Artigo
| Characterization of CeO2 doped MgAl2O4 prepared by the chelating agents-assisted impregnation method |
|
Alejandra C. Villagrán-Olivares; Mariana N. Barroso; Maria C. Abello*
Instituto de Investigaciones en Tecnología Química (INTEQUI-CONICET-UNSL), Almirante Brown 1455, 5700, San Luis, Argentina Recebido em: 16/04/2018 *e-mail: cabello@unsl.edu.ar MgAl2O4 spinel employed as support of reforming catalysts was doped by CeO2. The samples were prepared by wet impregnation from aqueous solutions of cerium nitrate, with and without chelating agents. The chelating agents used were ethylenediamine-tetraacetic acid (EDTA), nitrilotriacetic acid (NTA) and citric acid (CA) at different pH of impregnation. The solids were characterized by TG, XRD, SBET, TPR, SEM-EDX and CO2-TPD. Nanoparticles of CeO2 between 5 and 6.8 nm were obtained by the thermal decomposition in air at 600 °C. The surface cerium reduction occurred in a wide temperature range of 450° to 670 °C. The use of citric acid at pH= 8 led to the lowest crystallite size of CeO2 and the lowest interaction with the spinel. The H2 consumption and extent of CeO2-support interaction did not show a clear dependence with the CeO2 crystallite sizes. The use of chelating agents in the impregnation step did not modify the total basicity. Slight changes in the strength of weak basic sites were observed. INTRODUCTION The support employed in catalysts preparation usually affects the activity, selectivity and stability. In reforming catalysts, the support should fulfill with different requirements (i) favor the water dissociation in OH groups and their migration to the metallic particle; (ii) promote mainly the dehydrogenation reaction instead of the dehydration to mitigate the coke deactivation; and (iii) contribute to the metallic particles stabilization keeping the dispersion as high as possible during long reaction times. Complex oxides, spinel type (AB2O4, with a close-packed oxygen ion structure), have been employed as supports in many catalytic systems due to their lower acidity compared with Al2O3 and their high resistance to sintering and coking.1,2 In particular, the Mg spinel has shown to be a very good alternative to support Co and/or Ni for catalysts employed in the ethanol steam reforming reaction.3,4 The support can be doped with different additives which are incorporated for instance to change the metal-support interaction, the acid-base properties and to increase tolerance to sintering and coke. CeO2 is considered a powerful promoter to modify the structural and electronic properties of catalysts. It has been widely used due to the attractive acid-base and redox properties. Ceria could affect the thermal and structural stability of catalytic support and the dispersion of the active phase. Besides, as a consequence of the well-known capacity to deliver and store oxygen and to the presence of Ce3+/Ce4+ couple in oxidant atmospheres, CeO2 increases the tolerance to carbon deposition. The addition of CeO2 on Ni/MgAl2O4 catalysts has shown a positive effect for increasing the carbon deposition resistance.4 The deactivation in the ethanol steam reforming is a great inconvenience for technological applications. The influence of CeO2 addition will depend on its loading and the synthesis method, which affects the metallic dispersion and reducibility of active phase.5 In the literature, the use of chelating agents has been reported in the preparation of hydrodesulfurization catalysts,6,7 of reforming reactions8-11 and for the Fischer-Tropsch synthesis,12,13 among others. The use of aqueous solutions of chelated metal complexes in the wet impregnation method is very interesting, as it maintains the simplicity of the technique; it allows a high reproducibility of synthesis, uniform distributions of active phase and higher metallic dispersions. One characteristic of these solutions is the increase of viscosity during solvent removal in the drying step, inhibiting the distribution of surface species and forming poorly crystallized compounds with high dispersion.14 Several factors should be taken into account in the preparation with chelating ligands; for instance, the pH of solution which determines the complexing ability, the point of zero charge (PZC) of the support,13 the nature of the ligand, the thermal precursor decomposition and the drying procedure. The pH of the solution plays a significant role in the complex compound formation, in the surface speciation on the support and in the control of metal-support interactions.15 The chelating agents most widely used in preparation of supported catalysts are: ethylendiaminetetraacetic acid (EDTA), nitrilotriacetic acid (NTA) and citric acid (CA). This class of ligands forms soluble and very stable complexes with a wide range of metals. Besides, the obtained precursors contain C, H, N or O, thereby avoiding any contamination. The thermal decomposition of the complexes is completed at moderate temperatures, and the residual carbon contamination on the support surface is negligible.16 The existence of a correlation between the Ce3+/Ce4+ surface ratio and the carbon amounts deposited in the ethanol steam reforming reaction over Ni/CeO2-MgAl2O4 catalysts was reported in previous works.17,18 The reduction of surface ceria was facilitated by the presence of Ni.19 This synergetic effect was observed upon establishment of interactions between dispersed nickel oxide and ceria oxide. The NiO-CeO2 interaction and concomitantly the Ce3+/Ce4+ surface ratio could increase with an enhancement in CeO2 dispersion (lower particle sizes). Considering the advantages of using chelating agents in the impregnation step of catalysts and the possibility of Ce to form complexes with NTA,20 EDTA21,22 and CA,23 in this work the properties of Mg spinel doped by Ce complexes were studied. As far as we know, it has not been reported the effect of using Ce complexes on the interaction with Mg spinel. The results presented are part of a larger study related to the development of Ni catalysts supported in MgAl2O4 for obtaining hydrogen from the reaction of ethanol reforming with steam.
EXPERIMENTAL Samples preparation The MgAl2O4 used as support was synthetized using the citrate method under the experimental conditions described in reference 4. The CeO2 addition (nominal loading 5 wt.%) was carried out by the chelating agents-assisted impregnation method. Three chelating agents: EDTA (Sigma-Aldrich, 99%), NTA (Sigma-Aldrich, 99%) or CA (Sigma-Aldrich, 99,5%) with a metal to ligand molar ratio 1:1 were used. Two types of aqueous solutions of Ce(NO3)3·6H2O (Aldrich, 99%) with chelating agents were prepared: (i) acid solutions obtained by the dissolutions of chelating ligands with the cerium salt (pH between 1.5-3, Table 1) and (ii) basic solutions obtained from those prepared in (i) by the NH4OH addition (pH between 8-9.4, Table 1). These complex aqueous solutions were added to 300 mg of MgAl2O4 spinel under stirring at room temperature. The solvent was removed by evaporation in a rotavapor at 75 °C under a pressure between 8-10 mmHg. The sample was dried in a vacuum oven at 100 °C overnight. The calcination was carried out in static air from room temperature to 600 °C at 10 °C min-1, and then 1 h at 600 °C. The ceria-doped spinel carriers were named as CeL(x) being L = EDTA, NTA or CA and x = a or b, if the impregnation solution was acid or basic, respectively. Then, CeCA(a) means the MgAl2O4 spinel doped with a Ce complex obtained from Ce(NO3)3 and citric acid at an acid pH. As reference, a sample of MgAl2O4 doped with CeO2 from an aqueous solution of Ce(NO3) (free of chelating agent) was also prepared. This sample was designated Cenitrate.
Characterization of samples Point of Zero Charge: PZC value of the spinel was determined using the salt addition method.24 The pH of 25 mL KNO3 0.1 M solutions was adjusted with KOH and HNO3 in a pH range of 2 to 12. The pH of each solution (pHinitial) was measured by a pHmeter (Lutron PH-230 SD with a Van London glass electrode). 25 mg of powder sample were suspended in each solution under vigorous stirring of 175 rpm for 24 h in a universal stirrer (Edmund Buhler KL-2). After that, the pH of each suspension was measured (pHend). Then, ΔpH (estimated as pHend - pHinitial) was plotted as a function of pHinitial. The pHinitial value at ΔpH = 0 was taken as the PZC. Chemical species in the impregnation solution: The distinct species present in the impregnation solutions were determined using Medusa software.25 Ce3+, the deprotonated chelate and the ionic strength were considered. Specific surface area: Specific surface areas of samples (SBET) were determined by the BET method from adsorption measurement of N2 at -196 °C, using a Micromeritics Gemini V. The samples were previously degassed at 300 °C for 16 h in a N2 flow. Six points were recorded until P/P0= 0.3. Values of C constant varied between 83 and 120. Thermogravimetric analysis: The decomposition of precursors was studied using a TGA 51 Shimadzu equipment. The weight loss was recorded under an air flow (50 mL min-1) from room temperature until 1000 °C at 10 °C min-1. X-ray diffraction: Diffraction patterns of samples, XRD, were obtained using a diffractometer Rigaku, D-Max III C, operated at 30 kV and 20 mA. A radiation CuKα (γ = 0.15418 nm) with a Ni filter and a horizontal goniometer were employed. Step-scans were taken over a range of 2θ from 10° to 70° in step of 3° min-1. The data were collected every 0.05°. The crystalline phase identification was made by matching with JCPDS files. The crystallite sizes of CeO2 were determined using the Scherrer equation for the (111) diffraction peak at 2θ = 28.6°. These calculations were estimated from patterns recorded between 2θ = 25.5° and 31.5° at 0.01° at a fixed time of 5 s, using a Si monocrystal. Temperature programmed reduction (TPR): The redox properties of samples were examined using a conventional reduction equipment operated with a thermal conductivity detector. Before TPR experiments, the samples were previously treated under an O2/He flow (36 mL min-1) at 300 °C for 1 h. After cooling in an He flow until 25 °C, the samples were reduced in an H2(5 vol.%)/N2 flow from room temperature to 700 °C at 10 °C min-1. Temperature programmed desorption of CO2 (CO2-TPD): The basic properties of ceria doped spinel were studied by CO2-TPD employing a conventional desorption equipment with a thermal conductivity detector. 80 mg of sample were previously reduced in an H2(5 vol.%)/N2 flow at 650 °C for 30 min. The adsorption was carried out in a pure CO2 flow for 1 h. After a purge stage in He, the desorption was performed under an He flow (30 mL min-1) from room temperature to 700 °C at 10 °C min-1. The total integrated area of the CO2 band was considered as a measure of total basicity. CO2 uptake expressed as µmoles CO2 per gcat or µmoles CO2 per m2 were estimated after CO2 calibration. Scanning electron microscopy and energy dispersive X-ray spectroscopy (SEM-EDX). SEM images were obtained in a LEO 1450 VP equipped with an energy dispersive X-ray microanalyzer (EDAX Genesis 2000). A Si(Li) detector was used. The fresh samples were coated with a gold film.
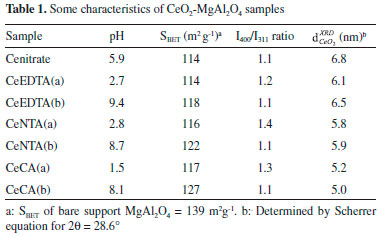
RESULTS AND DISCUSSION The diffraction pattern of Mg spinel obtained by the citrate method is shown in Figure 1. The peaks at 2θ = 19°, 31°, 37°, 45°, 56°, 59° and 65° were assigned to MgAl2O4 (JCPDS-21-1152). The XRD intensity ratio I400/I311 was 1.2. This value was higher than others in the literature (I400/I311= 0.65 for the pure stoichiometric spinel)26 and it is probably associated with defects in the spinel structure induced by the preparation method. The N2 adsorption-desorption isotherm (not shown) is type IV, characteristic of mesoporous materials with a hysteresis loop H1 type according to IUPAC classification. The specific surface area of prepared spinel was 139 m2 g-1. In the literature, values of specific surface area of 154 m2 g-1 (after a calcination at 800 °C) and 52 m2 g-1 (after a calcination at 900 °C) have been reported.27
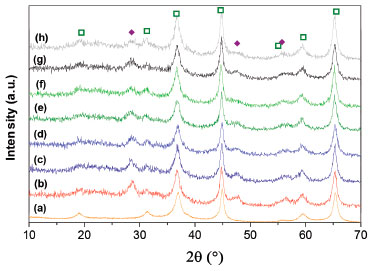 Figure 1. Diffraction patterns of calcined samples: a) MgAl2O4, b) Cenitrate, c) CeEDTA(a), d) CeEDTA(b), e) CeNTA(a), f) CeNTA(b), g) CeCA(a) and h) CeCA(b).  : MgAl2O4, : MgAl2O4,  : CeO2. The MgAl2O4 XRD was registered at 0.05° step fixed time of 5 s. : CeO2. The MgAl2O4 XRD was registered at 0.05° step fixed time of 5 s.
The spinel was doped by wet impregnation method using aqueous solutions of Ce complexes. The Ce3+ complexes in solution were obtained from different chelating agents and Ce(NO3)3. The main difference between the preparation with chelating agents and the reference sample (synthesized from an aqueous solution of Ce(NO3)3) is the nature of the impregnation ion, which is mainly anionic in the first case and cationic in the second one. For instance, Ce could be complexed with EDTA into the chelate [Ce(EDTA)]-. As it was mentioned, support-complex interactions depend on many factors as the isoelectric point of the support, the pH of the aqueous solutions and the nature of the impregnation reagents. PZC of prepared MgAl2O4 was determined by salt addition method. The ΔpH as a function of initial pH is shown in Figure 2. The PZC for the spinel was 9.4 at room temperature which is similar to others reported in the literature.28 This PZC value is between those of alumina and magnesium oxide and it is similar to PZC of Al2O3-(20%)MgO mixed oxides.28
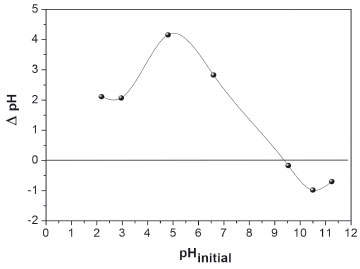 Figure 2. Determination of PZC value of MgAl2O4 spinel
The pH of impregnation solution used for preparing the ceria doped spinel was kept lower or equal to the spinel PZC to favor the absorption of the formed chelate, Table 1. Solutions with metal complex formed between Ce(NO3)3 and EDTA, NTA or CA were colorless except for the Ce-CA in a basic medium which was yellowish showing the complex formation. Figure 3 shows representative curves of TG analysis corresponding to dried samples. The weight loss until 100 °C indicates desorption of water whereas those until 600 °C could be attributed to decomposition/oxidation of the metal chelates and NO32- remain groups. The weight was almost constant from 600 °C. This temperature was selected as the appropriate one for the decomposition process, allowing the obtaining of supports with high surface areas with an acceptable residual carbonaceous contamination (as it was observed by FTIR, see Supplementary Material section). Besides, this temperature is in the temperature range where the ethanol steam reforming reaction is usually carried out. TGA showed weight losses of 16.8% for Cenitrate, 22.2% for CeEDTA(a) and 21.2% for CeCA(a). These values were lower than those expected considering possible structures of complex and the chelating nature. This behavior could be explained if the decomposition begins during the drying step. In fact, signals corresponding to CeO2 with fluorite structure (2θ = 28.6°, 33°, 47° and 56°, JCPDS-34-0394) were observed in the XRD pattern of a dried sample at 100 °C for 20 h in a vacuum (without calcination), Figure 4. Similar results have been reported by Daza et al. on Ni-containing Mg-Al mixed oxides obtained from hydrotalcite precursors and doped with different loads of [Ce(EDTA)]-.11
 Figure 3. Thermogravimetric analysis on samples after the drying step: a) Cenitrate, b) CeEDTA(a) and c) CeCA(a)
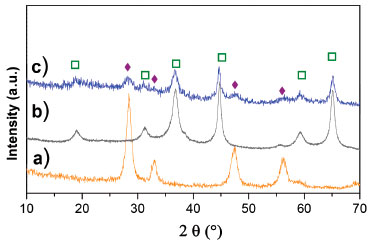 Figure 4. Diffraction patterns of dried Cenitrate sample (without calcinations). a) pure CeO2, b) MgAl2O4 (bare support), c) dried Cenitrate.  : MgAl2O4, : MgAl2O4,  : CeO2 : CeO2
SBET values corresponding to ceria doped spinel shown in Table 1 were high and did not present significant differences by changing the chelating agent. A decrease between 18% and 10% was observed after the addition of CeO2 as a dopant. SBET values are also not affected significantly by the pH change. Damyanova et al.29 have reported a decrease in the specific surface area around 15% for a CeO2(12 wt.%)/Al2O3 sample due to the plugging of the pores with cerium oxide species. In Figure 1, the diffraction patterns of calcined samples are shown. After the thermal decomposition in air the XRD reveal peaks corresponding to MgAl2O4 and CeO2. From the analysis of CeO2 characteristic peak at 2θ = 28.6°, moderate changes in crystallite sizes of CeO2 could be inferred due to the use of chelating agents in the impregnation step. The In Figure 5, the TPR profiles are shown. All samples exhibited broad peaks of consumption in the temperature range of 450 to 670 °C, assigned to the Ce4+ surface reduction to Ce3+. As it was expected, the reduction profile of spinel was flat in the used temperature range in TPR. It is known that surface Ce4+ species are reduced at lower temperature than the one predicted by thermodynamic for bulk CeO2 and its reduction is affected by the textural and morphological properties of oxide.30 The maximum temperatures in TPR considered as an indirect measurement of CeO2-spinel interaction extent are shown in Table 2. The support prepared by impregnation without chelating agent showed a Tmax= 570 °C. This temperature was changed between 538- 607 °C for the other samples. The sample prepared in the presence of citric acid at a basic pH presented the highest reducibility. The reducibility and H2 consumption did not show a clear dependence with the crystallite size,
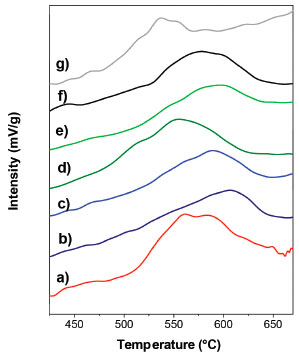 Figure 5. Temperature programmed reduction profiles: a) Cenitrate, b) CeEDTA(a), c) CeEDTA(b), d) CeNTA(a), e) CeNTA(b), f) CeCA(a) and g) CeCA(b)
The maximum temperature in TPR is higher when the species are anionic indicating a stronger interaction with the spinel whereas the temperature is lower in neutral and cationic species, in agreement with the PZC of MgAl2O4. The basic properties were investigated by CO2 desorption. The profiles of CO2-TPD illustrated in Figure 6 are qualitatively similar with a wide desorption peak in the temperature range of 25-700 °C. They suggest a heterogeneous distribution of basic sites, with predominant weak and medium-strength basic sites (surface species bicarbonate and bidentate carbonate).31 A slight change in the weak basicity strength could be inferred by the shift in temperature. This change was probably induced by the presence of chelating agents during preparation, despite the precursors are almost completely decomposed in the calcination step. There are not significant changes in medium and strong basicity. The total basicity determined by integration of CO2-TPD curves did not show substantial changes, Table 2.
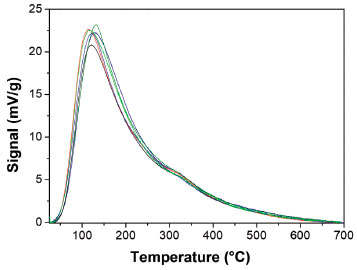 Figure 6. CO2-TPD profiles of reduced samples.  Cenitrate, Cenitrate,  CeEDTA(a), CeEDTA(a),  CeEDTA(b), CeEDTA(b),  CeNTA(a), CeNTA(a),  CeNTA(b), CeNTA(b),  CeCA(a) CeCA(a)
In Figure 7, SEM micrographs of representative samples are shown. In all SEM images irregular particles are present. The chemical mapping carried out by EDX spectroscopy in different zones of the sample reveals a quite uniform chemical composition which indicates a good distribution of Ce species on the spinel surface regardless the chelating agent used in preparation step.
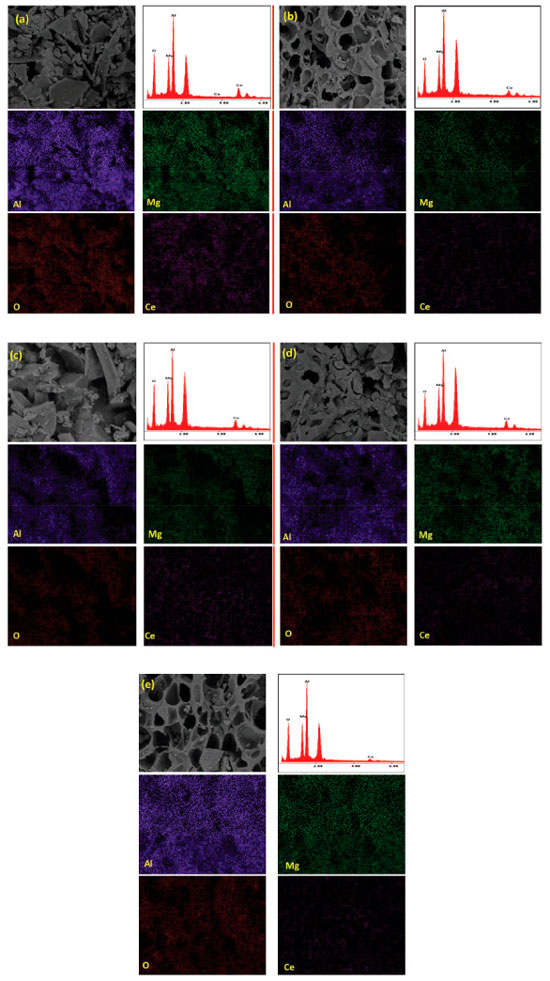 Figure 7. SEM images of samples and maps of Al, Mg, O and Ce. Cenitrate, (b) CeEDTA(a), (c) CeCA(a), (d) CeCA(b), and (e) CeNTA(a)
From the result analysis, it could be inferred that the use of chelating agents during the impregnation step decreased the CeO2 crystallite sizes with slight changes in the redox and basic properties of spinel support. The decreasing of was higher for the complex Ce-citric acid being of 25% lower than the reference sample. The samples prepared from citric acid at pH= 8 showed the lowest interaction with spinel support. The use of Ce complexes in the impregnation step did not lead higher changes in the physicochemical properties, likely due to the high calcination temperature required to thermally stabilize the support. Since CeO2 is partially formed during the drying step (Figure 4), the strategy of using the CeO2 doped spinel in dried form could induce more significant changes in the final catalysts. This study is under investigation in our laboratory.
CONCLUSIONS MgAl2O4 spinel was doped by the 5 wt.% CeO2. Aqueous solutions of Ce3+ in the presence of different chelating agents (EDTA, NTA or CA) were used in the impregnation step. Ceria nanoparticles between 5 to 6.8 nm were obtained after a thermal decomposition in air at 600 °C. The use of citric acid at pH=8 led to the lowest crystallite sizes of CeO2 being also low the interaction with the spinel. In all the samples surface CeO2 was reduced in a temperature range of 450 to 670 °C. It was not observed a clear dependence of H2 consumption under reduction conditions or in the degree of CeO2-spinel interaction with the crystallite size. The use of chelating agents did not change the total basicity although a slight increase in weak base strength could be inferred.
ACKNOWLEDGMENTS Financial supports are acknowledged to CONICET, ANPCyT and Universidad Nacional of San Luis. The authors would like to thank to Lic. Esther Fixman for her assistance in CO2-TPD determinations. SUPPLEMENTARY MATERIALIR spectroscopy spectra confirming carbon contamination in calcined samples (Figure 1S) can be found at http://quimicanova.sbq.org.br in PDF format with free access.
REFERENCES 1. Sehested, J.; Gelten, J. A. P.; Remediakis, I. N.; Bengaard, H.; Norskov, J. K.; J. Catal. 2004, 223, 432. 2. Barroso, M. N.; Galetti, A. E.; Gomez, M. F.; Arrúa, L. A.; Abello, M. C.; Chem. Eng. J. 2013, 222, 142. 3. Barroso, M. N.; Gomez, M. F.; Arrúa, L. A.; Abello, M. C.; Int. J. Hydrogen Energy 2014, 39, 8712. 4. Galetti, A. E.; Barroso, M. N.; Gomez, M. F.; Arrua, L. A.; Monzón, A.; Abello, M. C.; Catal. Lett. 2012, 142, 1461. 5. Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A.; Appl. Catal., A 2015, 500, 12. 6. Escobar, J.; Barrera, M. C.; de los Reyes, J. A.; Toledo, J. A.; Santes, V.; Colin, J. A.; J. Mol. Catal. A: Chem. 2008, 287, 33. 7. Peña, L.; Valencia, D.; Klimova, T.; Appl. Catal., B 2014, 147, 879. 8. Karnjanakom, S.; Guan, G.; Asep, B.; Dua, X.; Hao, X.; Samart, C.; Abudula, A.; Energy Conversion Manage. 2015, 98, 359. 9. Lu, X.; Chen, J-F.; Tan, Y.; Zhang, Y.; Catal. Commun. 2012, 20, 6. 10. Bang, Y.; Park, S.; Han, S-J.; Yoo, J.; Song, J-H.; Choi, J. H.; Kang, K. H.; Song, I. K.; Appl. Catal., B 2016, 180, 179. 11. Daza, C. E.; Gallego, J.; Mondragón, F.; Moreno, S.; Molina, R.; Fuel 2010, 89, 592. 12. Mochizuki, T.; Hara, T.; Koizumi, N.; Yamada, M.; Appl. Catal., A 2007, 317, 97. 13. Girardon, J-S.; Quinet, E.; Griboval-Constant, A.; Chernavskii, P. A.; Gengembre, L.; Khodakov, A. Y.; J. Catal. 2007, 248, 143. 14. van Dillen, A. J.; Terorde, R. J. A. M.; Lensveld, D. J.; Geus, J. W.; de Jong, K. P.; J. Catal. 2003, 216, 257. 15. Suarez Toriello, V. A.; Santolalla Vargas, C. E.; de los Reyes, J. A.; Vazques Zavala, A.; Vrinat, M.; Geantet, C.; J. Mol. Catal. A: Chem. 2015, 404-405, 36. 16. Wullens, H.; Leroy, D.; Devillers, M.; Int. J. Inorg. Mater. 2001, 3, 309. 17. Tarditi, A.; Barroso, M. N.; Galetti, A. E.; Arrúa, L. A.; Cornaglia, L.; Abello, M. C.; Surf. Interf. Anal. 2014, 46, 521. 18. Villagrán-Olivares, A.; Gomez, M. F.; Barroso, M. N.; Abello, M. C.; IJIC 2018, 9, 61. 19. Galetti, A. E.; Gomez, M. F.; Arrúa, L. A.; Abello. M. C.; Appl. Catal., A 2010, 380, 40. 20. Suzuki, Y.; Nankawa, T.; Francis, A. J.; Ohnuki, T.; Radiochim. Acta 2010, 98, 397. 21. Muñoz, M.; Moreno, S.; Molina, R.; Catal. Today 2013, 213, 33. 22. Abbaspour, A.; Mehrgardi, M.; Talanta 2005, 67, 579. 23. Verma, A.; Bakhshi, A. K.; Agnihotry, S. A.; Sol. Energy. Mater. Sol. Cells 2006, 90, 1640. 24. Mahmood, T.; Saddique, M. T.; Naeem, A.; Westerhoff, P.; Mustafa, S.; Alum, A.; Ind. Eng. Chem. Res. 2011, 50, 10017. 25. Puigdomenech, I.; Medusa Software, Chemical Equilibrium diagrams 2, 2013, available at http://www.kth.se/en/che/medusa/chemeq-1.369367, accessed July 2018. 26. Zhang, H.; Jia, X.; Liu, Z.; Li, Z.; Mater. Lett. 2004, 58, 1625. 27. Sanjabi, S.; Obeydavi, A.; J. Alloys Compd. 2015, 645, 535. 28. Ganesh, I.; Olhero, S.; Torres, P.; Ferreira, J.; J. Am. Ceram. Soc. 2009, 92, 350. 29. Damyanova, S.; Perez, C. A; Schmal, M.; Bueno, J. M. C.; Appl. Catal., A 2002, 234, 271. 30. Boaro, M.; Vicario, M.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A.; Catal. Today 2003, 77, 407. 31. Di Cosimo, J. I.; Diez, V. K.; Xu, M.; Iglesias, E.; Apesteguía, C.; J. Catal. 1998, 178, 499. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access






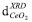 varied between 5 and 6.8 nm being 25% lower for the spinel doped by Ce-citric acid complex, Table 1. The presence of amorphous particles or very small crystals lower than 4 nm undetectable by XRD cannot be ruled out.
varied between 5 and 6.8 nm being 25% lower for the spinel doped by Ce-citric acid complex, Table 1. The presence of amorphous particles or very small crystals lower than 4 nm undetectable by XRD cannot be ruled out.