Artigo
| A new validated method for determination of roflumilast in tablet by CZE-UV |
|
Tufan Güray*,#
Department of Chemistry, Faculty of Letters and Sciences, Eskisehir Osmangazi University, F-5 block, 26480, Eskisehir, Turkey
Recebido em 15/01/2019 *e-mail: tufanguray@gmail.com This paper reports a new and validated capillary electrophoresis method to determine Roflumilast (RFL) with an internal standard in tablet. The separation was made on an uncoated fused-silica capillary column 50 cm effective length and 75 µm i.d. 20 mmol L-1 of Na2B4O7 buffer including 15% (v/v) methanol (pH=9.5) was used as background electrolyte. A potential of 20 kV was applied. The developed method was linear in the range of 0.75 µg mL-1 and 15.0 µg mL-1. LOQ and LOD values were 0.074 µg mL-1 and 0.022 µg mL-1 for inter-day, respectively. The precision value was 0.78% for inter-day. The accuracy was between 98.63 and 100.97 as recovery. This study was the first report for the determination of RFL in Daxas® solid dosage form using CE. The validated method was found as rapid, cheap, easy to apply and uses small sample volume, very little organic solvent, and high efficiency and resolution. INTRODUCTION Chronic obstructive pulmonary disease (COPD) is a common and progressive illness which is characterized by airflow limitation of lungs.1 Studies have focused on the development of new drugs, especially phosphodiesterase inhibitors that reduce chronic inflammation.2 Roflumilast (RFL) has been included as a novel treatment option for COPD in 2010 update of "The Global Initiative for Chronic Obstructive Lung Disease (COPD)". It is noted that the addition of RFL to long-acting bronchodilators in patients with severe and very severe COPD, acute exacerbations, and chronic bronchitis tumors reduce exacerbations.1 RFL (3-cyclo-propylmethoxy-4-difluoromethoxy-N- [3,5-di-chloropyrid-4-yl]-benzamide) (Figure 1) is a novel and selective PDE4 inhibitor3 and not yet an official drug in any of the famous pharmacopoeias.4,5 Few methods can be used for the determination of RFL3,6-21 such as HPLC-UV (High Performance Liquid Chromatography-Ultraviolet detection),3,6-11 HPLC-DAD (High Performance Liquid Chromatography-Diod Array Detector),12 HPLC-FL(High Performance Liquid Chromatography-Fluorescence detection),11 HPLC-MS/MS (High Performance Liquid Chromatography-Mass Spectrometry/ Mass Spectrometry),13,14 HPTLC (High Performance Thin Layer Chromatography)15 and spectrophotometry.16-22 Capillary electrophoresis (CE) has a lot of advantages when compared with the chromatographic methods including its higher efficiency, speed, resources utilization, small sample amount, unique separation mechanism, environmental friendliness, simple and numerous buffers and additive usage.23,24 The sensitivity of HPLC method is generally better when compared with the CE - UV, because the path taken by the sample in CE is short.23,25 But, fused silica tubing widespread used for CE has a UV cut-off approximetaly 170 nm, which is suitable for UV detection. Thanks to on-column detection, the capillary is illuminated during detection in order to reduce stray light.23 At low UV wavelength, because most organic analytes have some absorbance, detection of molecules without obvious chromophores become possible.26 CE applications have been used in bioscience,27 drug determination,28,29 ion analysis,30 food analysis31-33 and environmental science.30 As far as I am aware, there is no literature example about CE method for RFL determination.
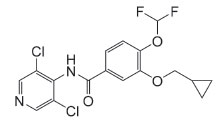 Figure 1. The chemical structure of RFL (pKa=8.74)
EXPERIMENTAL Standards, reagents and samples Luminal (Internal standard, IS), Roflumilast (RFL), CH3OH, and CH3CN were purchased from Sigma-Aldrich (St. Louis, MO, USA). Na3PO4 and Na2B4O7 anhydrous were obtained from Merck GmbH (Darmstadt, G). Ultrapure water with a resistivity of 18.2 MΩ cm (Millipore, Molsheim, France) was used. Daxas® was supplied from Takeda (Pharmaceutical Company Limited, Japan). Apparatus CE analysis were performed with Capillary Electrophoresis coupled with DAD (Agilent, G1600 A equipped with ChemStation software, Oregon, USA). Agilent fused silica capillaries dimensions were 75 µm (i.d.), 50 cm (effective length) and 57 cm (total long). The system was thermostated at 25 °C. pH measurements were carried out using Isolab Laborgeräte GmbH model pH meter (Bahnhofstrasse 10, D-97877 Wertheim Germany). The samples were centrifuged with 4000 rpm by using Sigma, 1-6P Centrifuge. Chromofil® RC-45/25 (MACHEREY-NAGEL GmbH & Co. KG, Germany) brand regenerated cellulose (RC) membrane filters (0.45 µm) were used before all injections. Preparation of solutions and sample To prepare a stock solution of RFL (2.48 x 10-3 mol L-1), 10 mg was weighed and dissolved in 10 mL CH3OH. 10% (v/v) CH3OH/H2O was used for all serial dilutions. The standard solutions were stable for at least fifteen days when they were stored in a freezer at -20 °C. Luminal (IS, 5.5 mg) was dissolved in 10 mL of ultrapure water. A stock solution of Na2B4O7 buffer solution (100 mmol L-1) was prepared with ultrasonication and used for the dilution of other buffer solutions. Each tablet (Daxas®) contains 500 µg of RFL and specific inactive ingredients, such as magnesium stearate, lactobiose, maize starch, hypromellose, macrogol 4000, povidone (K90), titanium dioxide (E171) and iron oxide yellow (E172). Daxas® tablets (10) of each solid dosage form were reduced to a homogeneous fine powder in a mortar. Exact mass of 1 tablet (267.2 ± 0.5 mg) was extracted with 10 mL CH3OH. 1 mL aliquot of the sample solution was diluted to 10 mL with H2O. 1 mL of this was filtered through a 0.45 µm RC and then 10 µL of IS at 2.35x10-3 mol L-1 was added before injection. CE system The capillary cartridge temperature was fixed at 25 °C and capillary was conditioned for 15 minutes in succession with 1.0 mol L-1 NaOH, 0.1 mol L-1 NaOH, H2O and running buffer, respectively by flushing at 9.35x104 N m-2. Between two successive injections, the capillary was flushed with deionize water for 2 minutes, 0.1 mol L-1 NaOH for 1 minute, and deionize water for 3 minutes, and then with a running buffer for 5 minutes. Membrane filters (0.45 µm porous RC) were used before all injections to prevent contamination. All the samples and solutions were sonicated for 5 minutes before the injection step. The amount of samples injection in CE was controlled by time (10 s) and hydrodynamic method (low-pressure 5x103 N m-2). The recorded migration times at 200 nm were 4.32 and 5.25 minutes for RFL and IS, respectively. Analytical method validation The validation of the method was done according to the International Council on Harmonization (ICH) guideline.34 A ratio of corrected peak (rPN) for both RFL and IS was calculated as follows and used for evaluation of the results. 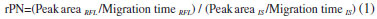 System suitability tests (SST) These tests contain fundamental analytical parameters including theoretical plate, capacity factor, retention time, resolution, peak tailing and repeatability. Thus, system suitability of the suggested method via SST using Agilent Software was evaluated. Calibration Calibration experiments were fulfilled by considering the standard solution of RFL in the linear range of 0.75 µg mL-1 (1.86 x 10-6 mol L-1) and 15.0 mg µL-1 (3.72 x 10-5 mol L-1) at three sets and five dilutions (n=6). A standard curve, also known as a calibration curve, was constructed by linear least squares methods and as intra-day and inter-day. LOQ and LOD values were calculated from the Eq. 2 and Eq. 3, respectively by considering the ratio of corrected peaks.  where, sblank = standard deviation of blank, m = slope of regression equation Precision and accuracy Precision in an analytical method under standard operation is described as the measure of the repeatability degree. In the developed method, precision was tabulated by both intermediate precision (inter-day) and repeatability (intra-day). Repeatability was investigated by considering the standards at the equal concentrations for six times over three consecutive days. The RFL standard solution (1.86x10-5 mol L-1) and IS solution at constant concentration (2.35x10-5 mol L-1) were used for the precision experiments. Certain amount of the standard RFL solutions of 1.86x10-4 mol L-1 (0.01 mL; 0.02 mL and 0.03 mL) was spiked into nearly 267.2 mg of Daxas®. It was extracted with 10 mL of CH3OH. 1 mL aliquot of the sample solution was diluted to 10 mL with H2O and then a 1 mL of this solution was filtered through a 0.45 µm RC (Final concentrations were 1.86x10-6, 3.72x10-6, and 5.58x10-6 mol L-1, respectively). After 10 µL of 2.35x10-3 mol L-1 IS was added, the solution was injected. Determination of RFL in solid dosage form The sample (Daxas® solid dosage form) was prepared as described in the section 'Preparation of solutions and sample' and analyzed for the determination of RFL by the validated CE method.
RESULTS AND DISCUSSION Analytical method optimization Acid ionization constant value of RFL is 8.74.11 RFL moves with electroosmotic flow at pH 7.5. It moves against the electroosmotic flow at pH values higher than 7.5. The optimum pH of the buffer solution was determined as 9.5, because the rPN value at this pH is maximum). RFL is present as negatively charged ionic form at this pH value. In order to select the optimum buffer solution, certain buffers (Na2B4O7 and Na3PO4) were tried. Na3PO4 was not a convenient buffer because it creates an extra noise signal in the electropherograms and high current. Thus, Na2B4O7 buffer was selected as the running buffer. The organic modifier can change zeta potential, electrolyte viscosity and selectivity. Na2B4O7 including CH3OH and CH3CN were tested as organic modifiers. All solutions of RFL were prepared in CH3OH since the RFL solution was not fully dissolved in CH3CN. The optimum conditions were determined by examining the effect of the concentration of Na2B4O7 (10-25 mmol L-1), organic modifier volume (CH3OH, %, 10-20), pH value (9.0-10.5), applied potential (10-25 kV), injection time (5-10 s at 5 x 103 N m-2) and wavelength (between 200 nm and 260 nm). Optimal conditions were a running buffer of 20 mmol L-1 Na2B4O7 solution, 15% (v/v) CH3OH at pH = 9.5, 10 s at 5 x 103 N m-2 injection time, 20 kV of applied potential, fixed temperature of 25 °C and 200 nm wavelength in terms of peak shape, retention time, sensitivity and resolution. To determine a suitable IS, butyl parahydroxybenzoate, nicotine amide, methyl parahydroxybenzoate, luminal, ethyl parahydroxybenzoate, p-hydroxybenzoic acid, and propyl parahydroxybenzoate were tested as the candidates for internal standards. Luminal was found as a convenient IS for the method. The electropherogram of the standard RFL solution (1.86 x 10-5 mol L-1) and IS (2.35 x 10-5 mol L-1) under the optimum conditions is shown in Figure 2.
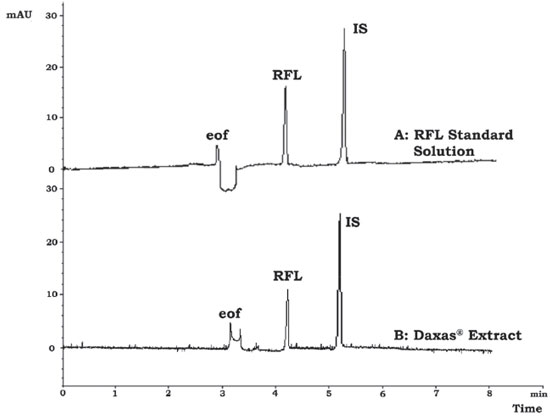 Figure 2. (A) The electropherogram of RFL (1.86x10-5 mol L-1) and IS (2.35x10-5 mol L-1) in the employment of 20 mmol L-1 sodium tetraborate buffer, 15% (v/v) MeOH, at pH=9.5 in the use of 20 kV of applied potential, 10 s at 5x103 N m-2 of injection time, 200 nm of wavelength and 25 °C of fixed temperature. (B) A typical electropherogram of Daxas® tablet under the optimum conditions
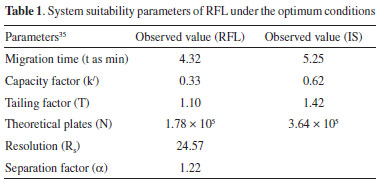
As seen from Figure 1, RFL and IS migrated in 4.32 (RSD of 0.24%) and 5.25 (RSD of 0.19%) minutes, respectively under the optimum conditions. The mean electrophoretic mobility from cathode to anode (m2 s-1V-1) of RFL and IS was calculated as -1.84 x 10-8 (RSD: 1.38%) and -2.83 x 10-8 (RSD: 0.88%), respectively (n=3). Validation of the method The standard solution containing 1.86 x 10-5 mol L-1 RFL and IS (2.35 x 10-5 mol L-1) was used for the method validation studies. The obtained system suitability parameters under the optimum conditions are given in Table 1 and approved the successfully determination of RFL.
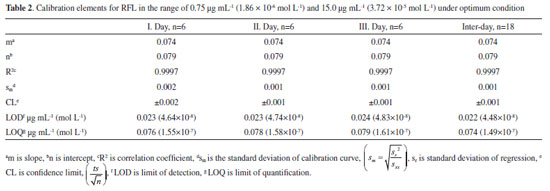
Linear range results for RFL in Table 2 indicated that the calibration equation is linear in the range of 0.75 µg mL-1 (1.86 x 10-6 mol L-1) and 15.0 µg mL-1 (3.72 x 10-5 mol L-1) with R2 = 0.9997 for inter-day (rPN = 7.42 x 10-2 CRFL + 7.98 x 102) on the basis of inter-day results, with a good determination coefficient (0.9997). LOQ and LOD values were 0.074 µg mL-1 (1.49 x 10-7 mol L-1) and 0.022 µg mL-1 (4.48 x 10-8 mol L-1) for RFL, respectively, considering inter-day results. These values of LOQ and LOD in Table 2 are pretty well low.
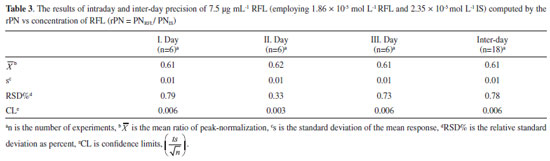
Precision results for RFL in Table 3 show that the method is precise with the RSD values of 0.33-0.79% as intra-day precision and 0.78% as inter-day precision.
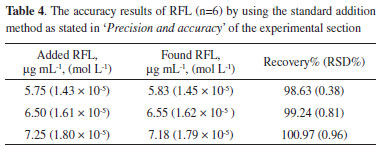
The results of accuracy explained in the experimental section of 'Precision and accuracy' are presented in Table 4. According to the accepted criteria (85-115%),5 the calculated recovery% (98.63-100.97%) values indicated that the developed method has perfect accuracy.
For robustness, effects of certain parameters including pH (9.4, 9.5, 9.6), Na2B4O7 concentration (19.9, 20.0, 20.1 mmol L-1), CH3OH % (14.9, 15.0, 15.1, v/v, %), applied voltage (19.8, 20.0, 21.2 kV), column temperature (24, 25, 26 °C), wavelength (199, 200, 201 nm), and injection time (9.9, 10.0, 10.1 s) were investigated. The method was not affected by small but intentional changes in the optimization parameters and it is extremely reliable with small standard errors of the mean (between 0.00-0.02, SE<1) and the RSD% value (between 0.37-1.28, RSD% < 2) for each parameter. These findings indicated that the suggested method is highly robust. Application to solid dosage form for RFL determination A typical electropherogram of Daxas® analyzed for the determination of the RFL was given in Figure 1. According to the analysis results of Daxas® solid dosage form in Table 5, the determined RFL amount was 499.75 µg with a relative error of 0.05% (500 µg of certified RFL value).
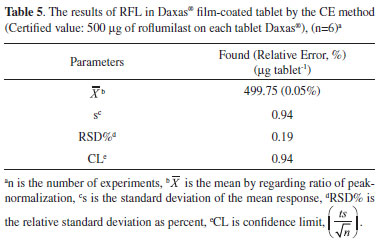
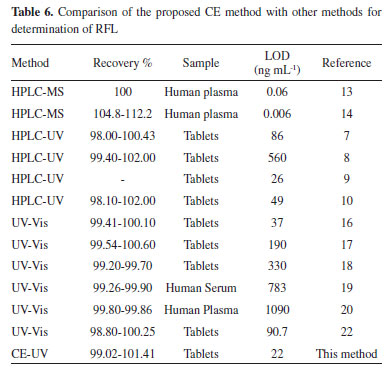
No monograph has been reported for this new drug, RFL, in the pharmacopeias. According to the USP 34-NF 29, the maximum allowed acceptance criteria is a 15% deviation.5 The deviation of result in this study (0.28%) is within limits for RFL. Furthermore, USP 34-NF 29 states that the RSD% value of any drug preparation in the final dosage units should not exceed 2%.5 The calculated RSD% value (0.19) in the present study is within the USP 34-NF 29 limit (2%) (Table 5). The developed method is reliable and valid. When compared with the literature (Table 6), the LOD value of the developed and validated new CE method for the determination of RFL are higher than HPLC-MS methods.13,14 However, HPLC-MS methods are expensive. Furthermore, complicated cleanup procedures, the necessities of pre-concentration steps and expensive equipment are important disadvantages of these methods. LOD value of suggested method is lower than those of HPLC-UV7-10 and UV-Vis. methods 16-20,22 reported in the literature.
CONCLUSIONS The suggested CE method in the present study targeting the RFL determination has considerable advantages like cheapness, accuracy, selectivity, reliability and environmentally friendly. As far as I know, this validated method is the first example based on the determination of RFL by CE. It has been verified according to the ICH guideline. All the ICH parameters give good results. Finally, the RFL in the Daxas® solid dosage form was successfully determined by using this method.
ACKNOWLEDGEMENTS The author is grateful to Eskisehir Osmangazi University Scientific Research Projects Commission (Project No. 201719039) for the financial support of this work and Prof. Dr. Muzaffer TUNÇEL and Prof. Dr. Ülkü Dilek UYSAL for their valuable contributions.
REFERENCES 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Report, 2017. Available at http://www.goldcopd.org/gold-2017-global-strategy-diagnosis-managementprevention-copd. Accessed May, 2019. 2. Sezgi, C.; Şenyiğit, A.; Tuberculosis and Thorax 2011, 59, 285. 3. Barhate, V. D.; Deosthalee, P.; Indian J. Pharm. Sci. 2010, 72, 401. 4. European Pharmacopoeia - 9th edition; European Department for the quality of medicines, Strasbourg, France, 2016. 5. United States Pharmacopeia XXIX, US Pharmacopeial Convention, Rockville, MD, 2011. 6. Barhate V. D.; Deosthalee, P. C.; J. Chem. Pharm. Res. 2011, 3, 770. 7. Shah, A. D.; Patel, N. C., World J. Pharm. Pharm. Sci. 2014, 3, 2281. 8. Belal T. S.; Ahmed H. M.; Mahrous M. S.; Daabees H. G.; Baker, M. M.; Bulletin of Faculty of Pharmacy, Cairo University 2014, 52, 79. 9. Fang, T.; Advanced Mat. Res., 2013, 781-784, 68. 10. Ladani J. J.; Bhimani R. D.; Vyas K. B.; Nimavat, K. S.; Int. J. Pharm. Res. Scholars 2012, 1, 28. 11. Daxas, International Nonproprietary Name: Roflumilast - European Medicines Agency - Europe EU, EMA/464905/2010, CHMP assessment report, Procedure No. EMEA/H/C/001179, page 12. 12. Pinheiro M. S.; Marinsa, R. C. E. E.; Cabrala L. M.; Sousa, V. P. D.; J. Liq. Chromatogr. Relat. Technol. 2018, 41, 223. 13. Knebel, N. G.; Herzog, R.; Reutter, F.; Zech, K.; J. Chromatogr. B 2012, 893, 82. 14. Cui, X. E.; Huang, J.; Zheng, X.; Jiang, J.; Kuang, Y.; Hu, P.; J. Chromatogr. B 2016, 1029, 60. 15. Suganthi, A.; Arthi, K.; Ravi, T. K.; Indian J. Pharm. Sci. 2017, 79, 287. 16. Patel, U. B.; Patel, P. U.; Int. Res. J. Pharm. 2016, 7, 58. 17. Patel P. U.; Patel, U. B.; Int. J. Pharm. Pharm. Res. 2016, 5, 68. 18. Babu G. R.; Kumar G. V.; Krishna B. R.; Bhavani M. D.; Brahmini M.; Subbarao D.; V.Ramesh, G.; Indo Am. J. Pharm. Res. 2016, 6, 4332. 19. Raveendra B. G.; Kiran S. S. B.; Kumari V. M.; Jyothi R. K.; Bahavani, D. M.; Pharm. Anal. Acta 2016, 7, 1. 20. Babu G. R.; Bhavani M. D.; Krishna, B. R.; Int. J. Chem. Sci., 2016, 14, 1263. 21. Ladani J. J.; Bhimani R. D.; Vyas K. B.; Nimavat, K. S.; J. At. Mol. 2012, 2, 369. 22. Patel K. R.; Desai D. G.; Zanwar A.; Sen A. K.; Seth A. K.; Pharma Sci. Monit. 2013, 4, 274. 23. Li, S. F. Y.; Capillary electrophoresis: principles, practice and applications, Elsevier: Amsterdam, 1992. 24. Schmitt-Kopplin, P.; Capillary electrophoresis: methods and protocols, Springer Science & Business Media: Neuherberg, 2008. 25. Shintani, H.; Polansky, J.; Handbook of capillary electrophoresis applications, Blackie Academic and Professional: Tokyo, 1997. 26. Marina, M. L.; Rios, A.; Valcárcel, M.; Analysis and detection by capillary electrophoresis, Elsevier: Amsterdam, 2005. 27. Vlckova, M.; Stettler, A. R.; Schwarz, M. A.; J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1047. 28. Güray, T.; Turan, T.; Tuncel, M.; Uysal, U. D.; J. AOAC Int. 2017, 100, 206. 29. Güray, T.; Tunçel, M.; Uysal, U. D.; J. Food Drug Anal. 2018, 26, 842. 30. Kratii, E.; Nikonorov, V.; Nikitina, T.; Microchem. J. 2017, 130, 198. 31. D’Orazio, G.; Asensio-Ramos, M.; Fanali, C.; Hernandez-Borges, J.; Fanali, S.; TRAC --Trends Anal. Chem. 2016, 82, 250. 32. Leon, C.; Garcia-Canas, V.; Gonzalez, R.; Morales, P.; Cifuentes, A.; J. Chromatogr. A 2011, 1218, 7550. 33. Uysal, U. D.; Aturki, Z.; Raggi, M. A.; Fanali, S.; J. Sep. Sci., 2009, 32, 1002. 34. Guideline, Validation of Analytical Procedures: Text and Methodology ICH Q2 (R1); ICH: Geneva, Switzerland, 2005. Available at https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2_Guideline.pdf, Accessed May, 2019. 35. Lauer, H. H.; Rozing, G. P.; High Performance Capillary Electrophoresis, Agilent Technologies: Germany, 2009. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access