Artigo
| Preparation and characterization of a quercetin-tetraethyl ether-based photoprotective nanoemulsion |
|
Marlucy da Cruz GonçalvesI; Viviane Martins R. dos SantosI,*; Jason Guy TaylorI; Fernanda Barçante PerasoliII; Orlando David H. dos SantosII; Ana Carolina S. RabeloIII; Joamyr Victor Rossoni JuniorIII; Daniela Caldeira CostaIII; Thiago CazatiIV
I. Departamento de Química, Instituto de Ciências Exatas e Biológicas, Universidade Federal de Ouro Preto, Campus Morro do Cruzeiro, 35400-000 Ouro Preto - MG, Brasil Recebido em: 17/09/2018 *e-mail: vivianesantos@ufop.edu.br Although Quercetin absorbs in the UVA/UVB electromagnetic region, it is limited for applications as a UV filter due to its low lipophilicity and capacity to penetrate the epidermis. In order to overcome this limitation, we synthetized and evaluated the photo protective properties of a derivative obtained from Quercetin. The derivative was prepared by alkylation of Quercetin with iodoethane and characterized by IR and NMR spectroscopy. The in vitro Solar Protection Factor was determined by the Mansur method and the cytotoxicity was evaluated using hepatocellular cell (Hep G2) cells. Finally, Quercetin and the corresponding derivative were incorporated in nanoemulsions. Nanoemulsions with particles sizes between 53 and 73 nm were obtained, and polydispersity indexes were around 0.1, indicating good homogeneity of the nanoemulsion particles. The cell viability study for the Quercetin derivative indicated a very low cytotoxicity profile. The chemical modification of Quercetin resulted in a promising compound with improved properties desirable for skin penetration and incorporation into sunscreen formulations. INTRODUCTION The ultraviolet (UV) radiation that reaches the earth's surface is one the main factors responsible for causing skin cancers in many people, and its frequency has increased in recent years.1 UV rays burn the skin, induce premature aging and skin cancer. For this reason, the use of sunscreens and sunblocks is a common method for providing sunprotection to the skin.1 The growth in commercially available products containing sunscreens indicates that, even if a tan is still desired, people are conscious of the possible danger of photo-aging and skin cancer, occurring as a result of overexposure to the sun.2 The search for sun protection has intensified in recent decades as the sun's harmful effects have become more known and publicized.3 Recent studies aimed towards the development of new compounds with photoprotective applications have included otocrylene analogues,4 bis(indolyl)methane derivatives,5 vaniline derivatives,6 bile acids/azastilbenes conjugates,7 N-acyl hydrazone compounds8 and benzophenone derivatives.9 The photoprotective action of a sunscreen is measured universally by the sun protection factor (SPF) which establishes a correlation between the dose of sun exposure with the concentration of photoprotective product applied without the occurrence of erythema. Thus, the higher the SPF the greater the time allowed for safe exposure. The SPF is applied exclusively to UVB radiation, because it causes erythema.10 Topical sunscreens can have several pharmaceutical forms, like: oils, gels, emulsions, among others.11 Emulsions are the most suitable vehicle for the preparation of sunscreens, since they have a number of advantages, such as: the affinity for the epicutaneous mantle, the formation of thick film on the skin, ensuring a better fixation of the photoprotectors, increasing the resistance to water, compatibility with different ranges of pH and the possibility for incorporation of water soluble and liposoluble filters that act in synergy.12-15 The use of nanoemulsions in cosmetics becomes attractive because of the small size of the globules, which reduces the possibility of sedimentation, flocculation and coalescence, allowing the system to remain dispersed and without phase division, guaranteeing greater stability.16 Choquenet and co-workers have previously shown that Quercetin could potentially be used in sunscreen products and successfully incorporated this natural product in oil-in-water emulsions at a concentration of 10% (w/w).17 Moreover, Quercetin has exhibited photostabilizing properties that render it a useful additive for the formulation of effective broad-spectrum sunscreens containing butyl methoxy dibenzoyl methane and octylmethoxycinnamate.18 A limitation however for the use of Quercetin as a UV filter is its low lipophilicity and capacity to penetrate the epidermis. Adequate percutaneous absorption is an essential requirement for topically applied photoprotective agents and Quercetin penetrates very poorly in to the stratum corneum. Given this limitation, we have synthesized a more lipophilic derivative of Quercetin by alkylation of quercetins free hydroxyl groups.19 Herein, we report the preparation of Quercetin 3,7,3',4'-tetraethyl ether and evaluation of is in vitro photoprotective activity, photostability, cellular viability and incorporation it an O/W type nanoemulsion sunscreen formulation.
EXPERIMENTAL General considerations Reagents and solvents were purchased from Sigma Aldrich and used without further purification. The melting points were measured on the Büchi Melting Point B-540. NMR data were obtained with 200 MHz NMR instrument. Chemical shifts are reported in d (ppm) with reference to residual protons and 13C signals in deuterated solvent (CDCl3) The UV-vis absorption measurements were performed with a Bel Engineering UV-M51 Spectrophotometer. The MTT (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyl tetrazolium bromide) cytotoxicity assay was performed using Hep G2 cells. A mechanical stirrer used for the production of the nanoemulsion was Fisatom® Mod.713. The granulometry of the nanoemulsion was determined by Photon Correlation Spectroscopy on the Malvern Zetasizer model ZS. For the development of nanoemulsions were used: a) Sunflower oil (Liza) - food oil; b) Croduret 50 - Special® (Croda) - hydrogenated and ethoxylated castor oil, surfactant with HLB of 14.1; designated by the CTFA as PEG-40 Hydrogenated Castor Oil; c) Crill 3 - (Croda) - Sorbitan monostearate, surfactant with EHL of 4.7; named by CTFA Sorbitan stearate (C24H46O6); d) Newly obtained distilled waterand e) Substance of interest (Quercetin and/or derivative and EthylhexylMethoxycinnamate). Synthesis of quercetin 3,7,3',4'-tetraethyl ether 0.755 g (2.49 mmol) of Quercetin, 7.5 mL of DMF, 1.552 g (11.23 mmol) of potassium carbonate and 1mL (12.50 mmol) of iodoethane were added to a 50 mL round bottomed flask. Stirring was allowed for two days at room temperature. The reaction solution was diluted with 30 mL of dichloromethane and 20 mL of HCl (0.1 mol L-1) and the resulting organic phase was separated and washed three times with 20 mL of water. Anhydrous sodium sulfate was added to dry the solution and the organic solvent was concentrated on rotary evaporator. Thin layer chromatography (TLC) of the crude product was carried out using 2:8 ethyl acetate/hexane as the eluent and the product presented an Rf equal to 0.72 compared which was greater than the Rf of the staring material Quercetin. Next, the product was purified by flash column chromatography employing silica gel and ethyl acetate/hexane 2:8. The product was obtained in 0.150 g (14% yield) as a yellow solid with a melting point in the range of 119-120 ºC (lit = 116-120 ºC)20 and Octanol-Water partition coefficient (miLogP) of 4.61; IR (KBr) n (cm-1): 2500-3500 (intense and wide band of OH associated), 3030 (C-H of aromatics), 1,600, 1,580, 1,500 and 1,450 (C=C of aromatics).RMN1H (200MHz, DMSO-d6, ppm): 1.34 - 153 (m, 12H); 4.14 (q, J = 6.6, 4H); 4.23 (q, J = 6.6, 4H); 6,34 (s, 1H); 6.43 (s, 1H); 6.97 (d, J = 9.3 1H); 7.72 (d, J = 9.3, 1H) e 7.76 (s, 1H); 13CNMR (50 MHz, DMSO-d6, ppm): 14.8; 15.6; 64.1; 64.4; 64.8; 68.5; 92.5; 98.1; 109.5; 112.4; 113.8; 122.1; 123.2; 137.9; 148.2; 151.2; 156.1; 156.7; 162.0; 164.7; 178.8. In vitro determination of the Sun Protection Factor (SPF) The in vitro Solar Protection Factor (SPF) was determined by the spectrophotometric method developed by Mansur,20 using Eq. (1). 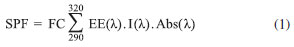 where: FC = 10 (constant), EE = erythemogenic effect, I = Intensity of the sun and Abs= absorbance of the sample. Absorption readings were performed in the range of 290 to 320 nm with intervals of 5 nm and added in equation 1. The constants EE and I were pre-defined by Mansur,20 according to Table 1.
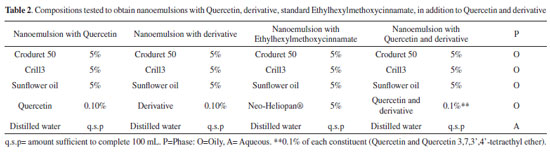
Log P- octanol-water partition coefficient21 The log P octanol-water partition coefficient was calculated using the online Molinspiration program Interactive log P calculator (software version v2015.01). Evaluation of photostability The photostability test was performed using a light chamber with a UVB lamp source. Solutions of 0.010 µg mL-1 Quercetin, derivative and a reference standard (Ethylhexylmethoxycinnamate) were prepared in methanol. The solutions were placed in volumetric flasks without a cap and exposed to radiation for total time of 2 h and evaluated at 20-minute intervals upon exposure to UV radiation. The system were open during the irradiation. Preparation of nanoemulsions The nanoemulsions were prepared according to the Emulsion Phase Inversion (EPI) method,22 However, to obtain the formulations, the distilled water and the oil phase were heated separately at 75±2 ºC. The water was slowly poured under the oil phase and the system was maintained under constant stirring at a speed of 520 rpm. Stirring was continued until the temperature was reached at 25±2 º C. After 24 h, all formulations were macroscopically evaluated. The compositions tested for nanoemulsions were the ones according to Table 2.
Determination of the granulometric distribution The formulations were analyzed for particle size distribution by Photon Correlation Spectroscopy (PCS). This technique is based on the incision of a laser beam on the sample to evaluate the light scattering resulting from the Brownian motion performed by the particles. The intensity of the scattered light forms a pattern of movement that allows to define the average diameter of these particles, whereas the smaller ones move faster, promoting greater modifications in this intensity.23,24 The samples were initially diluted at room temperature in ultrapure water (Milli-Q®) in a ratio of 1:1000, and then 3mL were transferred to a quartz cuvette for analysis. The samples were subjected to light scattering at a fixed angle of 90º at a temperature of 25 ºC, and the measurements were taken in triplicates.25 The results were given as mean particle diameter ± standard deviation (SD) and polydispersity index (PI) of nanoemulsions. The PI refers to the homogeneity of the particle size distribution present in the sample, and samples with PI values smaller than 0.3 are considered monodisperse, ie the particle size distribution in the sample is more homogeneous or narrow. Cell viability assay (MTT) Hepatocyte carcinoma (HepG2) cell line was acquired from the Cell Bank from the Federal University of Rio de Janeiro (UFRJ) and was cultured in DMEM medium supplemented with 10% FBS (fetal bovine serum), 1% glucose, 1% glutamine and 100 U/mL penicillin in a humidified atmosphere containing 5% CO2 in air at 37 ºC. Cell viability was determined using colorimetric MTT (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay). In brief, HepG2 cells (1x105) were cultured in 96 well-plates with or without different concentrations of concentrations of Quercetin or derivatives of Quercetin (10, 50, 100, 200, 400, 500, 600 and 800 µmol L-1) for 24 h. After incubation, medium was removed and 200 µL of 5 mg/mL MTT solution was added and incubated at 37 ºC for further 2 h. The MTT solution was then removed and 100 µl of DMSO were added to each well. The absorbance was read on ELISA reader at 570 nm. For the assays, the calculation used to evaluate the percentage of cell viability was: Absorbance of treated cells / absorbance of control x 100.
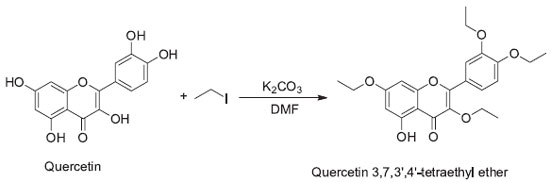
RESULTS AND DISCUSSION Synthesis of quercetin 3,7,3',4'-tetraethyl ether (derivative of quercetin) The reaction of the Quercetin with iodoethane occurred in the presence of inorganic base potassium carbonate and solvent dimethylformamide resulting in the formation of Quercetin derivative Quercetin 3,7,3',4'-tetraethyl ether. The phenolic hydroxyls present in Quercetin were alkylated according to Figure 1.
Quercetin 3,7,3',4'-tetraethyl ether is a known compound and comparison of its melting point and spectroscopic data (NMR and FT-IR) with literature26,27 values confirmed the formation of product. The spectrum in the infrared region showed absorption bands characteristic of the product. The absorption band in 3400 cm-1 indicated the presence of a hydroxyl, which did not undergo alkylation reaction with iodoethane, due to intramolecular hydrogen bonding with the ketone group present in the structure. IR bands ~3000 cm-1, 1650, 1500 e 1450 cm-1, which are, respectively, the CH band of aromatics and C = C of aromatics. The NMR spectra (1H and of 13C) showed resonance signals corresponding to the ethoxy substituents (OCH2CH3) formed by alkylation of the phenolic hydroxyls. The presence of a triplet integrating for eight hydrogens with chemical shift equal to 4.10ppm and a singlet in the 13C spectrum with chemical shift of 64.4 ppm was indicative of methylene group and a quartet integrating 12 hydrogens at 1.34 ppm with its corresponding singlet in the 13C spectrum at 14.8 ppm confirmed the presence of methyl group.28 In order to improve the liphophilicity of Quercetin, alkylation of the free hydroxyl groups with an iodoalkane was envisaged to improve the chemical properties so that the modified compound would more efficiently absorbed by the skin. The log P of Quercetin 3,7,3',4'-tetraethyl ether is 4.61 and Quercetin is 1.68. Log P> 1 the molecule has a lipophilic character and Log P < 1 the molecule has a hydrophilic character. The log P octanol-water partition coefficient was calculated using Molinspiration Interactive log P calculator.29 Solar Protection Factor The SPF was evaluated using the Mansur method.20 The analysis was carried out on an ultraviolet spectrophotometer, where the values of the obtained absorbances were placed in equation 1, and generated SPF values as presented in the Table 3. Ethylhexylmethoxy cinnamate (octinoxate) and 2-hydroxy-4-methoxylbenzophenone (oxybenzone) have been used as positive controls in previous studies. Octinoxate at a concentration of 0.010 mg mL-1 presented an SPF of 9.36 and the SPF of Oxybenzone at a concentration of 0.023mg mL-1 was reported to be 6.90.27 By analysis of the obtained data, we verified that the Quercetin and Quercetin 3, 7, 3', 4'-tetraethyl ether (QD) presented SPF proportional to the analyzed concentrations, that is, the higher non-toxic concentration (according to the cell viability test) the higher the Sun Protection Factor. Although chemical modification of Quercetin slightly lowered its SPF, the results are never the less promising and given the potential for improved skin absorption, the derivative compound would be more effective in acting as a UV filter.
Evaluation of photostability Quercetin, Quercetin 3,7,3',4'-tetraethyl ether and Ethyl-hexyl Methoxycinnamate were investigated separately in a photostability study in triplicate at room temperature. The temperature of the experiment was maintained constant throughout the total period of the experiment. The compounds were dissolved in methanol, forming solutions of concentration equal to 0.010 mg mL-1. The graphs depicted in Figure 2 show how the analyzed solutions behave, in terms of photostability, before and after exposure to UVB radiation. Quercetin showed minimal variation during the observed period of exposure to UV light. Quercetin 3,7,3',4'-tetraethyl ether showed a small decrease in absorbance after the first 20 minutes, but then stabilized over 40, 60 and 80 minutes respectively. Similar results were observed for standard sunscreen agent Ethyl-hexyl Methoxycinnamate. The absorbance increase after 20 min in the Figure 2a because occurred evaporation of the solvent and with that increased concentration.
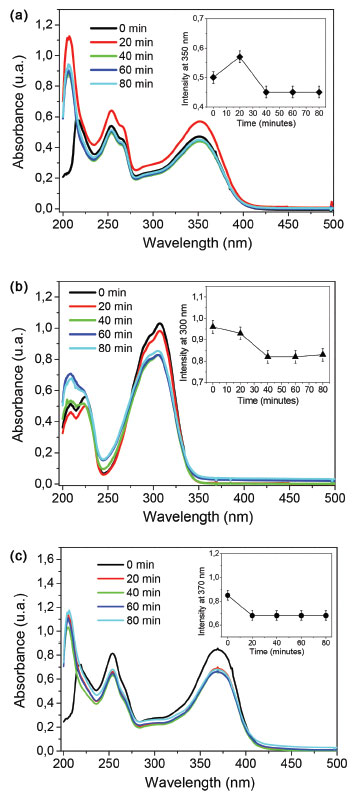 Figure 2. Graphs of photostability of Quercetin 3,7,3',4'-tetraethyl ether (a), standard Ethylhexylmethoxycinnamate (b) and Quercetin (c), in methanol and room temperature. The photostability of the compounds before and after exposure to UVB radiation are shown in the insert
Nanoemulsions Physical appearance of nanoemulsions and determination of granulometry The macroscopic analysis was performed and it was observed that the developed nanoemulsions presented transparent bluish aspects, characteristic of nanoemulsions.30 In addition, they were stable after 24 h of preparation. As can be seen in Table 4, the average particle diameters present in the formulations varied between 54.16 ± 0.36 e 75.27 ± 0.04. In all cases, the particle diameters are in the range that is characteristic for nanoemulsions.31,32 All nanoemulsions presented PI values lower than 0.3 and thus are considered monodispersed.33
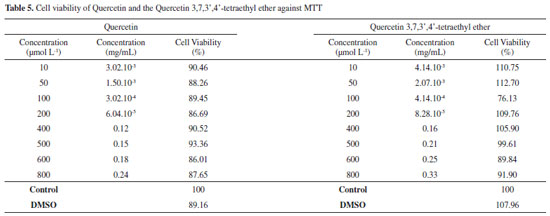
The size of the globules of an emulsion depends on the emulsification method employed. The results demonstrate the efficiency of the phase inversion emulsification method in obtaining nanoemulsions, since the presented deviations are small, characterizing a good homogeneity of the granulometric size of the nanoemulsions particles. In addition, the small particle size allows them to deposit evenly on the surface of the skin, creating a continuous film and consequently increasing the effectiveness of the nanoemulsion in protection against UVA and UVB rays. This type of formulation also protects photosensitive compounds, which is of great interest in cases where the asset degrades during exposure to visible or UV light.34-37 Formulations were also evaluated for the addiction of different concentration of Quercetin, (5, 1, 0.2 and 0.1%). A precipitate formation could be observed for the three highest concentrations tested, showing the instability of these formulations and the difficulty of solubilizing quercetin even in an oily vehicle. Thus, as previously mentioned, a nanoemulsion with 0.1% Quercetin was prepared, in which there was no precipitate formation after 24 h from the preparation time. This concentration was also used for the Quercetin 3,7,3',4'-tetraethyl ether. As it is more hydrophobic than Quercetin, it could be better solubilized within the oily phase, showing no sign of instability or precipitation. It was noticed the reduction of particle size of nanoemulsions with both additives without compromising system stability. Cell viability According to ISO2009 - 10993-5, a substance is considered cytotoxic when the cell viability is less than or equal to 70%. Therefore, the results obtained in the MTT test were compared according to the ISO. The MTT assay provided the data related to Quercetin and Quercetin 3,7,3',4'-tetraethyl ether according to Table 5. Based on the results obtained, it is observed that Quercetin and the derivative are non-toxic in the concentration range of 1 to 800 µmol L-1, since the cell viability was higher than 86.69% for Quercetin and 89.84% for Quercetin 3,7,3',4'-tetraethyl ether.
CONCLUSIONS The photoprotection study performed according to the Mansur methodology showed promising applications for Quercetin 3, 7, 3', 4'-tetraethyl ether which presented an SPF value similar to quercetin. The observed cytotoxicity values prove that, like Quercetin, the derivative is non-toxic at the maximum concentration assessed (800 µmol L-1). In addition, the photostability test suggests that the derivative is stable after an initial from a small drop in its absorption observed in the first 20 minutes of exposure to UV light. The nanoemulsions containing the 0.1% Quercetin 3,7,3',4'-tetraethyl ether, 0.1% Quercetin, 5% Ethyl-hexyl Methoxycinnamate and Quercetin with 0.1% Quercetin 3, 7, 3', 4'-tetraethyl ether were stable for 24 h after preparation and homogeneous in the size of the particles. Nanoemulsions were successfully obtained with particles sizes with the range of 53 to 75 nm. In addition to this, the EPI method consumes low energy in the process of forming emulsions, being economical and meeting industrial demand. Finally, Quercetin 3,7,3',4'-tetraethyl ether is a promising molecule to be incorporated into formulations of sunscreens, due to the greater liphophilicity when compared to Quercetin.
SUPPLEMENTARY MATERIAL NMR and IR spectra used in the characterization of the compounds are available from http://quimicanova.sbq.org.br, free of charge.
ACKNOWLEDGMENT UFOP, PROPP, CNPQ, FAPEMIG.
REFERENCES 1. de Souza, F. P.; Rev. Cienc. Farm. Basica Apl. 2013, 34, 69. 2. Dutra, E. A.; Da Costa, D. A. G.; Kedor-Hackmann, E. R. M.; Santoro, M. I. R. M.; Rev. Bras. Cienc. Farm. 2004, 40, 381. 3. Milesi, S. S.; Guterress, S. S.; Caderno de Farmácia 2002, 18, 103. 4. Polonini, C. P.; Lopes, R. S; Beatriz, A., Gomes, R. S.; Silva, A. O.; Lima, R. V. De; Nunes, G. A.; Brandao, M. A. F.; Raposo, N. R. B.; de Lima, D. P.; Quim. Nova 2014, 37, 1004. 5. Ergindemir, H. N.; Aker, A.; Hamitbeyli, A.; Ocal, N.; Molecules 2016, 21, 2. 6. Leite Filho, C. A.; Reis, S. A. G. B.; Rolim, L. A.; Araújo C. R. M.; Gonsalves, A. A.; Rev. Virtual Quim. 2016, 8, 2057. 7. Dos Santos, J. A.; Polonini, H. C.; Suzuki, É.Y.; Da Silva, A. D.; Steroids 2015, 98, 114. 8. Reis, J. S.; Correa, M. A.; Chin, C. M.; Dos Santos, J. L.; Bioorg. Med. Chem. 2014, 22, 2733. 9. Gonçalves, M. C.; Rossoni Jr., J. V.; Rabelo, A. C. S.; Costa, D. C.; Cazati, T.; Taylor, J. G.; dos Santos, V. M. R.; Rev. Virtual Quim. 2018, 10, 600. 10. Do Nascimento, M. S. T. Dissertaçao de Mestrado, Universidade Federal do Mato Grosso do Sul, Campo Grande, 2014. 11. Cabral, L. D. Da S.; Pereira, S. De O.; Partata, A. K.; Revista Científica do Itpac 2013, 5, 107. 12. Damiani, E.; Rosati, L.; Castagna, R.; Carloni, P.; Greci, L.; J. Photochem. Photobiol. B 2006, 26, 204. 13. Morrison, I. D.; Ross, S.; Colloidal Dispersions: Suspensions, Emulsions, and Foams, Wiley: New York, 2002. 14. Oliveira, A. G.; Scarpa, M. V.; Correa, M. A.; Cera, L. F. R.; Formariz, T. P.; Quim. Nova 2004, 7, 1196. 15. Flor, J.; Davolos, M. R.; Correa, M. A.; Quim. Nova 2007, 30, 153. 16. Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C.; Adv. Colloid Interface Sci. 2004, 109, 303. 17. Choquenet, B.; Couteau, C.; Paparis, E.; Coiffard, L. J. M.; J. Nat. Prod. 2008, 71, 1117. 18. Santos, S.; Matteo, M.; Photochem. Photobiol. 2010, 86, 273. 19. Vicent, F. T.; Simir, T. R.; Del Ciampo, J. O.; Wolga N. O.; Pitol, D. L.; Iyomasa, M. M.; Bentley, M. V.; Fonseca, M. J.; Eur. J. Pharm. Biopharm. 2008, 69, 948. 20. Picq, M.; Prigent, A. F.; Nemoz, G.; Andre, A. C.; Pacheco, H., J. Med. Chem. 1982, 25, 1192 21. Bouchemal, K.; Briançon S.; Perrier, E.; Fessi, H.; Int. J. Pharm. 2004, 280, 241. 22. Prigent, A.F.; Nemoz, G.; Andre, A.C.; Pacheco, H.; J. Med. Chem. 1982, 25, 1192. 23. Molinspiration Cheminformatics, v2015.01, Bratislava University, Slovak Republic, 1986, available at 01, Bratislava University, Slovak Republic, 1986, available at http://www.molinspiration.com/ , assessed at February 2019. 24. Seibert, J. B.; Rodrigues, I. V.; Carneiro, S. P.; Amparo, T. R.; Lanza, J. S.; Frezard, F. F.; Souza, G. H. B.; Santos, O. D. H.; Flavour Fragr. J. 2018, 00, 1. 25. Morais, J. M.; Tese de doutorado, Universidade de Sao Paulo, Ribeirao Preto, 2008. 26. Harborne, J. B.; The Flavonoids: advances in research since 1986, Chapman and Hall: London, 1994. 27. Agrawal, P. K.; Carbon-13 NMR of Flavonoids, Elsevier: Amsterdam, 1989. 28. Leite Filho, C. A.; Reis, S. A. G. B.; Rolim, L. A.; Araújo, C. R. M.; Gonsalves, A. A.; Rev. Virtual Quim. 2016, 8, 2057. 29. Pedersen, B.; Kristensen, K.; Eur. J. Nucl. Med. 1981, 6, 521. 30. Janjic, J. M., Shao, P., Zhang, S.; Biomaterials 2014, 35, 4958. 31. Zhang, L.; Kosaraju, S. L.; Eur. Polym. J. 2007, 43, 2956. 32. Sadurní, N.; Solans, C.; Azemar, N.; García-Celma, M. J.; Eur. J. Pharm. Sci. 2005, 26, 438. 33. Klassen, P. L.; George, Z.; Warwick, J.; Georgiadou, S.; Colloids Surf., A 2014, A.455, 1. 34. Li, X.; Anton, N.; Ta, T. M. C.; Int. J. Nanomed. 2011, 6, 1313. 35. Rebolleda, S.; Sanz, M. T.; Benito, J. M.; Food Chem. 2015, 167, 16. 36. Gorain, B.; Choudhury, H.; Kundu, A.; Colloids Surf., B 2014, B.115, 286. 37. Bouchama, F.; Aken, G.; Van, A. A. J.; Koper, G. J.; Colloids Surf., A 2003, 231, 11. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access