Artigo
| PhnSnCl4-n supported on activated carbon as novel tin-based catalysts for acetylene hydrochlorination |
|
Yibo Wu; Longjie Cui; Rong Zhang; Rujing Pei; Sufang Hu; Ruyue Han; Huimin Yang; Fuxiang Li*; Jianwei Xue; Zhiping Lv
College of Chemistry and Chemical Engineering, Taiyuan University of technology, Taiyuan, 030024, China Recebido em 19/04/2019 *e-mail: l63f64x@163.com In this work, a series of PhnSnCl4-n (n = 1, 2, 3, and 4) based catalysts were prepared by incipient wetness impregnation using activated carbon (AC) as support and triphenyltin chloride (Ph3ClSn) and tin(IV) chloride (SnCl4) as Sn precursors. The obtained catalysts were systematically characterized by X-ray diffraction (XRD), N2 physisorption, Scanning electron microscope (SEM), Thermogravimetric analysis (TG-DTG), and Inductively coupled plasma (ICP) analytical techniques. The catalytic performance of the prepared PhnSnCl4-n samples was evaluated in the acetylene hydrochlorination reaction. When a gas hourly space velocity (GHSV, C2H2 based) and a reaction temperature of 30 h-1 and 200 ºC, respectively were used for the reaction performed over the 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200 catalyst, an acetylene conversion of 98.0% with a selectivity to vinyl chloride of 98.5% were obtained. These results demonstrated that PhnSnCl4-n can effectively improve the catalytic activity and prolong the lifetime of SnCl4/AC catalysts. Moreover, by comparing with HgCl2/AC, PhnSnCl4-n-based catalysts exhibit much enhanced catalytic activity in the hydrochlorination of acetylene. It is assumed that the outstanding activity is due to trichlorophenylstannane (PhCl3Sn) active sites, resulted from the organotin redistribution reaction between Ph3ClSn and SnCl4. In addition, such sites also improved the SnCl4/AC catalyst stability during the reaction. INTRODUCTION Tin compounds include inorganic tin and organotin, the latter being represented by R4-nSnXn formula (R = alkyl or aryl, X = anionic species, n=1~4). Because of its various physical, chemical and biological properties, organotin has been used as stabilizers, biocides, anticancer therapy and catalysts.1-4 In the past few years, tin(IV) chloride, one of the inorganic tin compounds, has attracted considerable attention for many applications,5-15 such as hetero Diels- Alder reaction,10,11 organotin redistribution reaction,12 and acetylene hydrochlorination reaction.13-15 Acetylene hydrochlorination is the main reaction involved in the production polyvinyl chloride (PVC). In the past few years, the traditional mercury-based catalysts commonly used to catalyze acetylene hydrochlorination. However, due to the toxicity and sublimation of mercury chloride, its use will not be allowed in the future, reason why the exploration of non-mercury catalysts in acetylene hydrochlorination is strongly promoted, at least in regions with large reserves of coal (e.g., China).16,17 By compared with the precious metal catalysts exhibiting outstanding catalytic activity,18-21 the environmentally friendly non-precious metal catalysts has high potential to replace HgCl2 and be used at largescale production of PVC. For instance, it is already shown that the multicomponent inorganic tin materials can catalyze the acetylene hydrochlorination.13-15,22,23 On the other hand, tin(IV) chloride catalysts show relatively low stability due to the low boiling point, but the investigation of Sn based catalysts exhibiting high activity, selectivity, and stability in acetylene hydrochlorination is strongly encouraged. The acetylene hydrochlorination at industrial scale is performed with a space velocity and reaction temperature in the ranges of 30 - 50 h−1 and 130 - 180 °C, respectively.24 However, it was previously reported that the Sn-C bonds are stable at temperature up to 200 ºC.4 Regarding the use of organotin as catalysts for the hydrochlorination of acetylene is scarcely reported. Therefore, the purpose of this work is to investigate the organotin as catalysts for the acetylene hydrochlorination. To reach this aim, the triphenyltin chloride (Ph3ClSn) was selected as precursor for the organotin catalysts.2 Hence, a series of PhnSnCl4-n-based catalysts were prepared based on triphenyltin chloride (Ph3ClSn) and tin(IV) chloride (SnCl4) through the redistribution reaction and then loaded on activated carbon (AC) by incipient wetness impregnation. The resulted PhnSnCl4-n-based catalysts were systematically characterized by techniques, such as N2 physisorption, SEM-EDS, XRD, TG-DTG, and ICP. The well-characterized samples were used as catalysts for the acetylene hydrochlorination. According to the experimental results, three parameters, i.e., the Ph3ClSn and SnCl4 mole ratio, total amount of Sn(IV), and calcination temperature, have a strong influence on the acetylene conversion. Furthermore, it was noticed that the main active sites are the trichlorophenylstannane (PhCl3Sn) groups with higher boiling point, which significantly increase the catalytic activity and stability of the complex samples in comparison with those of SnCl4/AC.
EXPERIMENTAL Catalyst preparation The PhnSnCl4-n/AC-based catalysts were prepared by an incipient wetness impregnation method and using triphenyltin chloride (Ph3ClSn) and tin(IV) chloride (SnCl4) as Sn active sites precursors.25 The support was a coal-based columnar activated carbon pre-treated with HCl (0.01 mol L-1) to remove impurities. After filtration, the obtained sample was washed with distilled water until neutral pH, followed by drying at 120 ºC for 24 h. The obtained activated carbon was denoted as AC. The solution of Sn precursors, containing triphenyltin chloride and tin (IV) chloride, was prepared in ethanol, then mixed with the AC (a reference sample was prepared using water as solvent). The adsorption of precursor on AC was performed at 80 ºC. The mixture was dried in an oven at 100 ºC for 12 h, followed by calcination at various temperatures, i.e., 100, 200, 300, and 400 ºC for 4 h (flowed with a nitrogen atmosphere). The calcined samples were denoted as PhnSnCl4-n-based catalyst. Using a similar procedure, samples with constant loading degree of 10 wt.% and various Ph3ClSn : SnCl4 molar ratios (i.e., 1 : 1, 1 : 2, 1 : 3, 1 : 3.5, and 1 : 4) as well as various total loading degrees (5, 10, 12, and 15 wt.%) at constant Ph3ClSn: SnCl4 molar ratio of 1 : 3.5 were prepared. Catalyst characterization The textural properties of samples were evaluated by N2 physisorption on a NOVA 2000e instrument from Quantachrome. Before analysis, samples were degassed under vacuum at 200 ºC for 12 h. X-ray diffraction of catalysts was performed Shimadzu XRD-6000 diffractometer at 2θ between 10° and 80°. The SEM were performed on a ZEISS MERLIN Compact microscope at an acceleration voltage of 15 kV. The weight loss and stability of the catalysts were analyzed by TG-DTG (NETZSCH STA 449F3) in the range of 25-800 ºC under nitrogen gas with flow rate of 30 mL/min and a heating rate of 30 ºC min-1. Inductively Coupled Plasma (ICP) was performed on Agilent 720. Catalytic test The acetylene hydrochlorination was performed in a fixed-bed glass reactor (i.d. = 10mm) at atmospheric pressure. Hydrogen chloride was first fed through the reactor to remove trace impurities and activate the catalyst.26 In a typical experiment, 4 ml catalyst was added to the reactor containing the reaction mixture made of HCl and C2H2 in a mole ratio of 1.1:1.0. The C2H2 gas hourly space velocity wad maintained at 30 h-1 via a calibrated mass flow controller. Before analysis of products, the gas was passed through the medical sodalime to absorb the unreacted hydrogen chloride. Then, the product was analyzed by a gas chromatography using a GC900 apparatus equipped with a GDX-301 column.
RESULTS AND DISCUSSION The effect of the Ph3ClSn and SnCl4 mole ratio on the textural and catalytic properties of PhnSnCl4-n-based catalysts The N2 physisorption isotherms of AC support and different PhnSnCl4-n-based catalysts supported on AC are shown in Figure 1S while the textural properties obtained based on the corresponding isotherms and applying specific equations are listed in Table 1.
It can be seen that all samples exhibit the characteristic isotherm of type I/IV with a narrow hysteresis loop according to IUPAC classification (Figure 1S), indicating the coexistence of micropores and mesopores in the investigated samples.27,28 Additionally, the different visible hysteresis loop in catalysts shows the change in pore volume and pore size, which is good accord with Table1. The values of specific surface area and pore volume of all catalysts changed in comparison with those of AC support in agreement with the treatment applied to obtain a certain catalyst. Hence, the AC displays the highest specific surface area (987 m2g-1) and total pore volume (0.48 cm3 g-1). However, with the increase in content of tin active sites, the decrease in specific surface area is obvious in the range of 740 and 601 m2g-1, demonstrating the successful loading of tin ingredients on AC support. Figure 1 displays the catalytic results obtained for the acetylene hydrochlorination over the prepared catalysts.
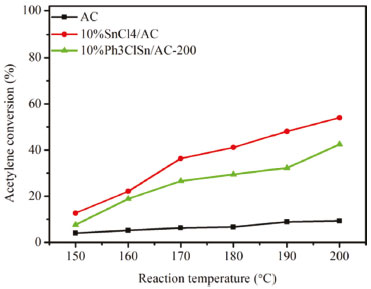 Figure 1. Catalytic activity vs reaction temperature over different catalysts
As noticed, a very low acetylene conversion was obtained over AC support irrespective the reaction temperature. Interesting, when the reaction was performed over 10%SnCl4/AC-200 and 10%Ph3ClSn/AC-200 catalysts, the conversion increased with temperature increase from 150 to 200 ºC. Hence, the maximum conversions obtained for these two samples at 200 °C are 58.3 and 42.5%, respectively, indicating that Ph3ClSn can catalyze the conversion of acetylene into vinyl chloride. Because both Ph3ClSn and SnCl4 were active in acetylene hydrochlorination, it was interesting to verify whether a catalyst obtained by co-impregnation of AC with both tin compounds ((Ph3ClSn+SnCl4)/AC) exhibit improved catalytic performance than those of monocomponent samples. Therefore, bi-component samples with various Ph3ClSn and SnCl4 mole ratios were prepared and their catalytic activity in acetylene hydrochlorination was assessed. The catalytic activity and selectivity to vinyl chloride are illustrated in Figure 2.
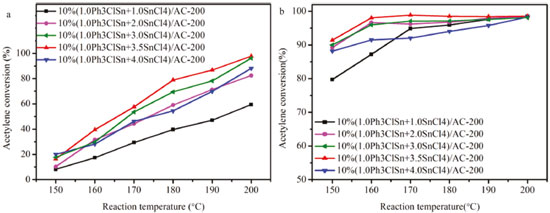 Figure 2. (a) Catalytic activity vs reaction temperature and (b) selectivity to vinyl chloride over PhnSnCl4-n-based catalysts with different Ph3ClSn and SnCl4 mole ratios
As shown in Figure 2a and b, the increase in Ph3ClSn and SnCl4 mole ratio results in the increase in both catalytic activity and selectivity to vinyl chloride up to optimal values (97.5%) then decreases. Particularly, for the Ph3ClSn and SnCl4 mole ratio of 1.0:3.5 (10% (1.0Ph3ClSn+3.5SnCl4)/AC-200 catalyst), an acetylene conversion of 97.5% with a selectivity toward vinyl chloride (VCM) of 98.5% were obtained when the reaction was performed at 200 °C. It is worth mentioning that these values are the best in the series. Interesting, the micropore specific surface area and micropore volume of 10% (1.0Ph3ClSn + 3.5SnCl4)/AC-200 display higher values of 582.9 m2/g and 0.22 cm3/g, respectively, except for 10% (1.0Ph3ClSn + 1.0SnCl4)/AC-200 among these PhnSnCl4-n-based catalysts (Table 1). Additionally, the organotin redistribution reaction can be operated in Ph3ClSn and SnCl4.2 On the basis of these results, it inferred that Ph3ClSn reacted with SnCl4 to produce the novel active sites and the appropriate amount of micropore display positive influence on the improvement of acetylene conversion and selectivity. To verify if the activity can be tuned by carefully changing the temperature of pretreatment, the 10% (1.0Ph3ClSn+3.5SnCl4)/AC sample was calcined at different temperatures. The results are discussed in the following sub-chapter. The effect of the calcination temperature on the textural and catalytic properties of 10% (1.0Ph3ClSn +3.5SnCl4)/AC The textural properties of 10% (1.0Ph3ClSn+3.5SnCl4)/AC calcined at 100, 200, 300, and 400 °C were evaluated by N2 physisorption, ant the obtained isotherms are displayed in Figure 2S. It is obvious that all isotherms keep the shape of type I/IV after these thermal treatments. The values of corresponding textural properties of each sample are listed in Table 2.
As shown in Table 2, the values of specific surface area of catalysts were affected by the calcination temperature, but not proportionally. Hence, the surface area increased in the order of 100 < 400 < 200 < 300 °C. In line with these results, it can be assumed that the calcination temperature influenced the formation of new active sites as well as their dispersion on the surface. Interestingly, its optimal calcinations temperature keeps agreement with the organotin redistribution reaction (200 °C).2 Figure 3 displays the catalytic results obtained for the acetylene hydrochlorination over the catalysts calcined at different temperatures.
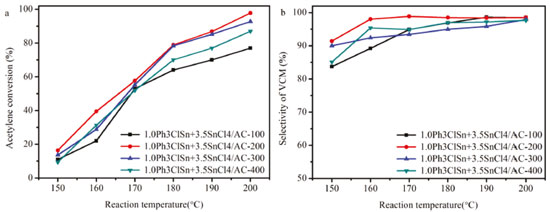 Figure 3. (a) Catalytic activity vs reaction temperature and (b) selectivity to vinyl chloride over 1.0Ph3ClSn+3.5SnCl4/AC catalysts calcined at different calcinations temperatures
Figure 3a shows the effect of the calcination temperature on the acetylene conversion as a function of reaction temperature. As a first observation, it can be seen that the reaction temperature impacted the catalytic conversion of acetylene. Secondly, the conversion of acetylene obtained at 200 °C was different depending on the calcination temperature. Interesting, the sample calcined at 200 °C exhibited the highest catalytic activity, i.e., 97.5% suggesting this temperature as optimal for the obtaining of the most active sites. Apparently, at this temperature, triphenyltin chloride reacted with tin (IV) chloride and generated enough available active sites for the conversion of acetylene. In addition, this sample has the highest mesopore volume value (0.08 cm3 g-1) among the samples (Table2). Furthermore, the small mesopore in catalysts display a positive effect on the acetylene conversion of original catalysts.29 Therefore, the mesopore volume is one of reason to affect the catalytic performance of 10% (1.0Ph3ClSn+3.5SnCl4)/AC catalysts. Figure 3b shows the selectivity toward vinyl chloride obtained over the catalysts calcined at different temperatures. It is worth mentioning that at lower reaction temperatures (i.e., 150-170 °C), the selectivity was influenced by the calcination temperature. In this range of temperatures, the sample calcined at 200 °C exhibits the highest selectivity toward the reaction product. However, as the reaction temperature increases (180-200 °C), the effect of the calcination temperature is less obvious, so that at 200 °C, the selectivity toward vinyl chloride is practically the same for all sample (98.5%). This behavior shows either a heterogeneity of active sites at lower reaction temperatures or they are differently activated in this temperature range. At higher reaction temperature, particularly 200 °C, the selectivity results indicate that the active sites are similar in nature. To further improve the activity of 1.0Ph3ClSn + 3.5SnCl4/AC-200 catalyst, the effect of total amount of active sites, while keeping Ph3ClSn:SnCl4 ratio constant, on the catalytic properties of 1.0Ph3ClSn+3.5SnCl4/AC-200 was studied. The effect of the total amount of active sites on the textural and catalytic properties of 1.0Ph3ClSn+3.5SnCl4/AC-200 The N2 physisorption isotherms of (1.0Ph3ClSn+3.5SnCl4)/AC with total amount of Ph3ClSn + SnCl4 of 5, 10, 12, and 15 wt.% are displayed in Figure 3S. As noticed, the isotherm shape did not change after loading various amounts of Ph3ClSn + SnCl4. However, the values of textural properties listed in Table 3 show a change in the surface areas and pores volumes depending on the amount of Ph3ClSn + SnCl4 loaded on AC support. To note, the specific surface area and total pore volume of catalysts gradually decreased with the increase in the amount of Ph3ClSn + SnCl4 loaded on the support. This phenomenon was mentioned as the dilution effect.30 Therefore, these results reveal that the precursors of the active site were successfully loaded on support (Figure 3S). Figure 4 illustrates the catalytic results obtained for the acetylene hydrochlorination over the catalysts with different amounts of Ph3ClSn + SnCl4.
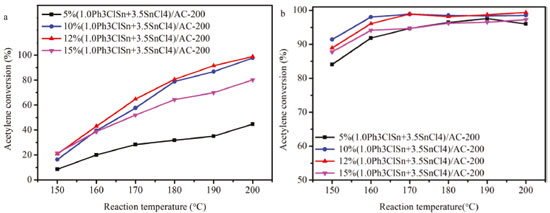 Figure 4. (a) Catalytic activity vs reaction temperature and (b) selectivity to vinyl chloride over 1.0Ph3ClSn+3.5SnCl4/AC-200 with different amounts of Ph3ClSn+ SnCl4
As seen in Figure 4a, the acetylene conversion is influenced by the amount of Ph3ClSn + SnCl4. If the reaction temperature of 200 °C is taken as an example, it can be noticed that the acetylene conversion increases from 40 to 98% for the samples with 5 and 10 wt.% Ph3ClSn + SnCl4, respectively. The conversion keeps constant when the loading degree increases to 12%, but it decreases to 78.5% for a loading degree of 15%. Regarding the selectivity to vinyl chloride (Figure 4b), a similar phenomenon was noticed as for the influence of the calcination temperature. Hence, the selectivity is different at lower reaction temperatures (i.e., 150-170 °C) while it is almost the same at higher reaction temperatures (i.e., 180-200 °C) irrespective of the amount of Ph3ClSn + SnCl4 loaded on AC support, indicating active sites similar in nature at higher reaction temperature, particularly, 200 °C. Once the optimal conditions in relation to the loading degree, ratio between the active components, calcination temperature, and reaction temperature were identified, the stability of the best catalyst (i.e., 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200) was studied. Catalytic performance of 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200 The catalytic activity and stability of 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200 sample evaluated for 40 h of reaction. The conversion curve as a function of reaction time is illustrated in Figure 8. It was previously shown that the acetylene conversion over 20%SnCl4/AC decreases from 93% to 90% after 4 h or reaction.13 For the sample investigated herein (12% (1.0Ph3ClSn+3.5SnCl4)/AC-200), the activity keeps above 90% for at least 10 h of reaction although a decreasing trend is noticed (Figure 5). After 10 h, the conversion continuously decreased to 62.5% as the reaction time increased to 40 h. This result suggests that the sample is quite stable and maintains a rather high activity for longer reaction time.
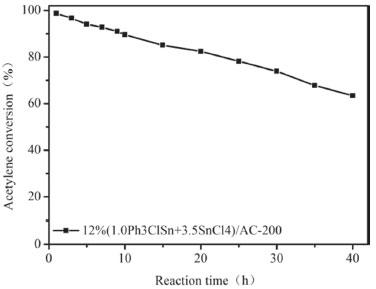 Figure 5. Acetylene conversion vs reaction time over 12%(1.0Ph3ClSn+3.5SnCl4)/AC-200 catalyst
Physico-chemical characterization of samples In the next step, a systematic characterization of this sample was performed aiming at observing the structural and morphological properties. Hence, the structural properties were first investigated by XRD. Figure 6 shows the XRD pattern of AC support and 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200 sample. Both XRD patterns display two obvious diffraction peaks at 26.4 and 44.4°, which correspond to the (002) and (101) crystal planes of AC (PDF#41-1487).31 The absence of diffraction peaks typical for Ph3ClSn+SnCl4 suggests their high dispersion on AC support.30
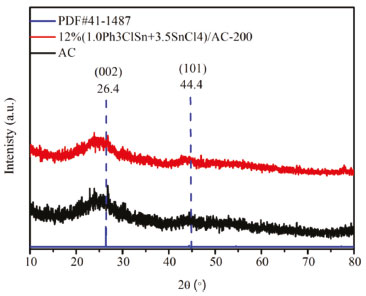 Figure 6. XRD pattern of 12%(1.0Ph3ClSn+3.5SnCl4)/AC-200 and AC
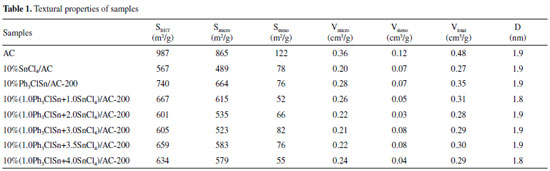
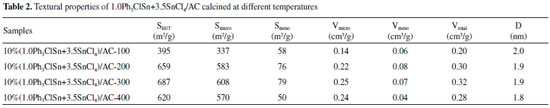
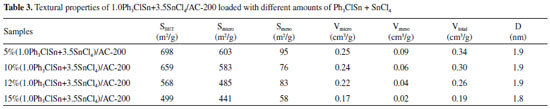
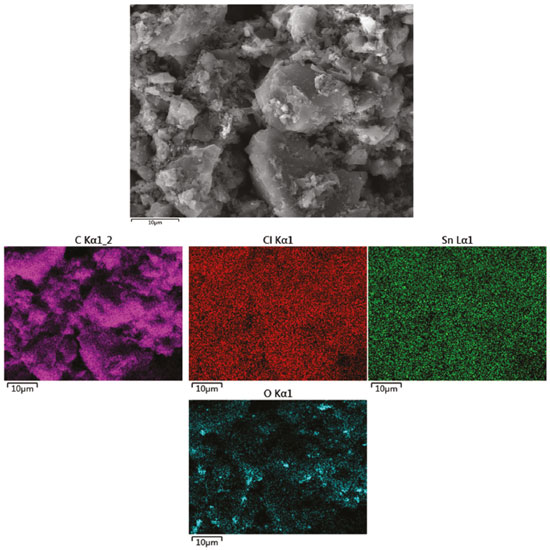
The morphology of the sample was further analyzed by SEM. A representative image is depicted in Figure 7. The elemental mapping was carried out to assess the distribution of the main elements in the sample. Thus, Figure 7 also illustrates the elemental maps for C, Cl, Sn, and O, which are homogeneously distributed in the catalyst. The existence of oxygen in catalysts is associated with the functional group on the surface of AC or the SnCl4 reacted with vapor during preparation process.32-36 In addition, the amount of Sn (4wt.%) was calculated. It was observed the that real amount of Sn is 3.66 wt.% (ICP), which is very close to the theoretical one of 4 wt.%.
TG-DTG was carried out to simulate the calcination process and to identify the main thermal transformation occurring in the sample during calcination at different temperatures. Figure 8 displays the TG curve for 12% (1.0Ph3ClSn+3.5SnCl4)/AC recorded between 25 and 800 °C. It can be observed that the mass loss up to 150 ºC is about 6.88% and corresponds to the evaporation of physically adsorbed ethanol (78.0 ºC), water (100.0 ºC) and partial sublimation of SnCl4 (114.3 ºC). As the temperature increases to 200 ºC, a weight loss of 1.62% was noticed. At same time, a small peak at around 200 ºC appeared in the DTG curve. The transformation occurring at this temperature is attributed to the organotin redistribution reaction between Ph3ClSn and SnCl4.2,12 When the temperature exceeds 250 ºC, a rapid weight loss of 11.14% was recorded. Along with the weight loss, the two peaks at around 237.2 ºC and 313.2 ºC appeared in the DTG curve, inferring that the pyrolysis of tin active sites may be operated in the range 250-400 ºC.
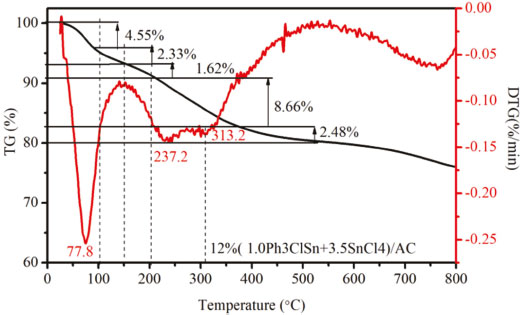 Figure 8. TG-DTG curve of 12%(1.0Ph3ClSn+3.5SnCl4)/AC
All samples studies in this work were prepared by using ethanol as solvent for the impregnation solution. However, a sample was also prepared using water as solvent aiming at verify the role of solvent on the activity of the resulted active sites. Therefore, to eliminate the effect of vapor on the catalytic activity of 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200,12% (1.0Ph3ClSn+3.5SnCl4)/AC-200-H2O was prepared by the same method and using water as solvent. Figure 9 shows the catalytic activity of 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200 prepared with ethanol and water in comparison with those of HgCl2 and PhCl3Sn/AC-200 catalysts.
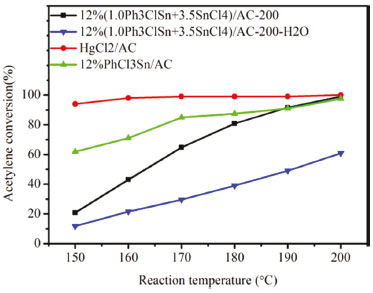 Figure 9. The acetylene conversion vs reaction temperature of 12%(1.0Ph3ClSn+3.5SnCl4)/AC-200,12%(1.0Ph3ClSn+3.5SnCl4)/AC-200-H2O, 12% PhCl3Sn /AC, and HgCl2/AC
As observed, the solvent has a strong effect on the catalytic activity, the sample prepared using water (12% (1.0Ph3ClSn+3.5SnCl4)/AC-200-H2O) exhibiting the lowest acetylene conversion (i.e., 58.5% at 200 °C). This result suggests that water favored the oxidation of tin precursor, which is detrimental for the catalytic activity catalysts. Thus, the solvent is crucial for the formation of tin active sites. Interestingly, 12% PhCl3Sn/AC-200 display a similar acetylene conversion (98.0%) with 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200 at 200°C. Based on the pyrolysis temperature of tin active site at ~237.2 ºC (Figure 8) and the organotin redistribution reaction between Ph3ClSn and SnCl4, we inferred that PhCl3Sn (257.4 ºC) is the possible mainly active sites in 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200. The PhCl3Sn /AC catalyst shows a high activity, but it is expensive. Moreover, 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200 also exhibits good acetylene conversion (98.0%) when compared to that of HgCl2/AC (99.5%) catalysts at 200 ºC. On the basis of these results, it can be stated that 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200 prepared with ethanol as solvent is a suitable catalyst for acetylene hydrochlorination and with high potential of scale-up.
CONCLUSION In this study, the PhnSnCl4-n-based catalysts were prepared by incipient wetness impregnation method using AC as support and triphenyltin chloride and tin(IV) chloride as precursors of the active sites. The catalytic performance of the resulted materials was evaluated in the acetylene hydrochlorination. The highest acetylene conversion of 98% with a selectivity to vinyl chloride of 98.5% was obtained at 200 °C for the catalyst with 12 wt.% total loading in Sn precursors and a Ph3ClSn : SnCl4 molar ratio of 1 : 3.5 after calcination at 200 °C under N2 (i.e., 12% (1.0Ph3ClSn+3.5SnCl4)/AC-200 catalyst). In addition, the experimental results revealed that the PhnSnCl4-n-based catalysts exhibit higher activity and stability than SnCl4/AC catalyst. On the basis of the results of physico-chemical characterization and catalysis, it was assumed that the active sites are mainly associated with the trichlorophenylstannane (PhCl3Sn) resulted from the redistribution reaction of triphenyltinchloride in the presence of SnCl4. It was also showed the solvent used for the preparation of catalyst plays a crucial role in generation of active catalysts. The experimental results revealed that water favors the oxidation of Sn active sites, which has a negative impact on the catalytic activity while ethanol was beneficial for obtaining a material with outstanding catalytic performance in acetylene conversion. Moreover, it can be stated that the cost-effective PhnSnCl4-n-based catalysts proposed in this work have a high potential to replace the more expensive PhCl3Sn /AC and the toxic HgCl2/AC catalysts for the hydrochlorination of acetylene.
SUPPLEMENTARY MATERIAL Figures 1S-3S are freely available online at http://quimicanova.sbq.org.br in PDF format.
REFERENCES 1. Hoch, M.; Appl. Geochem. 2001, 16, 719. 2. Ingham, R. K.; Rosenberg, S. D.; Gilman, H.; Chem. Rev. 1960, 60, 459. 3. Silva, M. A. D.; Santos, A. S. S. D.; Santos, T. V. D.; Catal. Sci. Technol. 2017, 7, 5750. 4. Ruiz, S. G.; Kaluđeroviđ, G. N.; Prashar, S.; Hawkin, E. H.; Eric, A.; Zizak, Z.; Juranic, Z. D.; J. Inorg. Biochem. 2008, 102, 2087. 5. Danishefsky, S.; Kerwin, J. F.; Kobayashi, S.; J. Am. Chem. Soc. 1982, 104, 358. 6. Nie, Y.; Hou, Q.; Li, W.; Bai, C.; Bai, X.; Ju, M.; Molecules 2019, 24, 594. 7. Howald, R. A.; Willard, J. E.; J. Am. Chem. Soc. 1955, 77, 2046. 8. Jing, H.; Nguyen, S. B. T.; J. Mol. Catal. A: Chem. 2007, 261, 12. 9. Dossetter, A. G.; Jamison, T. F.; Jacobsen, E. N.; Angew. Chem. 2010, 30, 2309. 10. Gademann, K.; Chavez, D. E.; Jacobsen, E. N.; Angew. Chem. 2010, 33, 144. 11. Espinet, P.; Echavarren, A. M.; Angew. Chem., Int. Ed. 2010, 43, 4704. 12. Gao, S.; Sun, X.; Lv, Z.; Qin, Y.; Zhang, X.; Song, L.; Li, J.; J. of Petrochem. Univ. 2016, 29, 1. 13. Deng, G.; Wu, B.; Li, T.; Liu, G.; Wang, L.; Zhou, W.; Chen, R.; Polyvinyl Chloride 1994, 6, 5. 14. Xiong, Q.; Wu, G.; Ling, S.; Xiong, Z.; Hu, Z.; Zou, Y.; Mod. Chem. Ind. 2017, 37, 66. 15. Lin, Y.; Wang, S.; Wu, Q.; Larssen, T.; Environ. Sci. Technol. 2016, 50, 2337. 16. Schoberth, H.; Chem. Rev. 2014, 114, 1743. 17. Hutchings, G. J.; ACS Cent. Sci. 2018, 4, 1095. 18. Conte, M.; Davies, C. J.; Morgan, D. J.; Davies, T. E.; Elias, D. J.; Carley, A. F.; Johnston, P.; Hutchings, G. J.; J. Catal. 2013, 297, 128. 19. Malta, G.; Kondrat, S. A.; Freakley, S. J.; Davies, C. J.; Lu, L.; Dawson, S.; Thetford, A.; Gibson, E. K.; Morgan, D. J.; Jones, W.; Wells, P. P.; Johnston, P.; Catlow, C. R. A.; Kiely, C. J.; Hutchings, G. J.; Science 2017, 355, 1399. 20. Ye, L.; Duan, X.; Wu, S.; Wu, T.; Zhao, Y.; Roberston, A. W.; Chou, H.; Zheng, J.; Ayvali, T.; Day, S.; Tang, C.; Soo, Y.; Yuan, Y.; Tsang, S. C. E.; Nat. Commun. 2019, 10, 914. 21. Zhang, L.; Jiang, H.; Wang, H.; Dong, S.; Ding, Q.; Song, L.; J. of Petrochem. Univ. 2013, 26, 6. 22. Guo, Y.; Liu, Y.; Hu, R.; Gao, G.; Sun, H.; Appl. Chem. 2014, 31, 624. 23. Zuckerman, J. J.; ACS Symp. Ser. 1978, 24, 388. 24. Li, X.; Pan, X.; Yu, L.; Ren, P.; Wu, X.; Sun, Li.; Jiao, F.; Bao, X.; Nat. Commun. 2014, 5, 3688. 25. Conte, M.; Carley, A. F.; Attard, G.; Herzing, A. A.; Kiely, C. J.; Hutchings, G. J.; J. Catal. 2008, 257, 190. 26. Conte, M.; Carleya, F.; Heirence, C.; Willock, D. J.; Johnston, P.; Herzing, A. A.; Kiely, C. J.; Hutcgings, G. J.; J. Catal. 2007, 250, 231. 27. Shen, Z.; Hong, Z.; Yue, L.; Ze, Kan.; Ping, X.; Jing, Z.; Biao, J.; React. Chem. Eng. 2018, 3, 34. 28. Li, X.; Zhang, J.; You, H.; Zhu, M.; Shang, S.; Li, W.; J. Mater. Sci. 2018, 53, 4913. 29. Chen, K.; Kang, L.; Zhu, M.; Dai, B.; Catal. Sci. Technol. 2015, 5, 1035. 30. Dong, Y.; Zhang, H.; Li, W.; Sun, M.; Guo, C.; Zhang, J.; J. Ind. Eng. Chem. 2015, 35, 177. 31. Qian, H. S.; Han, F. M.; Zhang, B.; Guo, B.; Yan, Y.; Peng, B.; Carbon 2004, 42, 761. 32. Zhang, H.; Li, W.; Jin, Y.; Sheng, W.; Mao, C.; Wang, X.; Zhang, J.; Appl. Catal., B 2016, 189, 56. 33. Gurrath, M.; Kuretzky, T.; Boehm, H. P.; Okhlopkova, L. B.; Lisitsyn, A. S.; Likholobov. V. A.; Carbon 2000, 38, 1241. 34. Dumbuya, K.; Cabailh, G.; Lazzari, R.; Jupille, J.; Ringel, L.; Pistor, M.; Lytken, O.; Steinruck, H. P.; Gottfried, J. M.; Catal. Today 2012, 181, 20. 35. Kobayashi, Y.; Lizmarzán, L. M.; Chem. Mater. 2001, 13, 1630. 36. Kim, J. H.; Choi, S. M.; Sang, H. N.; Seo, M. H.; Chol, S. H.; Kim, W. B.; Appl. Catal., B 2008, 82, 89. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access