate is used in small quantities to accentuate citrus notes. The geraniol molecule possesses a double bond, and its esterification with a fatty acid in the presence of a strong catalyst such as a mineral acid requires protection and deprotection steps. Alternatively, the synthesis of geranyl butanoate from geraniol and butanoic acid can be achieved by an immobilized biocatalyst (lipase), which can be easily separated from the reaction mixture.16 Geranyl butanoate has been produced in yields between 85 and 99.9% using free or immobilized lipases;16-18 however, there is a gap in the literature on the use of homemade polyurethane immobilized enzymes for this synthesis. Based on the aspects mentioned above, the main objective of this work was to investigate the solvent-free enzymatic production of geranyl butanoate using geraniol and butanoic acid as substrates. A kinetic study was also performed to assess the influence of the substrates' molar ratio, enzyme concentration, and temperature on reaction conversion. Two immobilized lipases (a commercial one and a homemade one) were used with the purpose of reducing the cost of the process (at least tenfold less), maintaining a high yield when obtaining the product.
EXPERIMENTAL Materials Geraniol (Vetec, 97% purity) and butanoic acid (Quimex, 97% purity) were used as substrates for the esterification reactions. Molecular sieves of 4 Å were purchased from Sigma-Aldrich. The commercial lipase used in this work was Novozyme 435 from Candida antarctica immobilized on an acrylic resin, kindly donated by Novozymes, Brazil (Araucária, PR, Brazil), and Novozyme NZL-102-LYO-HQ from Candida antarctica B (Cal B) immobilized in a homemade support based on polyol and isocyanate, both from Mannes (Sao Paulo, SP, Brazil). Immobilization of Novozyme NZL-102-LYO-HQ Immobilization of Novozyme NZL-102-LYO-HQ in polyurethane foam (Cal-B PU) was performed using 6 mL of polyol and 4 mL of isocyanate (60-40% v/v), with 2 mL of enzymatic solution (0.8 g of free enzyme and 5 mL of distilled water).19 In situ polymerization was conducted at 20 ºC until the growth of PU foam (5 min), which was kept stationary for 3 h and then crushed, giving a homogeneous product. The enzyme leaching from the support was evaluated by measuring the esterification activity of the wash solution using the methodology described below. Preliminary kinetic evaluation of geranyl butanoate production To determine the reaction time for the study of the synthesis of geranyl butanoate, a preliminary kinetic experiment was performed under the experimental optimized conditions defined by Paroul et al. using both Novozyme 435 and Cal-B PU as catalysts.20 The reaction mixture of geraniol, butanoic acid (alcohol to acid molar ratio of 3:1), enzyme (concentration of 5 wt%, based on the total amount of substrates), and molecular sieve (20 mg mL-1 of substrates) was kept under mechanical agitation at 150 rpm and 50 °C during 6 h of reaction. Aliquots of 100 µL were withdrawn from the reaction medium at 0, 0.5, 1, 1.5, 2, 3, 4, 5, and 6 h. An experiment without addition of enzyme was also carried out using only the substrates and molecular sieve. Experimental procedure for enzymatic esterification The esterification reactions were performed by preparing a reaction mixture of geraniol, butanoic acid (at molar ratios employed in the experimental design), and molecular sieves (20 mg mL-1 of substrates) in a 50-mL Erlenmeyer flask. After complete dissolution of the substrates, the enzyme was added to the mixture. All experiments were carried out in a closed system in an orbital shaker at constant agitation of 150 rpm. After completion of the reaction time, the biocatalyst was filtered for later measurement of enzyme activity and samples were kept at 5 ºC for further analysis and determination of the reaction conversion. Optimization of geranyl butanoate production To determine the experimental conditions that optimize the synthesis of geranyl butanoate, a 23 central composite rotatable design (CCRD) was carried out. Eleven runs were performed, varying the substrate molar ratio (0.36:1 to 6.36:1), enzyme concentration (0.025 to 13.06 wt%), and temperature (16.4 to 83.4 °C) for Novozyme 435 and Cal-B PU. The planning was executed with a triplicate central point to assess the error associated with the process. The software Statistica 5.0 (Statsoft Inc., USA) was used to assist the design and statistical analysis of experimental information, adopting in all cases studied a confidence level of 95% (p < 0.05). Kinetic evaluation of enzymatic production of geranyl butanoate After optimizing the experimental conditions, reaction kinetic experiments were performed with alcohol to acid molar ratios of 1:1, 5:1, 10:1, and 15:1, enzyme concentrations of 0, 5, 10, and 15 wt% (by weight of substrates), and temperature ranging from 40 to 70 °C for Novozyme 435 and Novozyme NZL-102-LYO-HQ immobilized in polyurethane. In all cases, destructive experiments, without sampling, were carried out at times of 0.25-3 h. The initial reaction rate was determined by Equation 1 as:21  where v is the initial reaction rate (g g-1 h-1), M is the mass of converted substance (g), Mc is the mass of catalyst (g), and t is time (h). Determination of reaction conversion Quantitative analyses of geranyl butanoate produced were conducted in a gas chromatograph (Shimadzu GC-2010) equipped with a data processor using an Inowax capillary column of fused silica (30 m length x 250 µm i.d. x 0.25 µm thickness), flame ionization detector, with the following temperature program: 40-180 ºC (3 ºC/min), 180-230 ºC (20 ºC min-1), and 230 ºC (20 min) with an injector temperature of 250 ºC, the detector at 275 ºC, injection in the split mode, and ratio of split of 1:100. H2 (56 KPa) was used as carrier gas and a volume of 0.4 µL of sample diluted in n-hexane (1:10) was injected. Reagent retention times were 20.8 and 26.5 min. for butanoic acid and geraniol and 28.0 min. for the geranyl butanoate product. The reaction conversion was calculated based on the reduction of the area of limiting reagent on the basis of reaction stoichiometry.20
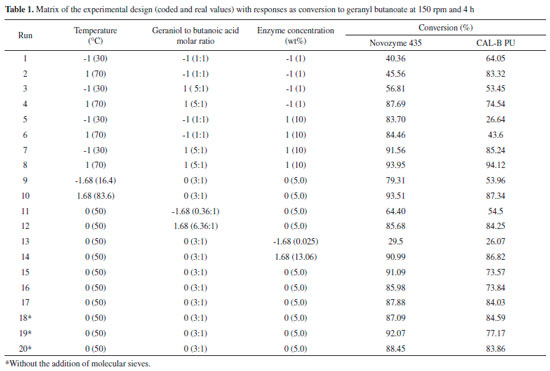
RESULTS AND DISCUSSION Preliminary kinetic evaluation of geranyl butanoate production To determine the reaction time to maximum production of geranyl butanoate using both immobilized lipases, a preliminary kinetic experiment was carried out at the experimental conditions optimized by Paroul et al.: 50 °C, a geraniol to butanoic acid molar ratio of 3:1, and enzyme concentration of 5 wt%.20 The results obtained are presented in Figure 1, where one can verify conversions above 90% after 2 h for Novozyme 435 and 80% after 4 h for Cal-B PU.
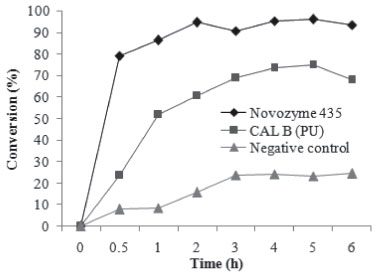 Figure 1. Preliminary kinetics of geranyl butanoate production using Novozyme 435, Cal-B PU, and control (without enzyme) (50 °C, geraniol to butanoic acid molar ratio of 3:1, and enzyme concentration of 5 wt%
The reaction carried out without catalyst led to a maximum conversion of 20% geranyl butanoate after 3 h of reaction. Taking into account the results presented in this figure, the reaction time was kept constant at 4 h in the next step. In the production of geranyl oleate, the results of Paroul et al. showed conversions higher than 90% after 1 h of reaction in a solvent-free system under conditions of 40 ºC, 10 wt% of Novozym 435, and a geraniol to oleic acid molar ratio of 1:5.12 Optimization of geranyl butanoate production To assess the effects of the alcohol to acid molar ratio, enzyme concentration, and temperature on geranyl butanoate production, a 23 CCRD with a center point triplicate was adopted. The matrix of the experimental design with coded and real values and the response in terms of geranyl butanoate production is presented in Table 1. To evaluate the effect of molecular sieves used for absorption of water produced during the reaction, three additional experiments were conducted under the condition of the central point of the experimental design without the use of enzyme. Higher yields (~95%) were obtained at 70 °C, an alcohol to acid molar ratio of 5:1, and an enzyme concentration of 10 wt% (Table 1, experiment 8) for both enzymes.
Temperature plays an important role in reaction systems, firstly due to the increase in the number of collisions among the substrates and biocatalyst and secondly due to the fact that enzymes have an optimum temperature of action: between 40 and 70 °C for Novozyme 435 and Novozyme NZL-102-LYO-HQ.22 The alcohol to acid molar ratio is another important parameter for the esterification reversible reaction. An increase in the amount of one of the substrates shifts the chemical equilibrium in the direction of product formation and will result in an increase in reaction conversion. High alcohol concentration (nucleophilic/acyl receptor) generally leads to higher conversions due to the availability of nucleophilic excess to acid transfer. On the other hand, as cited by Güvenç et al., an excess of nucleophilic above critical levels can result in lower initial rates.23 This can indicate enzyme inhibition as a function of the bonds between alcohol molecules and the active site of the enzyme. This effect was observed in run 12 of Table 1. Related to the enzyme concentration, higher conversions were achieved using 10 wt% of both Novozyme 435 and Novozyme NZL-102-LYO-HQ immobilized in polyurethane (run 8, Table 1). Higher levels of enzyme did not lead to increases in reaction conversion (run 14, Table 1). Runs 18, 19, and 20 (Table 1) were performed without adding molecular sieves. No significant differences were found on comparing runs 15-17 and 18-20 for both enzymes. The absence of effect of the sieves may be linked to the evaluated experimental condition (central point) which uses excess of one of the reagents, with this effect being more significant than that of the water in the ester hydrolysis, favoring the displacement of the chemical equilibrium toward the formation of the geranyl butanoate. According to Halting et al.,24 the amount of molecular sieve usually differs for each molar ratio. Bartling et al. studied the synthesis of geranyl acetate in n-hexane catalyzed by a commercial lipase from Candida antarctica.25 The equilibrium conversion was 94% for a substrate molar ratio of 1:1 and 30 °C. For selective removal of water produced during the esterification reaction, the authors tested membranes with ceramic/cellulose acetate composites, which allowed the reaction to be shifted towards the products, reaching a conversion of 100%. The results for geranyl butanoate conversions using both Novozyme 435 and Cal-B PU (Table 1) were treated statistically. After 4 h of reaction, all variables presented significant effects (p < 0.05) on the production of geranyl butanoate with Cal-B PU catalyst but the coded model was not validated for variance analysis. Regarding the Novozyme 435, after the statistical analysis of the results obtained in the experimental design, an optimized coded model for geranyl butanoate production was validated. The analysis of variance (ANOVA) led to calculated values of the F test for regression higher than the listed one and an R squared (coefficient of determination) value of 0.97. This implies a very satisfactory representation of the process by the empirical model, within the range evaluated for each variable. The coded model fitted by the regression analysis is given by Equation 2. 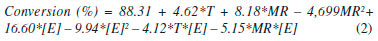 where conversion (%) denotes the reaction conversion to geranyl butanoate (%), MR is the geraniol/butanoic acid molar ratio, T is temperature, and [E] represents the enzyme concentration (wt%). The model expressed by Equation 2 shows that the linear effect of temperature, molar ratio, and enzyme concentration presented significant positive effects (p < 0.05) and the interactions between temperature and enzyme concentration and between molar ratio and enzyme concentration showed negative effects on geranyl butanoate conversion. This model had good ability to represent the experimental data of conversion of geraniol and butanoic acid in the ranges of variables investigated here, with a high correlation between predicted and observed conversions (y = 0.967x + 2.973; R2 = 0.963) and the response surface shown in Figure 2.
 Figure 2. Response surface contour for geranyl butanoate production using Novozyme 435 as a function of temperature and enzyme concentration (a), molar ratio and enzyme concentration (b), and molar ratio and temperature (c)
As we can observe in Figure 2(a), when using a Novozyme 435 concentration from 9 to 1 wt%, it is possible to obtain maximal conversions using even lower temperatures (25 to 40 °C), leading to considerable energy savings during the esterification process. The effects of varying the molar ratio and enzyme amount on esterification are shown in Figure 2(b). Better responses in terms of conversion were obtained for enzyme concentrations from 7 to 10 wt% and geraniol to butanoic acid molar ratio of 4:1-5:1. Figure 2(c) shows that better conversions as a function of molar ratio and temperature were obtained at higher temperature (70 °C) and higher alcohol excess (5:1-6:1). Some works about the enzymatic esterification of monoterpene alcohols in solvent-free and organic systems can be found in the literature.9,13,25-29 The synergism of microwave irradiation and enzyme catalysis in the transesterification of ethyl cinnamate and geraniol was investigated by Shinde and Yadav.22 The effects of different operating parameters such as biocatalyst, solvent, and temperature were studied. The optimal conditions obtained via the Taguchi approach were as follows: an enzyme loading of 60 mg, temperature of 65 °C, speed of agitation of 300 rpm, and substrate molar ratio of 1:2. The analysis of the initial rate data established the validity of the ternary complex ordered bi-bi mechanism with inhibition by geraniol. Direct esterification of tertiary alcohol (α-terpineol) with acetic anhydride was investigated by Liaw and Liu using immobilized lipases from Candida rugosa type VII, Amano PS, Amano AP-6, Amano G, and Lipozyme RM IM as catalysts in supercritical CO2 and n-heptane as co-solvent.27 A terpenyl acetate yield of 53.1% were obtained after 1.5 h of reaction at 50 °C and 10 MPa. Data from the initial reaction rate also showed that the biocatalysis followed the ping-pong bi-bi mechanism.5 Kinetic evaluation of enzymatic production of geranyl butanoate using Novozyme 435 and Novozyme NZL-102-LYO-HQ in polyurethane foam The effects of the substrate molar ratio, temperature, and enzyme concentration on the kinetics of geranyl butanoate production were investigated. As presented earlier, the execution of experimental designs with the abovementioned variables revealed the achievement of high conversions in 4 h of reaction at 70 °C with a substrate molar ratio of 5:1 and 10 wt% of enzyme. It may be important to mention that the kinetic results subsequently presented in this work are in fact mean values of triplicate runs, which resulted in an overall absolute deviation in terms of reaction conversion of around 5%. Data scattering observed in the kinetic esterification data may be explained in terms of associated experimental errors and the fact that destructive experiments were carried out without sampling, which may be viewed as an important test of the internal consistency of the results. Thus, the good behavior verified for the conversion versus time curves may ensure the reliability of the experimental measurements reported in the present work. Effect of geraniol to butanoic acid molar ratio In order to evaluate the effect of the geraniol to butanoic acid molar ratio on geranyl butanoate production, the conditions were set at a fixed temperature of 70 ºC, Novozyme 435 and Cal-B PU enzyme concentration of 10 wt%, and agitation speed of 150 rpm, making is possible to build conversion versus time curves. The initial rate of substrate consumption (g g-1 h-1) during geranyl butanoate production was also determined (Equation 1). Figure 3(1) presents the effect of the geraniol to butanoic acid molar ratio (1:1, 5:1, 10:1) (1) on the kinetics of geranyl butanoate production and on the initial rate (g g-1 h-1) for Novozyme 435 and Cal-B PU, respectively.
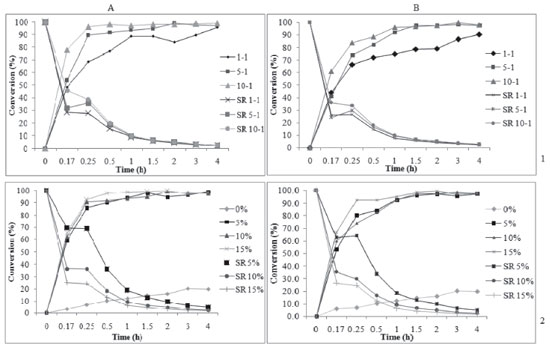 Figure 3. Effect of substrate molar ratio (mol geraniol: mol butanoic acid) (1) and concentrations of Novozyme 435 and Cal-B PU (2) on the production of geranyl butanoate and initial rate of consumption of substrate using Novozyme 435 (a) and Cal-B PU (b) under conditions of 70 ºC, an enzyme concentration of 10 wt%, and agitation speed of 150 rpm
The maximum geranyl butanoate conversion (~100%) was obtained after 30 minutes of reaction at a molar ratio of 10:1 and after 2 h for a ratio of 5:1 using Novozyme 435. The initial rates decreased with time from 28.37, 31.64, and 45.85 g g-1 h-1 after 5 min of reaction to 2.39, 2.43, and 2.48 g g-1 h-1 at 3 and 4 h for molar ratios of 1:1, 5:1, and 10:1, respectively (Figure 3 (1 a)). For Cal-B PU (Figure 3 (1 b)), the maximum conversion (96%) was reached after 1.5 h at molar ratios of 5:1 and 10:1. The initial rate decreased from 25.9, 24.11, and 33.8 g g-1 h-1 in the first 5 min to 2.26, 2.43, and 2.45 g.g-1·h-1 at 3 and 4 h for molar ratios of 1:1, 5:1, and 10:1, respectively. Data from the literature suggest the existence of a limit of maximum nucleophilic concentration until it presents a positive effect on the reaction conversion, favoring the product formation.11,13,23,30 The authors pointed out a decrease in the esterification conversion when a high excess of one of the substrates was used. A possible reason for this behavior is that at high concentration, the alcohol is bound to the active site of enzyme molecules by reversible competition and prevents the binding of ester molecules. The driving force for alcohol binding might be high polarity of the region around the active serine site of the enzyme.19,31,32 Effect of enzyme concentration The effect of enzyme concentration on geranyl butanoate conversion was evaluated at 70 ºC, keeping the alcohol to acid ratio and agitation speed constant at 5:1 and 150 rpm, respectively, while varying the enzyme concentration between 0, 5, 10, and 15 wt%. The kinetic curves and specific rate obtained in this step for Novozyme 435 and Cal-B PU are presented in Figures 3(a) and (b), respectively. When an enzyme loading of 15 wt% was used, high conversions (98%) were achieved in short reaction times (30 minutes). After 1 h of reaction, a correlation between enzyme concentration and conversion was not observed, and 100% product formation was reached using 5, 10, and 15 wt% of both enzymes. A possible explanation for this is the hypothesis that an excess of enzyme in the reaction medium cannot provide an increase in the conversion through the formation of aggregates and nonhomogeneous distribution of enzyme. The molecules of enzyme on the external surface of these agglomerates are exposed to a high concentration of substrates, but the mass transfer inside the particles of biocatalyst can drastically limit the concentration of substrates inside the particles.8 The experiment carried out without catalyst led to conversions of about 20% after 3 h of reaction. The specific rates presented a similar behavior, decreasing with time from 69.41, 36.47, and 25.49 g g-1 h-1 after 5 min to 4.94, 2.43, and 1.64 g g-1 h-1 after 4 h of reaction at enzyme concentrations of 5, 10, and 15 wt% Novozyme 435, respectively (Figure 3(a)). Cal-B PU presented a similar behavior (Figure 3(b)). Effect of temperature In order to evaluate the effect of temperature (40 and 70 ºC) on the geranyl butanoate production and specific rate, the molar ratio of alcohol to acid, enzyme concentration, and agitation speed were fixed at 5:1, 10 wt% (Novozyme 435 and Cal-B PU), and 150 rpm, respectively, making it possible to follow the time-course of reaction conversion, as presented in Figures 4(a) and (b).
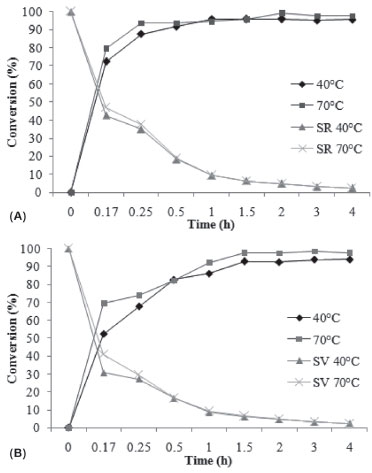 Figure 4. Kinetics of geranyl butanoate production using Novozyme 435 (A) and Cal-B PU (B) at different temperatures with an alcohol to acid ratio of 5:1, enzyme concentration of 10 wt%, and agitation speed of 150 rpm
From Figure 4(a) it can be observed that at 40 ºC, high conversions of about 70% were achieved after 15 minutes of reaction. After 1 h of reaction, a maximum conversion of ~95% was achieved at all temperatures. The specific rates obtained with Novozyme 435 decreased from 42.53 and 47.82 (5 min) to 2.3 and 2.4 (4 h) at 40 and 70 °C, respectively (Figure 4a). With Cal-B PU (Figure 4(b)), the temperature presented a more pronounced effect on the reaction kinetic. The maximum conversion (~94%) was obtained at higher temperature (70 °C) after 1 h of reaction. The same effect of temperature was observed by Paroul et al. in the production of geranyl propionate in solvent-free system.20 The operating conditions that optimized geranyl propionate production were determined to be 40 ºC, a geraniol to propionic acid molar ratio of 3:1, agitation speed of 150 rpm, and enzyme concentration of 10 wt% (Novozym 435), affording nearly complete reaction conversion (93%) after 30 min of reaction. A similar study was carried out by Karra-Chaabouni et al.33 The factors affecting the synthesis of geranyl butyrate by esterase 30.000 of Mucor miehei were studied in a solvent-free system. The effects of substrate molar ratio, temperature, agitation speed, and initial addition of water were investigated. The equimolar ratio was most interesting for ester production in batch. There were no diffusion limitations and the reaction could be performed at low agitation speed. The catalytic activity of the enzyme was irreversibly deactivated at 60 °C and the initial addition of water decreased the rate of conversion after 75 h of reaction. Microbial lipase produced by Rhizopus sp. was tested by Melo et al. for the synthesis of citronellyl butyrate and citronellyl valerate.9 Lipase showed more ability to catalyze the synthesis of citronellyl butyrate. This process was optimized using response surface methodology (RSM) based on a five-level, two-variable CCRD. The optimum conditions were a molar ratio of 2.41:1 (alcohol: acid) and lipase amount of 6.12% (w/w) and the yield achieved was 100% (molar conversion) in 48 h. Lipase was also immobilized onto celite and its activity improved, leading to 100% molar conversion in 24 h. From the results obtained previously and bearing in mind the aim of reducing energy costs during the production of geranyl butanoate, a kinetic study was performed at 40 °C with 5 wt% of Novozyme 435 and Cal-B PU (Figures 5(a) and (b), respectively). From Figure 5(a) one can observe that the best conditions for Novozyme-435-catalyzed geranyl butanoate production (> 97%) were defined as a geraniol to butanoic acid molar ratio of 3:1, temperature of 40 °C, enzyme concentration of 5 wt%, and 1 h of reaction.
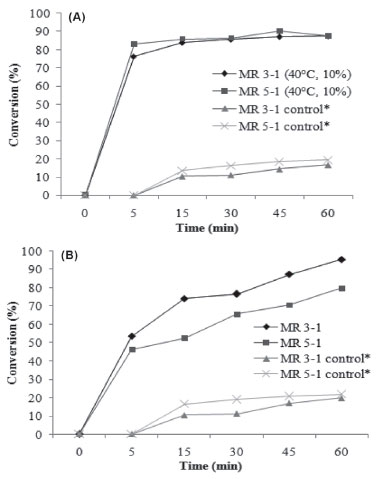 Figure 5. Kinetics of geranyl butanoate production using Novozyme 435 (A) and Cal-B PU (B) at a temperature of 40 °C, alcohol to acid ratios of 5:1 and 3:1, enzyme concentration of 5 wt%, and agitation speed of 150 rpm. Experiments marked with * were carried out without the addition of enzyme
Using Cal-B PU as catalyst, conversions of about 95% were achieved for higher temperatures and lower enzyme concentrations. The experimental condition that maximized geranyl butanoate production was defined as 70 °C, a geraniol to butanoic acid molar ratio of 3:1, 5 wt% of Cal-B PU, and 1 h of reaction (Figure 5(b)). A control experiment under this condition without the use of enzyme led to a maximum conversion of 20% for molar ratios of 5:1 and 3:1.
CONCLUSIONS The effects of temperature, substrate molar ratio, and enzyme concentration were evaluated in the process of enzymatic esterification of geraniol and butanoic acid in a solvent-free system. Two lipases were tested: commercial Novozym 435(r) and homemade Novozyme NZL-102-LYO-HQ immobilized in polyurethane. The experimental conditions that optimized the geranyl butanoate production were a geraniol to butanoic acid molar ratio of 3:1, 5 wt% of Novozyme 435, and temperature of 40 ºC and secondly a substrate molar ratio of 5:1, 5 wt% of Cal-B PU, and temperature of 70 ºC. Conversions higher than 95% obtained with 5% of both enzymes and the low cost of homemade immobilized lipase, shows the potential of the proposed method of immobilization.
ACKNOWLEDGMENTS The authors thank CAPES, CNPq and FAPERGS for the financial support of this work and scholarships.
REFERENCES 1. BBC Research, CHM034E, 2016, available at from http://www.bccresearch.com/market-research/chemicals/flavors-fragrances-markets-report-chm034e.html, accessed July 2018. 2. Aragao, V. C.; Anschau, A.; Porciuncula, B. D. A.; Thiesen, C.; Kalil, S. J.; Burkert, A. A. V.; Medeiros Burkert, J. F.; Quim Nova 2009, 32, 2268. 3. Hasan, F.; Shah, A. A.; Hameed, A.; Enzyme Microb. Technol. 2006, 39, 235. 4. Khan, N. R.; Rathod, V. K.; Process. Biochem. 2015, 50, 1793. 5. Sá, A. G. A.; Meneses, A. C.; Araújo, P. H. H.; Oliveira, D.; Trends Food Sci. Technol. 2017, 69, 95. 6. Hoang, H. N.; Matsuda, T.; Tetrahedron 2016, 72, 7229. 7. Berger, R. G.; Biotechnol. Lett. 2009, 31, 1659. 8. Karra-Chaabouni, M.; Ghamghi, H.; Bezzine, S.; Rekik, A.; Gargouri, Y.; Process Biochem. 2006, 41, 1692. 9. Mello, L. L. M. M; Pastore, G. M.; Macedo, G. A.; Process Biochem. 2005, 40, 3181. 10. Mahapatra, P.; Kumari, A.; Garlapati, V. K.; Nag, A. B.; J. Mol. Catal. B: Enzym. 2009, 60, 57. 11. Richetti, A.; Leite, S. G. F.; Antunes, O. A. C.; Souza, de A. L. F.; Lerin, L. A.; Dallago, R. M.; Paroul, N.; Di Luccio, M.; Oliveira, J. V.; Treichel, H.; Oliveira, D.; Appl. Biochem. Biotechnol. 2010, 160, 2498. 12. Paroul, N.; Grzegozeski, L. P.; Chiaradia, V.; Treichel, H.; Cansian, R. L; Oliveira, J. V.; Oliveira, D.; Bioprocess Biosyst. Eng. 2011, 34, 331. 13. Vanin, A. B.; Orlando, T.; Piazza, S. P.; Puton, B. M. S.; Cansian, R. L.; Oliveira, D.; Paroul, N.; Appl. Biochem. Biotechnol. 2011, 174, 1286. 14. Lerin, L. A.; Catani, M.; Oliveira, D.; Massi, A.; Bortolini, O.; Cavazzini, A.; Giovannini, P. P.; RSC Adv. 2015, 5, 76898. 15. Surburg, H.; Panten, J.; Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th ed.,Wiley-VCH: Weinheim, 2006. 16. Kanwar, S. S.; Gehlot, S.; Verma, M. L.; Gupta, R.; Kumar, Y.; Chauhan, G. S.; J. Appl. Polym. Sci. 2008, 110, 2681. 17. Shieh, C.-J.; Akoh, C. C.; Yee, L. N. Biotechnol. Bioeng. 1996, 51, 371. 18. Damnjanović, J. J.; Žuža, M. G.; Savanović, J. K.; Bezbradica, D. I.; Mijin, D. Z.; Bošković-Vragolović, N.; Knežević-Jugović, Z. D.; J. Mol. Catal. B: Enzym. 2012, 75, 50. 19. Nyari, N. D.; Fernandes, I. A.; Bustamante-Vargas, C. E.; Steffens, C.; Oliveira, D.; Zeni, J.; Rigo, E.; Dallagoa, R. M.; J. Mol. Catal. B: Enzym. 2016, 124, 52. 20. Paroul, N.; Grzegozeski, L. P.; Chiaradia, V.; Treichel, H.; Cansian, R. L.; Oliveira, J. V.; Oliveira, D.; J. Chem. Technol. Biotechnol. 2010, 85, 1636. 21. Hagen, J.; Industrial catalysis: A practical approach, 3rd ed., Wiley-VCH: Weinheim, 2006. 22. Shinde, S. D.; Yadav, G. D.; Appl. Biochem. Biotechnol. 2015, 175, 2035. 23. Güvenç, A.; Kapucu, N.; Mehmetoglu, Ü.; Process Biochem. 2002, 38, 379. 24. Halting, P. J.; Biotechnol. Bioeng. 1990, 35, 691. 25. Bartling, K.; Thompson, J. U. S.; Pfromm, P. H.; Czermak, P.; Rezac, M. E.; Biotechnol. Bioeng. 2001, 75, 676. 26. Shih, I. L.; Hung, S. H.; Chen, F. Y.; Ju, H. Y.; Shieh, C. J.; Food Chem. 2007, 100, 1223. 27. Liaw, E. T.; Liu, K. J.; Bioresour. Technol. 2010, 101, 3320. 28. Dhake, K. P.; Deshmukh, K. M.; Patil, Y. P.; Singhal, R. S.; Bhanage, B. M.; J. Biotechnol. 2011, 156, 46. 29. Sun, J.; Yu, B.; Curran, P.; Liu, S. Q.; Food Chem. 2012, 135, 2714. 30. Chiaradia, V.; Paroul, N.; Cansian, R. L.; Júnior, C. V.; Detofol, M. R.; Lerin, L. A.; Oliveira, J. V.; Oliveira, D.; Appl. Biochem. Biotechnol. 2012, 168, 742. 31. Puskas, J. E.; Chiang, C. K.; Sen, M. Y. In Green polymerization methods: renewable starting materials. Catalysis and waste reduction; Mathers, R. T., Meier, M. A. R., eds.; Wiley-VCH: Weinheim, 2011. 32. Yadav, G. D.; Devendran, S.; Process Biochem. 2012, 47, 496. 33. Karra-Chaâbouni, M.; Pulvin, S.; Thomas, D.; Touraud, D.; Kunz, W.; Biotechnol. Lett. 2002, 24, 1951. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access