Artigo
| Development of an analytical method for the determination of lead based on local surface plasmon resonance of silver nanoparticles |
|
Hamed AzimiI, Seyyed Hamid AhmadiII,*; Mohammadreza ManafiI; Syed Husain Hashemi MousaviI; Mostafa Najafic
I. Department of Applied Chemistry, Faculty of Science, South Tehran Branch, Islamic Azad University, Tehran, Iran Recebido em 12/04/2019 *e-mail: ahmadi@ccerci.ac.ir Lead is environmental pollutant that even in low trace is harmful for human health and wildlife. In this study we presented a colorimetric method for determination of Pb2+ in water by aggregation of silver nanoparticles capped citrate in the presence of deferoxamine as chelating agent. Silver nanoparticles were prepared by reduction of AgNO3 with NaBH4. In the presence of Pb2+, mixture of silver nanoparticles capped citrate and deferoxamine aggregated and color of solution changed from light yellow to violet that depends on Pb2+ concentration. As a result of aggregation, local surface plasmon resonance (LSPR) band of silver nanoparticles around 440 nm decreases and a new band appears at 580 nm that depend on Pb2+ concentration. Under optimized conditions, linear relation of concentration of Pb2+ and absorbance ratio (A580/A440), was from 0.19 to 1.29 µmol L-1 and limit of detection of 0.056 µmol L-1 was found. Control experiments with the addition of 10 other metal ions (Ni2+, Cu2+, Ca2+, Al3+, Co2+, K+, Ba2+, Zn2+, Mg2+, Hg2+) did not result in a distinct change in the color or in the absorption spectra. This approach was successfully tested for determination of Pb2+ in well and tap water. INTRODUCTION Heavy metals are generally described as metals with high densities, atomic weights or atomic numbers. Numerous applications of heavy metals in industry, medicine, agriculture, etc., caused concerning of their effects in health and safety in environment. Although heavy metals exist in all over the earth, most environmental contamination carried out in the mining and industrial producing. Environmental contamination by heavy metals also occurs by metal corrosion, atmospheric deposition and soil erosion of metal ions. Some of heavy metals like Fe, Mn, Cu and Zn considered as essential nutrients and required in biochemical and physiological performance. Other heavy metals like Pb, Cd, Hg and As considered as metals that have no essential biochemical and physiological roles and some of them have toxicity and harmful for human nature, therefore determination of these heavy metals in environment is necessary.1 Several of high sensitivity methods such as graphite furnace atomic absorption spectrometry,2 electrothermal vaporization inductively coupled plasma mass spectrometry (ETV-ICP-MS),3 solid-phase microextraction-gas chromatography-atomic emission detection (SPME-GC-AED),4 square wave anodic stripping voltammetry (SWASV)5 have been developed for the determination of Pb2+. However, these methods need to time consuming, high price and complicated instruments, thus it is necessary to develop a new method for determination of Pb2+ that fast, low price and there is no need to high price instruments for assay. Local surface plasmon resonance (LSPR) is a nanosize phenomenon that attracted attention in recent years. This phenomenon can create strong absorption spectra that using for detection of many of chemical and biological samples.6 Noble metals like gold and silver have free electrons, when a noble metal in form of nanoparticles, affected by the irradiation of light, free electrons of nanoparticles collectively oscillate at a resonant frequency in a phenomenon called surface plasmon resonance and when a noble metal nanoparticle affected under radiation of light, this phenomenon can create sharp absorption spectra.7 Local surface plasmon resonance is a powerful method for determination of chemical and biological analytes in the environmental samples. In the past decade, silver plasmonic nanostructures used for determination of metals like Ba2+, Mn2+, Fe3+, Co2+, Ni2+, Cd2+, Hg2+, Cu2+ and Cr3+.8-16 These experiments are based to plasmon resonance wavelength changes of silver nanoparticles as aggregation that takes place due to interaction between nanoparticles with samples. The goal of this work is development of a simple and low cost method for determination of Pb2+ in water by silver nanoparticles. We optimized effective factors in the assay like concentration of chelating agent, ionic strength, pH and concentration of stabilizer agent. Proposed method was successfully applied for the determination of Pb2+ in two real water samples.
EXPERIMENTAL Chemical and reagents Sodium borohydride (NaBH4), silver nitrate (AgNO3), sodium chloride (NaCl), sodium hydroxide (NaOH), hydrochloric acid (HCl 37%), tri sodium citrate (Na3C6H5O7) and lead (II) acetate (PbC4H6O4.3H2O) were purchased from Merck. Deferoxamine mesylate (C26H52N6O11S) was obtained from Sigma-Aldrich. Stock solutions of deferoxamine mesylate and Pb2+ were prepared in distilled water. All different salts were purchased from Merck. Preparation of silver nanoparticles Silver nanoparticles were synthesized by the reduction of AgNO3 with NaBH4. Under vigorous magnetic stirring, 0.012 g NaBH4 was added quickly to 100 mL aqueous solution of AgNO3 with concentration of 0.1 mmol L-1, producing a light yellow colored solution. Finally, the prepared AgNPs solution was stored at 4 °C in darkness before use.17 Apparatus UV-Vis absorbance spectra were acquired on a UV-Vis spectrophotometer with a 1.0 cm quartz cell from 350 to 650 nm (PG T80+, PG instruments, United Kingdom). Measurements of pH carried out by 827 Metrohm pH Meter (Metrohm AG, Switzerland). Size distributions of the particles were done in specific temperature with dynamic light scattering device (Horiba Sz-100, Horiba LTD, Japan). Transmission electron microscopy (TEM) was employed for recording the morphology and size of the nanoparticles (Philips CM30, Netherlands). Inductively coupled plasma-optical emission spectrometer (730-ES, Varian, USA) was used for obtaining ICP data. Colorimetric detection of Pb2+ For colorimetric of Pb2+, 3.3 mL silver nanoparticles, 50 µL deferoxamine (0.0001 mol L-1), 250 µL trisodium citrate (0.1 mol L-1), and 1.5 mL NaCl (0.1 mol L-1) were added in volumetric flask and then different amounts of Pb2+ were added to the solution, consequently an appropriate distilled water were added to reach a final volume of 10 mL. Finally, this solution was transferred to the spectrometric cell for measuring absorbance. Real sample analysis To investigate ability of this method for determination of Pb2+ in water samples, detection of Pb2+ was carried out in two different water samples, consisting of well and tap water. 160 µL of 0.001 mol L-1 Pb2+ spiked to 1 mL water then 25 and 37.5 µL from prepared sample individually added to mixture of silver nanoparticles and NaCl and trisodium citrate.
RESULTS AND DISCUSSION Mechanism of assay Preliminary experiments with silver nanoparticles were performed to reach the optimal conditions and improving sensitivity of the assay for Pb2+ determination in environmental water samples. The effect of silver nanoparticles, deferoxamine, trisodium citrate and sodium chloride concentrations, pH and reaction time were studied. The basis of Pb2+ determination is illustrated in the Scheme 1. This drug used to treatment of patients that suffering β-thalassemia for ability of this drug to create strong complexes with heavy metals. When deferoxamine was added to the solution contains citrate capped nanoparticles, silver nanoparticles were dispersed and aggregation of silver nanoparticles did not happen but when Pb2+ was added to the solution, due to chelation of Pb2+ with deferoxamine, aggregation of silver nanoparticles happened.
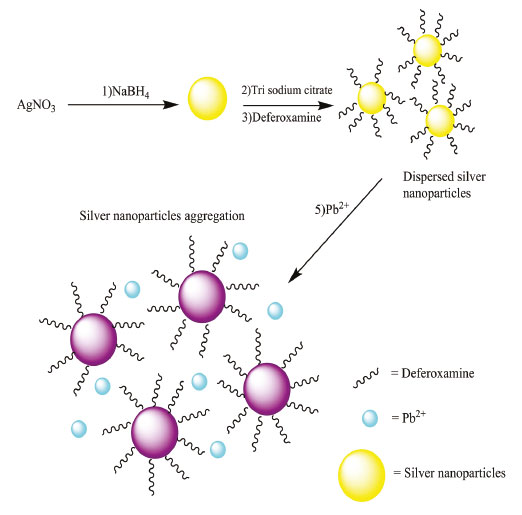 Scheme 1. The Strategy of DFO assay
Figure 1 shows absorption spectra of silver nanoparticles demonstrating that silver nanoparticles have a peak around 440 nm (blue). This Figure also shows when aggregation of silver nanoparticles with adding Pb2+ occurs, characteristic absorbance decreases, and a new broad absorption band appears in around 580 nm (red). Color of silver nanoparticles changes from light yellow to light violet or violet that depends on Pb2+ concentration. Aggregation of silver nanoparticles with adding Pb2+ and deferoxamine is supported with DLS and TEM images (Figure 1).
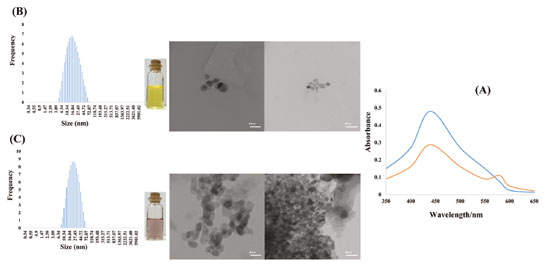 Figure 1. Absorption spectra in absence (blue) and in the presence (red) of 0.55 µmol L-1 Pb2+ (a). TEM images and color of silver nanoparticles and DLS analysis of silver nanoparticles (b) in absence and (c) in the presence of 0.92 µmol L-1 Pb2+
Optimization of assay condition We studied the effect of reaction time in range of 15-145 seconds in the assay and after 60 seconds there is no change in absorbance ratio and 60 seconds was selected as optimum reaction time for further experiments (Figure 1S). Effect of deferoxamine concentration in aggregation of silver nanoparticles from 0.5 to 2.5 µmol L−1 was also studied. Ability of silver nanoparticles for aggregation was decreased with increasing deferoxamine concentration (Figure 2S).
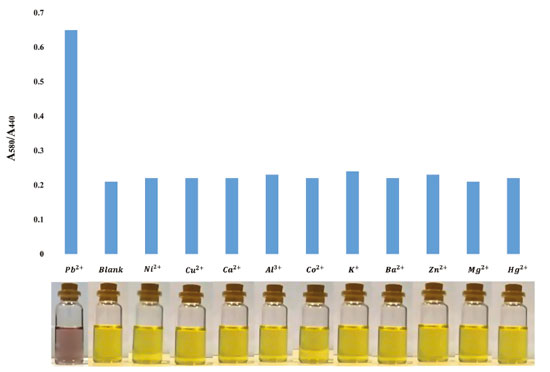 Figure 2. The absorption ratio value (A580/A440) and images of silver nanoparticles in the presence of interferences including metal ions (Ni2+, Cu2+, Ca2+, Al3+, Co2+, K+, Ba2+, Zn2+, Mg2+, Hg2+). Concentration of Pb2+ is 1.29 µmol L-1 and concentration of all metal ions considered 50 times higher than Pb2+
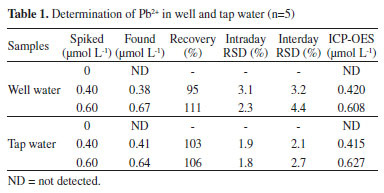
Also, the effect of NaCl concentration on aggregation of silver nanoparticles was explored because ionic strength has a critical role on aggregation of silver nanoparticles.18 Aggregation of silver nanoparticles did not take place in low concentration of NaCl (low ionic strength) but after increasing concentration of NaCl, aggregation carried out in the absence Pb2+ due to sodium chloride can constrict electrical double layer of citrate ions (Figure 3S). NaCl concentration was studied in range of 5-25 mmol L-1 and 15 mmol L-1 was selected as optimized concentration. Effect of tri sodium citrate concentration as stabilizer of silver nanoparticles was studied on aggregation of silver nanoparticles. As shown in Figure 4S, concentration of tri sodium citrate studied from 0.5 to 3 mmol L-1 and we observed that with increasing concentration of tri sodium citrate to 2.5 mmol L-1, ratio of absorption was increased and after that, ratio was stabilized, thus 2.5 mmol L-1 of tri sodium citrate was selected as optimum concentration of citrate. pH has a crucial effect on aggregation of silver nanoparticles, therefore effect of pH in the presence of 0.55 µmol L-1 Pb2+ was studied in the range of 3-10. In pH < 3, due to protonation of citrate molecules, surface charge of the silver nanoparticles was neutralized. In 3 < pH < 8, deferoxamine and citrate molecules were protonated and for this reason, aggregation of silver nanoparticles was increased. In pH > 8 tri sodium citrate becomes negative and deferoxamine is neutralized and for this reason, electrostatic attraction between silver nanoparticles decreased and then aggregation of silver nanoparticles was lowered.19 Consequently, pH=8 was selected as optimized pH for aggregation of silver nanoparticles in this assay (Figure 5S). Sensitivity and selectivity of the analytical method Colorimetric determination of Pb2+ by deferoxamine under optimum conditions was performed. The calibration curve shows a linear correlation at concentration from 0.19 to 1.29 µmol L-1. Limit of detection was calculated 0.056 µmol L-1. Color of the solution of silver nanoparticles is light yellow and when aggregation take place, color of solution firstly is changed to light violet and with increasing concentration of Pb2+ changed to violet (Figure 6S). Interferences By reason to verify selectivity of determination of Pb2+ in water, we studied interfering effect of some metal ions including Ni2+, Cu2+, Ca2+, Al3+, Co2+, K+, Ba2+, Zn2+, Mg2+ and Hg2+. The metal ions were individually added to silver nanoparticles colloid. Concentration of Pb2+ was 1.29 µmol L-1 and concentration of all metal ions considered 50 times higher than Pb2+. As shown in Figure 2, there is no distinct color change and additionally UV-visible absorption features. Analysis of spiked samples To evaluate performance of the colorimetric method, the proposed procedure was applied for the determination of Pb2+ in water samples and two different samples including tap water and well water were studied. Recovery tests were used to evaluate the matrix effects, accuracy and reliability of the method. The different standard amounts of Pb2+ were spiked into the samples and lead concentration was determined at optimum conditions for each sample. The results of study are summarized in Table 1, as can be seen, they indicate the potential of this method for simple and sensitive determination of Pb2+ in real water samples. The results indicate quantitative recoveries in tap and well water (95-111%). The results were in good agreement with those obtained by ICP OES, demonstrating the practical analytical utility of the method. Also, the relative standard deviations (RSD) were found to be <5% in tap and well water. The intra-day and inter-day precisions of the proposed method were used to evaluate the precision of the method. For this purpose, five similar experiments were carried out for the analysis of samples on the same day and on five consecutive days. The resulted RSDs for the analysis of intra-day and inter-day spiked samples were in the range of 1.8-3.1% and 2.1-4.4%, respectively.
Comparison with previous studies Our proposed analytical method showed satisfactory due to simplicity, speed, efficiency and no need to sample preconcentration compared with the previous studies as shown in Table 2. It can be successfully applied to determination of lead in tap water samples.
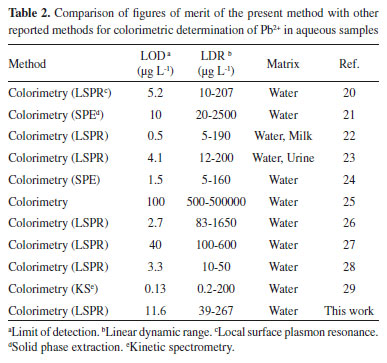
The limit of detection and the linear dynamic range of the proposed method for the lead are comparable with other methods.20-29 However, in comparison to the solid phase extraction-colorimetry, LSPR technique is faster.
CONCLUSION In this assay silver nanoparticles capped with citrate are used for determination of Pb2+ based on silver nanoparticles aggregation. We observed high sensitivity and good linear calibration curve. Under optimized conditions, DLR was from 0.19 to 1.29 µmol L-1 and limit of detection was 0.056 µmol L-1. The figures of merit obtained are in good agreement with reported colorimetric researches.20-29 The main advantages of the proposed method are simplicity, sensitivity, low cost, and ease of operation.
ACKNOWLEDGEMENTS The authors wish to state their thanks to Islamic Azad University South Tehran Branch, for supporting this work.
REFERENCES 1. Tchounwou, P. B.; Yedjou, C. G.; Patlolla, A. K.; Sutton, D. J.; Mol. Clin. Environ. Toxicol. 2012, 101, 133. 2. Spudich, T. M.; Herrmann, J. K.; J. Chem. Educ. 2004, 81, 262. 3. Li, L.; Hu, B.; Xia, L.; Jiang, Z.; Talanta 2006, 70, 468. 4. Crnoja, M.; Troyer, C. H.; Rosenberg, E.; Grasserbauer, M.; J. Anal. At. Spectrom. 2001, 16, 1160. 5. Pena, R. C.; Cornejo, L.; Bertottic, M.; Brett, C. M. A.; Anal. Methods 2018, 10, 3624. 6. Mayer, K. M.; Hafner, J. H.; Chem. Rev. 2011, 111, 3828. 7. Lu, X.; Rycenga, M.; Skrabalak, S. E.; Wiley, B.; Xia, Y.; Ann. Rev. Phys. Chem. 2009, 60, 167. 8. Khodaveisi, J.; Haji Shabani, A. M.; Dadfarnia, S.; Moghaddam, M. R.; Hormozi-Nezhad, M. R.; Quim. Nova 2015, 37, 896. 9. Zhou, Y.; Zhao, H.; Li, C.; He, P.; Peng, W.; Yuan, L.; Zeng, L.; He, Y.; Talanta 2012, 97, 331. 10. Zhan, J.; Wen, L.; Miao, F.; Tian, D.; Zhu, X.; Li, H.; New J. Chem. 2012, 36, 656. 11. Yao, Y.; Tian, D.; Li, H.; ACS Appl. Mater. Interfaces 2010, 2, 684. 12. Shang, Y.; Wu, F.; Qi, L.; J. Nanopart. Res. 2012, 14, 1169. 13. Li, H.; Yao, Y.; Han, C.; Zhan, J.; Chem. Commun. 2009, 32, 4812. 14. Fan, Y.; Liu, Z.; Wang, L.; Zhan, J.; Nanoscale Res. Lett. 2009, 4, 1230. 15. Ratnarathorn, N.; Chailapakul, O.; Henry, C. S.; Dungch, W.; Talanta 2012, 99, 557. 16. Wu, T.; Liu, C.; Tan, K. J.; Hu, P. P.; Huang, C. Z.; Anal. Bioanal. Chem. 2010, 397, 1273. 17. Wu, X.; Xu, Y.; Dong, Y.; Jiang, X.; Zhu, N.; Anal. Methods 2013, 5, 560. 18. Vaishnav, S. K.; Patel, K.; Chandraker J.; Korram, K.; Nagwanshi, R., Ghosh, K. K.; Satnami, M. L.; Spectrochim. Acta, Part A 2017, 179, 155. 19. Badawy, A. M. E.; Luxton, T. P.; Silva, R. G.; Scheckel, K. G.; Suidan, M. T.; Tolaymat, T. M.; Environ. Sci. Technol. 2010, 44, 1260. 20. Ding, N.; Cao, Q.; Zhao, H.; Yang, Y.; Zeng, L.; He, Y.; Xiang, K.; Wang, G.; Sensors 2010, 10, 11144. 21. Caliskan, E.; Tinas, H.; Ozbek, S.; Akman, N.; Anal. Sci. 2017, 33, 387. 22. Ratnarathorn, N.; Chailapakul, O.; Dungchai, W.; Talanta 2015, 132, 613. 23. Xu, H.; Liu, B.; Chen, Y.; Microchim. Acta 2012, 177, 89. 24. Al-Mallah, Z.; Amin, A. S.; J. Ind. Eng. Chem. 2018, 67, 461. 25. Yan, J.; Indra, E. M.; Anal. Chem. 2012, 84, 6122. 26. Qi, L.; Shang, Y.; Wu, F.; Microchim. Acta 2012, 178, 221. 27. Noh, K. C.; Nam, Y. S.; Lee, H. J.; Lee, K. B.; Analyst 2015, 140, 8209. 28. Annadhasan, M.; Muthukumarasamyvel, T.; Sankar Babu, V. R.; Rajendiran, N.; ACS Sustainable Chem. Eng. 2014, 2, 887. 29. Wu, T.; Ma. Z.; Microchim. Acta 2017, 184, 577. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





