Artigo
| Molecular dereplication of volatile oils from Saururus cernuus l. and evaluation of Snti-Trypanosoma cruzi activity |
|
Juliana R BritoI; Thais A Costa-SilvaII; Vinicius S LonderoI; João L BaldimIII; Andre G TemponeIV; Edgard A FerreiraV,*; João Henrique G LagoII,*
I. Instituto de Ciências Ambientais, Químicas e Farmacêuticas, Universidade Federal de São Paulo, 09972-270 Diadema - SP, Brasil Recebido em: 25/05/2021 *e-mail: joao.lago@ufabc.edu.br The present study reports the molecular dereplication and the evaluation of anti-Trypanosoma cruzi activity of volatile oils from inflorescences, leaves, branches, and roots of Saururus cernuus L. (Saururaceae). Chemically, the oils showed the predominance of sesquiterpenes in inflorescences (64.04%) and branches (63.82%), with β-sesquiphellandrene (25.50%) and (E)-caryophyllene (22.40%) corresponding to the main constituents of each oil. Furthermore, it was possible to detect safrole as the most predominant compound in the leaves oil (49.09%). On the other hand, the oil from roots was mainly composed by monoterpenes (84.60%), with limonene in higher concentration (38.41%), followed by α-pinene (20.19%), and camphene (14.71%). The oils from inflorescences and branches displayed higher antitrypanosomal potency with EC50 values of 7.1 and 8.8 μg mL-1, respectively, followed by the oils from the roots and inflorescences, with EC50 values of 17.3 and 30.4 μg mL-1, respectively. Additionally, branches and inflorescences oils displayed no toxicity in mammalian NCTC cells (CC50 > 200 μg mL-1). Using two PLS-DA methods, it was possible to suggest that the anti-T. cruzi activity of the tested oils could be associated with the presence of β-sesquiphellandrene, safrole, β-elemene, and α-zingiberene whereas threo-austrobailignan-5, β-sesquiphellandrene, α-humulene, germacrene D, and bicyclogermacrene play a role in the cytotoxicity against NCTC cells. INTRODUCTION Phytochemical studies conducted with species from Saururaceae have been shown a wide diversity of natural products,1-7 which are associated with biological properties such as anti-inflammatory, anti-tumoral, anti-osteoporotic, and hepatoprotective activities.8-12 Belonging to Saururaceae, Saururus cernuus L. is an ornamental plant with worldwide distribution but native to eastern North America, where is known as lizard's tail, water-dragon, or dragon's tail. In folk medicine, the roots of this plant have been used in the treatment of rheumatism and as poultice for healing skin wounds. The infusion of leaves has also been used for the treatment of back and breast pains as well as for treating stomach ailments.13 Chemically, S. cernuus is composed by lignans, alkaloids and terpenoids with antiviral,14 anti-inflammatory15 and antitumoral9,16 activities. The composition of S. cernuus volatile oils was demonstrated in a previous study17 with the identification of mono and sesquiterpenes from its aerial parts, with β-bisabolene as the main component. Although several phytochemical studies have been described, no antiprotozoal potential was evaluated to date, with exception to the occurrence of antileishmanial and antitrypanosomal neolignans isolated from leaves.18,19 American trypanosomiasis, also known as Chagas disease, is caused by the protozoan Trypanosoma cruzi. The disease is endemic in Latin America causing around 14,000 deaths per year, according to the Drugs for Neglected Disease Initiative - DNDi.20 The clinical treatment is currently based on two drugs (nifurtimox and benznidazole), with severe side effects and reduced efficacy.21,22 In this context, whereas available drugs are quite unsatisfactory, it is necessary a constant search for the development of prototypes to development of new chemotherapeutic agents for the treatment of the Chagas disease. As part of our continuous study concerning the discovery of natural products with antitrypanosomal activity,23,24 the present work aimed to analyze the chemical composition and evaluation of the antitrypanosomal potential and mammalian cytotoxicity of the volatile oils from inflorescences, flowers, branches and roots of S. cernuus. In addition, two partial least squares - discriminant analysis (PLS-DA) methods were built to explore and identify promising compounds associated to the antiparasitic activity of S. cernuus volatile oils.
EXPERIMENTAL Plant material Leaves, branches, roots and inflorescences of S. cernuus were collected on August, 2019 in Suzano City, São Paulo State, Brazil, from a producer of ornamental plants and received a registration code at SISGEN A4123E4. After botanical identification, a voucher specimen (E.A. Ferreira - 001) has been deposited at Herbarium of the Institute of Biosciences of the University of São Paulo (SPF). Volatile oils extraction The fresh plant material - leaves (400 g), branches (400 g), roots (400 g), and inflorescences (5 g) - were individually subjected to hydrodistillation in Clevenger apparatus for a period of five hours, in triplicate. After this period, the obtained oils were extracted with ethyl ether (3 X 3 mL), dried over anhydrous Na2SO4, filtered, concentrated under reduced pressure, and were kept at-20 °C for further analysis. The yield for each volatile oil was calculated using the equation - Y (%) = MO/MFm X 100, where MO is the mass of the extracted oil (g) and MFm is the mass of fresh plant material (g). Molecular dereplication Identification and quantification analysis of volatile oils were performed, in triplicates, using an Agilent 7890A GC gas chromatograph coupled to a 5975C MSD (Mass Selective Detector) equipped with a HP-5 fused silica capillary column (30 m x 0.25 mm, 0.25 μm film thickness). Helium was used as carrier gas with a flow rate of 1.0 mL min-1. Ionization energy was 70 eV and mass range detection from 40 to 500 Da. An automatic sampler (Agilent G4513A) was used for injection of 0.1 μL per sample, varying the split mode according to the plant tissues (leaves - 100:1; roots - 50:1; branches - 75:1; inflorescences - 5:1). The injector temperature was set at 260 °C. Initially the column temperature was maintained at 60 °C for 4 min, then gradually raised at a rate of 3 °C min-1 to 280 °C and then maintained at this temperature for 3 min. The identification of compounds was based on two methods: comparison of their mass spectra with data in NIST 11 (National Institute of Standards and Technologies, Mass Spectra Libraries) and by calculating arithmetic indexes (AI), determined relative to the retention times of a sequence of n-alkanes (C8 - C20), followed by comparison of obtained values with those reported in the literature.25,26 Determination of antitrypanosomal activity Volatile oils from inflorescences, leaves, branches and roots of S. cernuus were individually dissolved in DMSO, diluted in culture medium and incubated with the parasites to determine the respective EC50 (50% Effective Concentration) values. Briefly, each volatile oil was serially diluted (150 to 1.17 μg mL-1 - eight different concentrations) using RPMI-1640 medium in 96-well plates, and then trypomastigote forms of T. cruzi were added at a concentration of 1 x 106 parasites/well. Plates were kept at 37 °C at 5% CO2 for 24 h. After this period, to determine the viability of the parasites, 20 μL of 10% resazurin were added and the plates were incubated for another 20 h under the same conditions. The optical density was determined in Filter Max F5 (Multi-Mode Microplate Reader) at 570 nm.27 The standard drug benznidazole was used as positive control while untreated cells (without volatile oils samples) were used as negative control. Determination of cytotoxicity CC50 (50% Cytotoxic Concentration) values were determined in NCTC cells (clone 929) using the colorimetric method of MTT.28 Briefly, 6x104 cells/well were incubated with each volatile oil, serially diluted (200 to 1.56 μg mL-1 - eight different concentrations) in RPMI - 1640 medium and 10% SFB in 96-well plates. Then, the cells were incubated for 48 h at 37 °C with 5% CO2. The optical density was measured in FilterMax F5 (Molecular Devices) at 570 nm. To determine the cytotoxicity of each volatile oil with effective antiprotozoal activity, the Selectivity Indexes (SI) was calculated using the following expression: SI = CC50/EC50. Statistical analysis EC50 and CC50 data represent the mean of three independent representative assays tested in duplicate and were calculated using sigmoid dose-response curves in Graph-Pad Prism 6.0 software. Investigation of promising compounds using partial least squares - discriminant analysis (PLS-DA) Two PLS-DA methods were built using an in-house dataset associating compounds, the percentage of occurrence, and their respective source (inflorescences, branches, leaves or roots). Analysis were performed by the development of two supervised methods using the web-based platform Metaboanalyst29 based on concentration of compounds and values of EC50 and CC50, respectively, for T. cruzi and NCTC cells. Two groups were created for the EC50 analysis where the volatile oils of leaves, branches and roots were considered actives (EC50 values: 7.1 ± 3.3 μg mL-1; 8.8 ± 4.7 μg mL-1; 17.3 ± 1.5 μg mL1, respectively) whereas inflorescences oil was considered inactive (EC50 value: 30.4 ± 0.5 μg mL-1). The standard drug benznidazole was used as a positive control and resulted in an EC50 value of 12.4 ± 0.8 μg mL-1. Two groups were created for the CC50 values analysis where the volatile oils of inflorescences and branches were considered nontoxic (CC50 values > 200 μg mL-1) and the volatile oils of leaves and roots were considered toxic (CC50 values < 200 μg mL-1). These classes were created based on the CC50 value of the standard compound benznidazole (< 200 μg mL-1). The two PLS-DA methods were built using three components that explained at least 75% of the model variance. Validation values for EC50: component 1 - 69.0%; component 2 - 16.2%; component 3 - 14.8%. Accuracy - 0.75; 1; and 1 (for components 1, 2 and 3, respectively). R2 - 0.3; 0.93; and 1 (for components 1, 2 and 3, respectively), Q2 - 0.15; 0.84; and 1.0 (for components 1, 2 and 3, respectively). Validation values for CC50: component 1 - 60.2%; component 2 - 32.7%; component 3 - 7.1%. Accuracy - 0.75; 1; and 1 (for components 1, 2 and 3, respectively), R2 - 0.60; 0.97; and 1 (for components 1, 2 and 3, respectively); Q2 - 0.34; 0.96; and 1 (for components 1, 2 and 3, respectively). The results of coefficients to the classes were used in order to correlate promising compounds to each biological activity. The validation values for both methods suggest that important information can be retrieved from this analysis.30
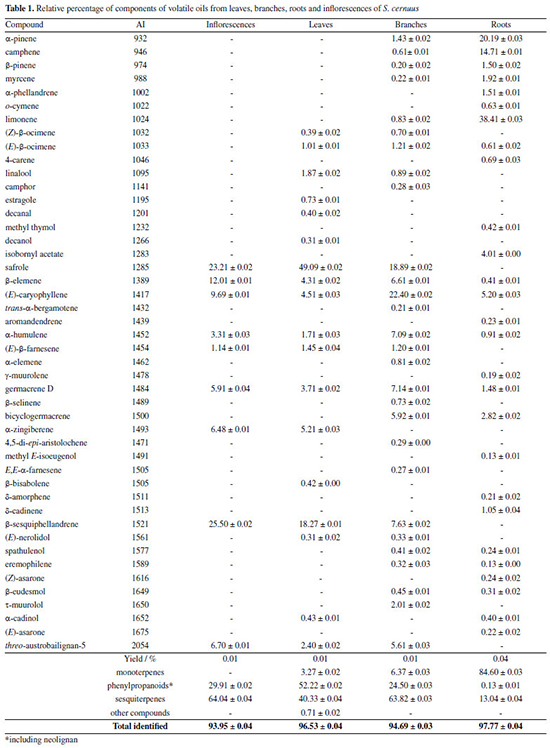
RESULTS AND DISCUSSION Hydrodistillation of leaves, branches, roots and inflorescences of S. cernuus, afforded volatile oils with yields of 0.01, 0.01, 0.01 and 0.04%, respectively. As could be seen in Table 1, 46 compounds were identified distributed in monoterpenes, phenylpropanoids and sesquiterpenes besides other minor metabolites. Inflorescences and leaves oils showed a reduced diversity of volatile compounds - nine and 18 compounds, respectively, corresponding to 93.95% and 96.53% of the total identified. On the other hand, in the branches and roots oils were observed a higher metabolic diversity with 28 (94.69%) and 27 (97.77%) compounds, respectively.
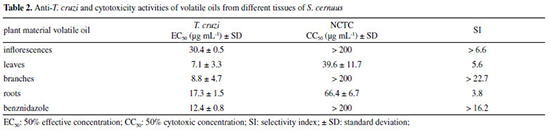
Sesquiterpenes were detected in high concentration compounds in all studied oils, except that from roots, with emphasis on inflorescences (64.04%) and branches (63.82%). Despite of the amounts of sesquiterpenes in the leaves oil (40.33%), the phenylpropanoids were found as predominant compounds (52.22%). On the other hand, the oil from roots showed to be composed mainly by monoterpenes (84.60%). Analyzing the relative percentage of each component in the studied oils, those obtained from inflorescences and leaves were composed mainly by β-sesquiphellandrene (25.50 and 18.3%) and safrole (23.21 and 49.09%), respectively, being this phenylpropanoid also detected in high amounts in branches (18.89%) but absent in roots oil. The sesquiterpenes (E)-caryophyllene and β-elemene were the only compounds found in all studied oils with relative concentrations ranging from 4.51 - 22.4% and 0.41 - 12.01%, respectively. The volatile oil from roots exhibited a different profile being composed, predominantly, by the monoterpenes limonene (38.4%), α-pinene (20.2%) and camphene (14.7%). It must be highlighted that the occurrence of the neolignan threo-austrobailigan-5, in the inflorescences (6.70%), leaves (2.40%) and branches (5.61%) oils. This compound, biosynthetically related to safrole, was also isolated from the MeOH extract from leaves of S. cernuus.18 In a previous study conducted with aerial parts of S. cernuus collected in United States of America, it was observed that the volatile oil is mainly constituted by sesquiterpenes with higher concentration of β-bisabolene (12.30%) and α-curcumene (7.03%).17 Comparatively, the oil from S. chinensis31 was predominately composed by sesquiterpenes being δ-cadinol, δ-cadinene and trans-phytol the main identified compounds, with 22.5, 19.7 and 13.7%, respectively. Considering other species of Saururaceae such as Houttuynia cordata, it was observed the predominance of terpenes and polyketides as volatiles from flowers, leaves, stems, rhizomes, and roots. As reported, the main identified compound in analyzed oils was 4-tridecanone whereas myrcene was the most frequent monoterpene in flowers, leaves and stems, while in rhizomes and roots was detected the predominance of β-pinene. 1-Decanal was the most common occurring polyketide in the leaves and stems oils but in reduced amounts in flowers, rhizomes and roots oils.32 According to the obtained results in the present study, the analyses of volatile oils from different tissues of S. cernuus showed an expressive chemical variety in their composition, in accordance with literature,17 since the sesquiterpenes were detected as predominant metabolites. The volatile oils from S. cernuus were submitted to evaluation of the in vitro anti-T. cruzi activity (trypomastigote forms) and cytotoxicity in mammalian cells (NCTC). The obtained results (Table 2) revealed that the oils were active against the parasite being those obtained from leaves and branches with higher potential (EC50 values of 7.1 and 8.8 μg mL-1, respectively) in comparison to inflorescences and roots oils (EC50 values of 17.3 and 30.4 μg mL1, respectively). Considering the cytotoxicity, leaves and roots oils exhibited CC50 values of 39.6 and 66.4 μg mL-1, respectively, while oils from branches and inflorescences were nontoxic to the highest tested concentration (200 μg mL-1). Based on these results, selectivity indexes to the studied oils were calculated as > 6.6 (inflorescences), 5.6 (leaves), > 22.7 (branches) and 3.8 (roots). Positive control (benznidazole) resulted in an EC50 of 12.4 μg mL-1 and a CC50 > 200 μg mL-1, with a SI > 16.2. Based on these results, it was possible to observe that the volatile oil from branches of S. cernuus exhibited a similar effect of benznidazole being, thus, relevant for future studies including non-clinical trials aiming at its uses for medicinal purposes.
In order to evaluate the individual components of each studied oil in the anti-T. cruzi activity and mammalian cytotoxicity, the qualitative (chemical composition) and quantitative (relative amount) data were organized as matrices containing instances (source) and attributes (compounds) as X axis. For each created PLS-DA method, the Y axis was added as nominal classes (active or inactive and low toxicity or toxic) based on the results of biological assays. This organization was carried out to identify promising compounds associated to the biological activities of volatile oils against the trypomastigote forms of T. cruzi. Two approaches were used to suggest important compounds: 1 - coefficients values to the classes (obtained by the PLS-DA results) and 2 - their distribution among sources. Once the method was validated according to the parameters obtained for each dataset (materials and methods section, item 2.6), coefficients values were obtained. Several components were associated to the active and inactive classes. The first four, and more important compounds, given the coefficient value to the method were β-sesquiphellandrene (highly correlated to the inactive class - Coeff. Value: 100), safrole (highly correlated to the active class - Coeff. Value: 87.3), β-elemene (highly correlated to the inactive class - Coeff. Value: 54.8), and α-zingiberene (highly correlated to the inactive class - Coeff. Value: 25.9), as showed in Figure 1. Additional compounds associated with anti-T. cruzi activity were also suggested, such as (E)-caryophyllene, bicyclogermacrene, limonene, α-pinene, linanol, (E)-β-ocimene, and camphene.
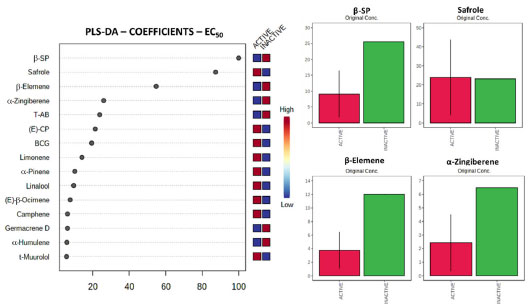 Figure 1. Coefficient values obtained from the PLS-DA method and the first four compounds exhibiting high coefficients to the classes for EC50. The first four compounds have their average concentrations among classes exhibited. Abbreviations: b-SP - β-sesquiphellandrene, T-AB - Threo-austrobailignan-5, E CP - (E)-caryophyllene, BCG - bicyclogermacrene
The concentration of each compound based on their classification exhibits that the active volatile oils contain lower percentages of β-sesquiphellandrene. On the other hand, higher concentrations of safrole as well as lower concentrations of β-elemene and α-zingiberene were associated to better EC50 values against trypomastigotes of T. cruzi (Figure 1). Considering the cytotoxicity, several compounds were suggested as important for the classification of volatile oils. The first four and more important compounds, given the coefficient value to the method, were limonene (highly correlated to increased toxicity - Coeff. Value: 100), safrole (highly correlated to increased cytotoxicity values - Coeff. Value: 95.5), (E)-caryophyllene (highly correlated to decreased toxicity - Coeff. Value: 69.3), and β-elemene (highly correlated to decreased cytotoxicity - Coeff. Value: 46.7), as showed in Figure 2. Additional compounds associated to the reduced cytotoxicity, with lower coefficient values, were also suggested, such as threo-austrobailignan-5, β-sesquiphellandrene, α-humulene, germacrene D, and bicyclogermacrene.
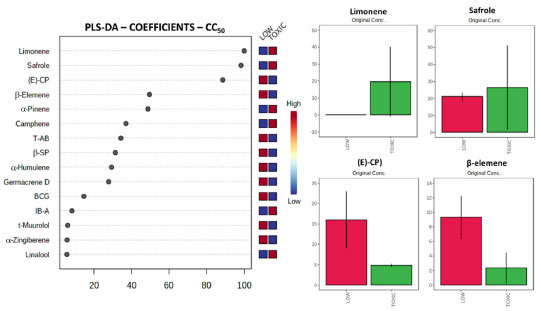 Figure 2. Coefficient values obtained from the PLS-DA method and the first four compounds exhibiting high coefficients to the classes for CC50. The first four compounds have their average concentrations among classes exhibited. Abbreviations: b-SP - β-sesquiphellandrene, E-CP - (E)-caryophyllene, BCG - bicyclogermacrene, T-AB - Threo-austrobailignan-5, IB-A - Isobornyl acetate
The concentration of each compound based on their classification allowed identification of important compounds in volatile oils with higher CC50 values, e.g., lower cytotoxicity against NCTC cells. The results showed that the decrease in cytotoxicity is associated with oils containing lower concentrations of limonene and safrole. According to the method, higher concentrations of limonene are associated to the toxicity. On the other hand, higher concentrations of (E)-caryophyllene and β-elemene are present in samples with lower cytotoxicity. Volatile oils are mixtures of compounds which can exhibit synergism, antagonism, or additive effects in biological assays.33 The influence and interpretation of each compound is complex in this scenario. In this sense, the use of multivariate statistical analysis, resembling discriminant approaches could suggest patterns that point to promising compounds in the entire composition.
CONCLUSIONS Chemical analysis of volatile oils from different parts of S. cernuus showed the predominance of sesquiterpenes in the inflorescences and branches, in which β-sesquiphellandrene and (E)-caryophyllene were the main compounds. It was also possible to detect phenylpropanoid derivatives, especially safrole, being one of the most predominant compounds in these tissues. On the other hand, the roots oil was predominantly composed by monoterpenes, with limonene, α-pinene, and camphene as main compounds. The presence of the threo-austrobailigan-5, a neolignan biosynthetically related to safrole, was also detected in inflorescences, leaves and branches. In addition, these oils showed different activities against trypomastigotes of T. cruzi, as well as toxicity against NCTC mammalian cells, indicated a promising antiparasitic source of bioactive molecules. The use of PLS-DA methods suggested that the anti-T. cruzi activity of tested oils could be associated to the presence of β-sesquiphellandrene, safrole, β-elemene and α-zingiberene whereas threo-austrobailignan-5, β-sesquiphellandrene, α-humulene, germacrene D, and bicyclogermacrene play a role in the cytotoxicity against NCTC cells. Therefore, our findings support a future application of these plant materials, especially the volatile oil from branches, for the development of new prototypes for the treatment of Chagas disease.
ACKNOWLEDGEMENTS The authors are grateful to FAPESP (2021/02789-7, 2018/07885-1, and 2018/10279-6) and MackPesquisa (151024) for the financial support for the development of this work. J.R.B., A.G.T. and J.H.G.L. thank CAPES and CNPq for fellowships.
REFERENCES 1. Rao, K. V.; Alvarez, F. M.; J. Nat. Prod. 1982, 45, 393. 2. Rao, K.V.; Puri, V. N.; Diwan, P.K.; Alvarez, F. M.; Pharm. Res. Commun. 1987, 19, 629. 3. Rao, K. V.; Reddy, G. C. S.; J. Nat. Prod. 1990, 53, 309. 4. Rao, K. V.; Rao, N. S. P.; J. Nat. Prod. 1990, 53, 212. 5. Rao, K. V.; Oruganty, R. S.; J. Liq. Chromat. Relat. Technol. 1997, 20, 3121. 6. Bauer, R.; Pröbstle, A.; Lotter, H.; Wagner-Redecker, W.; Matthiesen, U.; Phytomedicine 1996, 2, 305. 7. Kim, S. K.; Ryu, S. Y.; No, J.; Choi, S. U.; Kim, Y. S.; Arch. Pharm. Res. 2001, 24, 518. 8. Lee, E.; Haa, K.; Yook, J. M.; Jin, M. H.; Seo, C. S.; Son, K. H.; Kim, H. P.; Bae, K. H.; Kang, S. S.; Son, J. K.; Chang, H. W.; Biol. Pharm. Bull. 2006, 29, 211. 9. Hodges, T. W.; Hossain, C. F.; Kim, Y. P.; Zhou, D. G.; Nagle, J.; J. Nat. Prod. 2004, 67, 767. 10. Gao, Y.; Hongyu, Z.; Li, Y.; J. Biochem. Mol. Toxicol. 2018, 32, e22033. 11. Wang, L.; Cheng, D.; Wang, H.; Di, L.; Zhou, X.; Xu, T.; Yang, X.; Liu, Y.; J. Ethnopharmacol. 2009, 126, 491. 12. Liu, G.; Zhao, Z.; Shen, M.; Zhao, X.; Xie, J.; He, X.; Li, C.; Am. J. Chin. Med. 2020, 48, 47. 13. Parker, J. D.; Caudill, C. C.; Hay, M. E.; Oecologia 2007, 151, 616. 14. Cui, H.; Xu, B.; Wu, T.; Xu, J.; Yuan, Y.; Gu, Q.; J. Nat. Prod. 2014, 77, 100. 15. Lee, A. K.; Sung, S. H.; Kim, Y. C.; Kim, S. G.; Br. J. Pharmacol. 2003, 139, 11. 16. Brito, J. R.; Camilo, F. F.; Figueiredo, C. R.; Azevedo, R. A.; Romoff, P.; Buturi, F. O. S.; Fávero, O. A.; Lago, J. H. G.; Ferreira, E. A.; Quim. Nova 2018, 41, 778. 17. Tutupalli, L. V.; Brown, J. K.; Chaubal, M. G.; Phytochemistry 1975, 14, 595. 18. Brito, J. R.; Passero, L. F. D.; Bezerra-Souza, A.; Laurenti, M. D.; Romoff, P.; Barbosa, H.; Ferreira, E. A.; Lago, J. H. G.; J. Pharm. Pharmacol. 2019, 71, 1871. 19. Brito, J. R.; Costa-Silva, T. A.; Tempone, A. G.; Ferreira, E. A.; Fitoterapia 2019, 137, 104251. 20. Ioset, J. R.; Brun, R.; Wenzler, T.; Kaiser, M; Yardley, V.; Drug screening for kinetoplastids diseases. A Training Manual for Screening in Neglected Diseases 2009, available at https://dndi.org/wp-content/uploads/2009/04/kinetoplastid_drug_screening_manual_final.pdf accessed August 2021. 21. Paucar, R.; Moreno-Viguri, E.; Pérez-Silanes, S.; Curr. Med. Chem. 2016, 23, 3154. 22. Tiwari, N.; Gedda, M. R.; Tiwari, V. K.; Singh, S. P.; Singh, R. K.; Mini Rev. Med. Chem. 2017, 18, 26. 23. Conserva, G. A. A.; Quiros-Guerrero, L. M.; Costa-Silva, T.; Marcourt, L.; Pinto, E.G.; Tempone, A. G.; Fernandes, J. P. S.; Wolfender, J.; Queiroz, E. F.; Lago, J. H. G.; PLoS One 2021, 16, e0247334. 24. Londero, V. S.; Costa-Silva, T.; Antar, G. M.; Batista Junior, J. M.; Baitello, J. B. ; Oliveira, L. V. F. ; Camilo, F. F.; Batista, A. N. L.; Tempone, A. G.; Lago, J. H. G.; J. Nat. Prod. (2021), doi: 10.1021/acs.jnatprod.0c01303. DOI: http://dx.doi.org/10.1021/acs.jnatprod.0c01303 25. Adams, R. P.; Identification of essential oil components by gas chromatography/mass spectrometry. 4ª ed., Allured Publishing: Carol Stream, 2017. 26. Van Den Dool, H.; Kratz, P. D.; J. Chromatogr. A 1963, 11, 463. 27. Gehrke, S. S.; Pinto, E. G.; Steverding, D.; Pleban, K.; Tempone, A. G.; Hider, R. C.; Wagner, G. K.; Bioorg. Med. Chem. 2015, 21, 805. 28. Tada, H.; Shiho, O.; Kuroshima, K.; Koyama, M.; Tsukamoto, K.; J. Immunol. Meth. 1986, 93, 157. 29. Chong, J.; Wishart, D. S.; Xia, J.; Curr. Protoc. Bioinf. 2019, 68, 1. 30. Baldim, J. L.; Alcântara, B. G. V.; Domingos, O. S.; Soares, M. G.; Caldas, I. S.; Novaes, R. D.; Oliveira, T. B.; Lago, J. H. G.; Chagas-Paula, D. A.; Oxid. Med. Cell. Longevity 2017, 2017, 3789856. 31. Kim, C.; Lee, S. J.; Hyun, C. G., Lee, N. H.; Int. J. Pharmacol. 2013, 9, 258. 32. Asakawa, Y.; Tomiyama, K.; Sakurai, K.; Kawakami, Y.; Yaguchi, Y.; J. Oleo Sci. 2017, 66, 889. 33. Caesar, L. K.; Cech, N. B.; Nat. Prod. Rep. 2019, 36, 869. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access