Artigo
| Antioxidant naphthoquinones of Sinningia reitzii from Santa Catarina state, Brazil |
|
Vanessa WiniewskiI; Adson S. SilvaI; Kattleen D. C. AlvarezII; Eduardo L. de SáI; Marcos J. SalvadorII; Maria Élida A. StefanelloI,*
I. Departamento de Química, Universidade Federal do Paraná, 81530-900 Curitiba - PR, Brasil Recebido em 25/07/2020 *e-mail: elida@ufpr.br Sinningia reitzii (Hoehne) L. E. Skog (Gesneriaceae) is a subshrub native to Brazil, where it is distributed in two disjunctive populations around the 26°S and 23°S parallels. In this work, tubers of plants from the wild population growing at the 26°S parallel (Santa Catarina state) yielded two new naphthoquinones, 5,6-dihydroxy-7-methoxy-a-dunnione and 8-hydroxy-6,7-dimethoxy-a-dunnione. Three known compounds were also isolated: 6,8-dihydroxy-7-methoxy-a-dunnione, 5-hydroxy-6,7-dimethoxy-a-dunnione, and 6,8-dihydroxy-7-methoxy-2-O-methyldunniol. The naphthoquinones 6,8-dihydroxy-7-methoxy-2-O-methyldunniol, 6,8-dihydroxy-7-methoxy-a-dunnione, and 5,6-dihydroxy-7-methoxy-a-dunnione displayed antioxidant activity in the ORAC-FL method, with values of relative trolox equivalent in the range of 1.39-4.83 mmol g-1. INTRODUCTION The neotropical genus Sinningia (Gesneriaceae) comprises more than 70 species distributed from Mexico to Argentina, of which 75 species are recognized as native to Brazil. The genus is found in several biomes, but most species occur in the Atlantic Forest of the Southeast region of the country.1,2 The chemical constituents reported to date in Sinningia are typical for members of the Gesneriaceae family. Caffeoyl ethanoid glycosides, which are widely distributed in Gesneriaceae, have been reported from ethanolic extracts of several Sinningia species. Other compound classes, such as triterpenes, sesquiterpenes, flavonoids, anthraquinones, naphthoquinones and derivatives, have been isolated from less polar extracts of Sinningia and of other Gesneriaceae genera.3,4 Sinningia reitzii (Hoehne) L. E. Skog, known as "cachimbo", is a perennial subshrub with tubers endemic to Brazil. The plant measures 30-120 cm in height, and has dark green leaves with red veins on the abaxial surface; the red color also covers the entirety of the abaxial surface area in some specimens. The plants produce tubular magenta flowers from January to July.5 The geographic distribution of S. reitzii is discontinuous, with two isolated populations being reported.1 The first one, called S. reitzii "SC" in this work, occurs in Southern Brazil, in the area around the 26°S parallel, in the Santa Catarina State. The first description of S. reitzii (as Rechsteineria reitzii) was made from a specimen belonging to this population.6 The second population (referred to as S. reitzii "PR" in this work) is located around the 23°S parallel, with several records in the São Paulo State and one record in the Paraná State.5 Previous phytochemical studies of the tubers from S. reitzii "PR" have reported the isolation of twelve prenylated naphthoquinones with different framework, from the less polar extracts. Among them, two dunnione-type naphthoquinones deserve to be highlighted for their biological properties. Anti-inflammatory and anti-nociceptive activities were found for 8-hydroxydehydrodunnione,7 while 6,7-dimethoxydunnione showed cytotoxicity against PC-3 (prostate) and HeLa (cervix) human tumor cell lines.8 Considering that the geographic isolation of populations of plants from the same species can lead to development of chemical varieties within the species, we decided to study the less polar chemical constituents from tubers of S. reitzii "SC", which had not been investigated yet. In addition, antioxidant activity was evaluated for three isolated compounds.
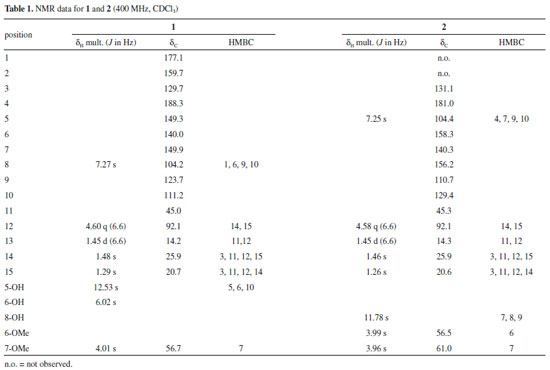
EXPERIMENTAL General procedures Optical rotations were measured in CHCl3 on a JASCO PTC-203 polarimeter (l = 589 nm, temperature = 20 oC). Optical density was measured using a Synergy 2 (Bio-Tek) spectrophotometer. UV spectra were obtained in CHCl3 on a Shimadzu UV-2401PC spectrophotometer. Circular dichroism (CD) spectra were recorded in a Jasco J-815 CD spectrometer. IV spectra were recorded on a Bruker Vertex 70/70v FTIR spectrophotometer over the range of 4000-500 cm-1. One-dimensional (1H, 13C) and two-dimensional (gHSQC, gHMBC) NMR spectra were recorded on Bruker spectrometers (Avance 400 and/or Avance 600) observing 1H at 400 or 600 MHz, and 13C at 100 or 150 MHz. Deuterated chloroform (CDCl3) was used as solvent. Chemical shifts are given in ppm (d), with coupling constants (J) in Hz. TMS was used as internal reference. High-resolution electrospray ionization mass spectrometry (HRESIMS) data were obtained on a Bruker Micromass ESI Q-TOF mass spectrometer. Geometry optimization and density functional theory (DFT) calculations on the electronic structure of the compounds employed B3LYP functional, having Los Alamos ECP as basis set as implemented in the Gaussian suite program.9 HPLC separations were performed in a Waters apparatus equipped with PDA detector and a semi-preparative Nucleosil 100-5 C18 column (250 × 10 mm). Acetonitrile:water (60:40 or 50:50, isocratic) was used as mobile phase, with a flow rate of 2.8 mL min-1 applied for 25 min at room temperature. The column effluent was monitored over the 210-400 nm range. Column chromatographic separations (CC) were carried on silica gel 60 (Merck, 230-400 mesh), while precoated silica gel 60 GF254 plates (Macherey-Nagel) were used for TLC analyses. Compounds were visualized by exposure under UV254/365 light and spraying with 5% (v/v) H2SO4 in ethanol solution, followed by heating on a hot plate. All solvents were analytical or spectroscopic grade, and the mixtures of solvents were prepared as v/v. Plant material Tubers of Sinningia reitzii (Hoehne) L. E. Skog were collected in Corupá, Santa Catarina State, Brazil (26°24'10.9'' S; 49º17'17.54'' W), in November/2018. The plant was identified by Mauro Peixoto, and a voucher specimen was deposited in the Herbarium of Universidade Federal do Paraná (UPCB 93050). The access was registered on SISGEN under number AF5C97F. Extraction and isolation Dried and powdered tubers of S. reitzii "SC" (53.2 g) were extracted with CH2Cl2 (four successive extractions employing, each time, 500 mL of solvent) at room temperature. The solvent was removed under reduced pressure to give the dichloromethane extract (387.1 mg). An aliquot (50 mg) of the extract was reserved. The remaining extract (337.1 mg) was submitted to CC eluted with mixtures of Hex:EtOAc (7:3; 3:2; 1:1; 3:7), EtOAc and MeOH, yielding 10 fractions after TLC analysis (F1-10). Fraction F4 (33.6 mg; eluted with Hex:EtOAc 7:3) was further purified by semi-preparative HPLC using H2O:MeCN 40:60 as mobile phase. The sample was dissolved in 4 mL of MeCN, and 20 aliquots of 200 µL were injected. This procedure yielded 3 (2.1 mg; Retention time 11.1 min), 2 (0.8 mg; Rt 17.44 min), 5 (1.7 mg; Rt 18.50 min) and 4 (1.0 mg; Rt 20.39 min). Fraction F5 (33.3 mg, eluted with Hex:EtOAc 7:3) yielded 3. Fraction F7 (17.6 mg; eluted with Hex:EtOAc 1:1) was purified by semi-preparative HPLC (H2O:MeCN 50:50). The sample was dissolved in 2 mL of MeCN, and 10 aliquots of 200 mL were injected to give 1 (3.2 mg; Rt 17.78 min) and 3 (1.1 mg, Rt 18.93 min). Antioxidant activity by ORAC-FL assay The antioxidant capacity of the samples was measured using the Oxygen Radical Absorbance Capacity (ORAC) assay, with fluorescein (FL) as the fluorescent probe and AAPH [2,2'-azobis(2-amidiopropane) dihydrochloride] as a free radical source. The experiments were carried on 96 wells plates as previously reported.10 Briefly, several dilutions of the samples (12.5-200 µM) were prepared in phosphate buffer. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as a standard, being analysed at concentrations of 12.5, 25, 50, 100 and 200 µM. The reading was performed using a fluorescent filter (excitation λ = 485 nm and emission λ = 528 nm) in a microplate reader, monitoring the reaction at 37°C every 2 min for a period of 70 min. Results were expressed as relative trolox equivalent ± standard deviation. Caffeic acid, chlorogenic acid and quercetin were used as experimental positive controls. All experiments were performed in triplicate. 5,6-dihydroxy-7-methoxy-a-dunnione (1) Orange solid; experimental [a]D20 -101.5 (c 0.03, CHCl3), calculated [a]D20 -132; UV-Vis (CHCl3) lmax/nm (log e) 268 (4.01), 338 (3.78), 431 (3.43); CD (c 0.001, CHCl3) lmax (q) 330 (-43.0), 381 (+10.7); IR (KBr) nmax/cm-1 3412 (OH, C-6), 2923 and 2851 (C-H), 1630 (C=O), 1453 and 1358 (C-H), 1194 (C-O); for 1H and 13C NMR data, see Table 1; HRESIMS, calcd. for C16H17O6 [M + H]+: 305.1025; observed: m/z 305.1010 (error = 4.9 ppm).
8-hydroxy-6,7-dimethoxy-a-dunnione (2) Yellow solid; experimental [a]D20 -158.4 (c 0.01, CHCl3), calculated [a]D20 -319; UV-Vis (CHCl3) lmax/nm (log e) 239 (3.77), 268 (4.09), 335 (3.88), 428 (3.34); CD (c 0.001, CHCl3) lmax (q) 330 (-23.5), 381 (+5.0); IR (CHCl3) nmax/cm-1 2923 and 2850 (C-H), 1640 (C=O), 1605 (C=C), 1462 and 1372 (C-H), 1261 and 1146 (C-O); for 1H and 13C NMR data, see Table 1; HRESIMS, calcd. for C17H19O6 [M + H]+: 319.1182; observed: m/z 319.1175 (error = 2.1 ppm).
RESULTS AND DISCUSSION The dichloromethane extract of tubers of S. reitzii "SC" yielded two new naphthoquinones (1-2) and three known compounds, which were identified as 6,8-dihydroxy-7-methoxy-a-dunnione (3),11 5-hydroxy-6,7-dimethoxy-a-dunnione (4),7 and 6,8-dihydroxy-7-methoxy-2-O-methyldunniol (5).12 (Figure 1). Compounds 4-5 had been previously reported in S. reitzii "PR" tubers;7,8 however, other naphthoquinones isolated from S. reitzii "PR" were not found in S. reitzii "SC", including the compounds previously described with biological effects. All isolated compounds were analyzed by NMR (1D and 2D), and the data were compared with the literature.
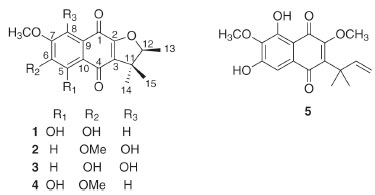 Figure 1. Chemical structures of isolated compounds of S. reitzii "SC"
Compound 1 was isolated as an orange solid, with molecular formula C16H16O6 deduced from NMR data (Table 1) and positive HRESIMS (m/z 305.1010 [M + H]+), which is consistent with nine indices of hydrogen deficiency. The IR spectrum of 1 showed absorption bands for hydroxy (3412 cm-1) and carbonyl groups (1630 cm-1).11,13 In the 1H NMR data, signals for one hydrogen (δH 7.27), a methoxy group (δH 4.01), an hydroxy group with an intramolecular hydrogen bond (δH 12.53), a second hydroxy group (δH 6.02, broad singlet), and a 2,3-dihydro-2,3,3-trimethylfuran group (quartet at δH 4.60, two singlets at δH 1.29 and 1.48, and a doublet at δH 1.45) were observed (Table 1). Considering previous studies on S. reitzii "PR", these data suggested a naphthoquinone type dunnione (1,2-naphthoquinone) or a-dunnione (1,4-naphthoquinone). These two types can be distinguished by analyzing the 13C NMR data. The carbons from the quinone moiety are observed at around δH123 (C-3), 168 (C-4), 175 (C-2), and 181 (C-1) for dunnione-derivatives, while in a-dunnione-derivatives they are observed at around δH130 (C-3), 158 (C-2), 177 (C-1), and 182 (C-4). Carbonyl groups involved in intramolecular hydrogen bonds are deshielded by 5-7 ppm.13 The 13C {1H} NMR data of 1 showed peaks for 16 carbons, including two of carbonyl groups at δH 177.1 and 188.3. The first one was typical of C-1 in the a-dunnione framework, while the second had the chemical shift compatible with a carbonyl group associated with a hydroxy group, which we assigned to C-4. Therefore, the hydroxy group with an intramolecular hydrogen bond was located at C-5. Other signals characteristic of the quinone group in a-dunnione-derivatives (δH 129.7 and 159.7) were also observed. In the HMBC spectrum, the hydrogen at δH 7.27 showed a cross-peak with C-1, and was consequently located at C-8. The hydrogen H-8 and the hydroxy group at C-5 also exhibited cross-peaks with carbons at δH111.2 (C-10) and 140.0 (C-6), while the methoxy group showed a cross-peak with a carbon at δH149.9. These correlations indicated that the methoxy group was at C-7, and the second hydroxy group at C-6. These and remaining correlations in HSQC and HMBC (Table 1, Figure 8S) led to identification of 1 as 5,6-dihydroxy-7-methoxy-a-dunnione. Compound 2, a yellow solid, had the molecular formula C17H18O6, with nine indices of hydrogen deficiency, as deduced from NMR data (Table 1), and an ion at m/z 319.1175 [M + H]+ in the positive HRESIMS. The IR spectrum of 2 exhibited an absorption band for a carbonyl group (1640 cm-1).11,13 The 1H NMR data of 2 were very similar to those of compound 1, showing signals for one hydrogen (δH 7.25), a hydroxy group with an intramolecular hydrogen bond (δH 11.78), two methoxy groups (δH3.96 and 3.99), and a 2,3-dihydro-2,3,3-trimethylfuran group. These data suggested that the main difference between 2 and 1 was the presence of a methoxy group in 2 replacing a hydroxy group in 1. It was not possible to record the 13C {1H} NMR for 2, since only 0.8 mg of it had been isolated. However, the 13C NMR chemical shifts could be obtained from HSQC and HMBC spectra, which showed cross-peaks for carbons at δH131.1, 158.3 and 181.0, indicating a a-dunnione-derivative. Besides, in the HMBC spectrum, the methyl groups at δH 1.26 (C-15) and 1.46 (C-14) showed correlation with C-3 (δH131.1), confirming the presence of a 1,4-naphthoquinone. However, unlike in 1, the hydrogen at δH 7.25 showed a cross-peak with C-4 (δH181.0) instead of C-1 (around δH177 for non-associated carbonyl group) in the HMBC. Therefore, this hydrogen was located at C-5, the hydroxy group with an intramolecular hydrogen bond was located at C-8, and the methoxy groups at C-6 and C-7. These and the remaining correlations observed in the HSQC and HMBC spectra (Table 1, Figure 15S) confirmed 2 as 8-hydroxy-6,7-dimethoxy-a-dunnione (Figure 1). In order to assign the absolute configuration of 1 and 2, the density functional theory (DFT)9 was applied, as previously reported for related compounds.4,8 Briefly, the theoretical optical rotations of specific enantiomers were calculated using DFT, and the calculated values were compared with experimental values, allowing the assignment of absolute configuration. Thus, the optical rotation calculated for 1 was 132, with negative signal for the S isomer. Considering that the experimental value was -101.5, compound 1 was represented as the isomer 12S. For compound 2, the optical rotation calculated was -319 for the S isomer, and the experimental value was -158.4. Therefore, compound 2 also was assigned as 12S. In another approach, the electronic circular dichroism (ECD), experimental and calculated by DFT, of 1 and 2 were obtained. The experimental ECD curves of both compounds exhibited the same profile (a negative Cotton effect at 330 nm and a positive Cotton effect at 381 nm), indicating the same absolute configuration. Furthermore, experimental and calculated ECD curves also were similar to each other, supporting the absolute configuration 12S for compounds 1 and 2 (Figures 16S, 17S). Compounds 1-5 contain at least one phenolic hydroxyl each, and consequently all the five are potential antioxidants.14 Therefore, the antioxidant capacity of compounds 1, 3 and 5, which were isolated with enough amount and purity, was evaluated using the ORAC-FL method. The three compounds displayed good antioxidant capacity, as their ORAC values were higher than 1.0 relative trolox equivalent (TE). Compound 1 was the most active, with ORAC of 4.83 TE, followed by compound 3 (ORAC 3.14 TE) and compound 5 (ORAC 1.39 TE) (Table 2). The antioxidant activity of 3 had already been previously determined by using the DPPH method.11
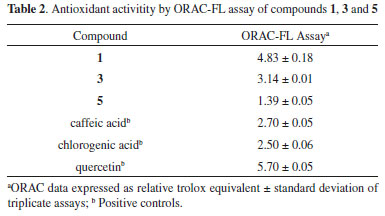
Antioxidant activity is dependent on the number and position of hydroxy groups, as well as on the type of framework and the presence of other substituents different from hydroxyl.14 Accordingly, compounds 1, 3 and 5 showed different degrees of antioxidant capacity that can be associated with their structures. All compounds have two hydroxy groups, but in 1 these substituents are in orto-relationship, a structural characteristic that gives higher antioxidant activity than hydroxy groups in meta-relationship, as those in 3. Moreover, compounds 1 and 3 exhibited higher activity than the positive control caffeic acid, suggesting an important contribution of the a-dunnione framework to the activity. In accordance, compound 5, a dunniol-derivative, showed minor antioxidant activity, in spite of having the same substitution pattern of 3 in the aromatic moiety.
CONCLUSIONS S. reitzii "SC" and S. reitzii "PR" share the characteristic of producing mainly naphthoquinones as less polar constituents, despite the geographic separation. However, plants from S. reitzii "PR" furnished, in previous studies, a higher number of naphthoquinones in significant amounts than S. reitzii "SC". Two naphthoquinones, with strong antioxidant activity, were isolated only from S. reitzii "SC". On the other hand, compounds with anti-inflammatory and cytotoxic activities, previously described in S. reitzii "PR", were not isolated from S. reitzii "SC". These results suggest that the biological properties of less polar extracts from S. reitzii tubers can be dependent on its collection area.
SUPPLEMENTARY MATERIAL Data and spectra of NMR of isolated compounds from S. reitzii "SC" tubers are available in http://quimicanova.sbq.org.br, in PDF format, with free access.
ACKNOWLEDGMENTS The authors are grateful to Dr. Mauro Peixoto (Instituto Plantarum, Nova Odessa, Brazil) for the help with collection and plant identification, to the Chemistry Department (UFSM) for Gaussian09, and to CAPES (Finance Code 001), FAPESP (process number 15/03726-8), and CNPq (process 304266/2017-5; 427859/2018-2; 309411/2019-0) for financial support. E. L. Sá is grateful to Laboratório Central de Processamento de Alto Desempenho (LCPAD/UFPR) for the computational facilities.
REFERENCES 1. Ferreira, G. E.; Ferreira, P. M. A.; Chautems, A.; Waechter, J. L.; Flora 2016, 222, 86. 2. Chautems, A.; Dutra, V. F.; Fontana, A. P.; Peixoto, M.; Perret, M.; Rossini, J.; Candollea 2019, 74, 33. 3. Verdan, M. H.; Stefanello, M. E. A.; Chem. Biodiversity 2012, 9, 2701. 4. Sales, K. A.; Silva, E. F.; Figueiredo, P. T. R.; Costa, V. C. O.; Scotti, M. T.; Agra, M. F.; Tavares, J. F.; Silva, M. S.; Biochem. Syst. Ecol. 2018, 80, 76; Winiewski, V.; Serain, A. F.; Sá, E. L.; Salvador, M. J.; Stefanello, M. E. A.; Quim. Nova 2020, 43, 181. 5. Chautems, A. In Flora Fanerogâmica do Estado de São Paulo; Wanderley, M. G. L.; Shepherd, G. J.; Melhem, T. S.; Giulietti, A. M.; Kirizawa, M., eds.; Rima: São Paulo, 2003, v. 3; Hinoshita, L. K. R.; Dissertação de Mestrado, Universidade Federal do Paraná, Brazil, 2017. https://acervodigital.ufpr.br/handle/1884/47717. 6. Hoehne, F. C.; Sellowia 1958, 9, 37. 7. Soares, A. S.; Barbosa, F. L.; Rüdiger, A. L.; Hughes, D. L.; Salvador, M. J.; Zampronio, A. R.; Stefanello, M. E. A.; J. Nat. Prod. 2017, 80, 1837. 8. Silva, A. S.; Amorim, M. S.; Fonseca, M. M.; Salvador, M. J.; Sá, E. L.; Stefanello, M. E. A.; J. Braz. Chem. Soc. 2019, 30, 2060. 9. Hay, P. J.; Wadt, W. R.; J. Chem. Phys. 1985, 82, 284; Becke, A. D.; J. Chem. Phys. 1993, 98, 5648; Pedersen, T. B.; Hansen, A. E.; Chem. Phys. Lett. 1995, 246, 1; Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B. G.;Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery-Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millan, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J.; Gaussian 16, Revision C01, Gaussian Inc., Wallingford, CT, 2016. 10. Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.; J. Agric. Food Chem. 2002, 50, 1815; Alencar, D. C.; Pinheiro, M. L. B.; Pereira, J. L. S.; Carvalho, J. E.; Campos, F. R.; Serain, A. F.; Tirico, R. B.; Hernandez-Tasco, A. J.; Costa, E. V.; Salvador, M. J.; Nat. Prod. Res. 2015, 30, 1088. 11. Cai, X.-H.; Luo, X.-D.; Zhou, J.; Hao, X.-J.; J. Nat. Prod. 2005, 68, 797. 12. Zhong, Y.-J.; Wen, Q.-F.; Li, C.-Y.; Su, X.-H.; Yuan, Z.-P.; Li, Y.-F.; Helv. Chim. Acta 2013, 96, 1750. 13. Inoue, K.; Ueda, S.; Nayeshiro, H.; Inouye, H.; Phytochemistry 1983, 22, 737. 14. Gulcin, I.; Arch. Toxicol. 2020, 94, 651. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access