Nota Técnica
| An adapted unmanned aerial vehicle for environmental water sampling |
|
José C. D. NetoI; Ian S. ResqueI; Rodrigo A. AvelinoI; Vagner B. dos SantosI,*; Lucas S. LeiteII; Lucas O. CesarI; Paulo H. da Silva BeloI; Jones AlbuquerqueII,III; José L. de Lima FilhoII
I. Departamento de Química Fundamental, Universidade Federal de Pernambuco, 50740-560 Recife - PE, Brasil Recebido em 27/01/2022 *e-mail: vagner.bsantos@ufpe.br In this work an unmanned aerial vehicle (UAV) was adapted to be used as an environmental tool to collect water samples of sea, estuary, and mangrove for further analysis by gas chromatography with a mass detector (GC-MS). The sampling automated system was based on a microcontroller, a solenoid valve, and a peristaltic mini-pump that was coupled to a quadcopter UAV. The apparatus was properly calibrated to acquire 100 mL of environmental water at 4.6 min. After, the concentration of BTEX (benzene, toluene, ethylbenzene, and o, m, p-xylenes) was analyzed in laboratory using GC-MS and the BTEX concentration ranged from 0.3 to 5.0 μg L-1 with a recovery from 83.9 to 118% with a relative standard deviation lower than 7.0%. Tests of cross-contamination were performed by simultaneous sampling and laboratory blank was carried out and no contamination was found for BTEX analysis. Based on the results, the adapted UAV presented a very useful ability to collect samples in difficult access areas such as mangroves, points between rocks, corals reefs, without health risk and without impacting the fragile ecosystem being an interesting tool for environmental purposes. INTRODUCTION The BTEX acronyme is referred to the benzene, toluene, ethylbenzene and the o, m, p-xylenes, a group of volatile organic compounds (VOCs). The BTEX are all volatile, being hydrocarbons with low molecular weight and present in large concentrations in oil or in its refining products, such as gasoline and kerosene. 1 Their high solubility in water reveals the danger of an environmental disaster as the spill of oil or their derivatives.2 The solubility of benzene in water is 1700 mg L-1, but its maximum allowable limit for freshwater is 5 μg L-1, according to the 357/2005 resolution CONAMA (National Environment Council from Brazil).3 For toluene, ethylbenzene and xylenes are 2 μg L-1, 90 μg L-1 and 300 μg L-1, respectively.3 For comparison purpose, the United States Environmental Protection Agency (USEPA)4 establishes the maximum allowable limits for benzene as 5 μg L-1 for drinking water and according to the World Health Organization (WHO)5 this concentration is 10 μg L-1. These quantities in trace levels reveal the hazardousness that such components can present for heath and ecosystem with the effects of its pollution last for several years.6,7 According to Werder et al.1 the BTEX was found in the blood of part of the population exposed to oil contaminated water, in which 49% of those infected still had at least one neurological problem. In another study, the asthma symptoms in children were correlated at long-term effect and the BTEX is cited as one of the toxic groups correlated with symptoms. 7 In the end of the 2019, tons of spilled oil were reported on the beach fringes of the Brazilian coast, which continued to be observed throughout 2020.8 In fact, portions of these are undergoing resuspension from the depth of the seas and rivers reach the beach shore and causes contamination of the ecosystem and its environmental impact are few know.8 The oil spill reached a sensible class of microorganisms such as phytoplankton, zooplankton, fundamental to life in estuaries, mangroves, coral reefs ecosystem. Nowadays, few research data have been reported about this disaster in Brazil. In fact, due to the SARS-COV-2 pandemic and its effects, few information was obtained and greatly compromised the studies of the real impact of this oil spill disaster.8 The BTEX can be determined by different analytical methods for their identification and quantification. Due to the physics and chemistry features of BTEX, the gas chromatography (GC) with mass detection (GC-MS) is the analytical method most used.9-11 For environmental research, it is very important to perform a systematic and periodical sampling to evaluate possible changes or impact on the ecosystem. For surface water samplers based on a van Dorn are generally used with the tube immersed in the water using a rope.12 This procedure is laborious; it involves several professionals, and is relatively expensive.13 In addition, this methodology could expose directly and frequently the researchers to toxic compounds. Moreover, the continuous displacement of the researchers could lead to a biological, organic and inorganic contamination,14,15 and impacting an already fragile ecosystem such as estuary, and mangrove. Thus, to overcome these drawbacks, new methodologies as the use of unmanned aerial vehicles (UAV) could be an interesting solution. UAVs are characterized as rotary wing aircraft, such as helicopters or multicopters. Quadcopter (four rotors) or hexacopter (six rotors) are the most common. They are highly foldable, compact, maneuverable and they have sensors to prevent collisions. The duration of flights is on average 20-30 min, ranging from 2 to 8 km of distance and weigh up to 6 kg for the most common models.16-24 The use of UAVs as sampling technology aims greater security, once the aircraft could have contact with hazardous compounds, it is possible to obtain samples in inaccessible ecosystems or dangers.19 However, the coupling of a sampling system to an UAV could lead to an overload on the aircraft, flight instability, reducing flight time, and thus, generally sophisticated or dedicated UAV are required. On the other hand, these UAV presented a high cost for acquisition, i.e., some models can reach more than 50 thousand dollars, and/or maintenance, or they are few accessible to be used for environmental applications.16-24 Thus, here we proposed for the first time a simple, compact, lightweight and low cost adaptation on a common quadcopter UAV to collect sea, estuary, and mangrove waters for further BTEX analysis by GC-MS in laboratory. This adaptation is based on a peristaltic mini-pump, and solenoid valve controlled by a microcontroller to collect surface water in distinct geolocations. The proposed sampling methodology allows to obtain environmental water samples in inaccessible places without contact by researchers with hazardous compounds, no impacting fragile ecosystems such as mangrove and coral with a fast and a secure procedure.
EXPERIMENTAL Chemicals, manual sampling and sample preparation All reagents and solvents were of analytical grade. Benzene (CAS Number 71-43-2), toluene (CAS: 108-88-3), ethylbenzene (CAS: 100-41-4), o-xylene (CAS: 95-47-6), m-xylene (CAS: 108-38-3), p-xylene (CAS: 108-38-3), hexane (CAS: 110-54-3), benzaldehyde (CAS: 100-52-7) were acquired from Sigma-Aldrich (Brazil). Hexane was used as solvent. Hydrochloric acid (CAS: 7647-01-0) was acquired from Dinâmica (Brazil). A deionized water was obtained using a Mili-Q (Direct -Q) purification system, with resistivity higher than 18.2 MΩ cm. All polypropylene and glassware were previously cleaned before use with an acid bath based on HCl 10% (w/w) solution and they were washed with surfactant as detergent and after with deionized water. All aqueous solutions used were absent of organic compounds as recommended by standard method.25,26 After, all samples were transferred to a glass (PYREXTM) bottle, sealed with septum of PTFE-faced silicone and protected from light.14 The manual water sampling was performed by displacement of our team to the local with a speedboat. The same samples were collected by manual and automated sampling using the GPS coordinates obtained. The surface water samples were collected with the glasses flasks aforementioned, previously degreased and cleaned. Approximately 500 mL was collected per sample. Latex gloves were used to avoid the contact with the sample flasks. Soon after, the samples were placed in Styrofoam with ice packs to reach 0°C. After approximately 2 h the samples can be measured in the laboratory after road transport. The storage time was less than 1 month. All the samples were filtered in laboratory using a glass-fiber filter paper, grade 25, Whatman®, except the sea water in which it was filtered in flow during the in-flight sampling using the glass microfiber membrane, pore size 0.45 μm (Whatman®). The samples and standard solutions of BTEX returned to the freezer (-10ºC) as soon as possible. The effect of temperature and sunlight on samples can lead to loss of volatile compounds and its degradation. Thus, a fast sampling and adequate store is required. Similar procedure was reported by Cincinelli et al.27 After this, the ion conductivity and pH measurements were performed. After thawing, a liquid-liquid microextraction (LLME) was performed based on the work developed by Desideri et al.28 in which it was compared to standard methods based on purge and trap methodology for sea water samples with adequate recovery percentage for VOCs as alkylbenzenes.28 Thus, the LLME used hexane, and the organic phase was pre-concentrated by vaporizing the solvent using a low flow of N2. For this, 5 mL of each collected sample and 3 mL of hexane were added to a closed glass decanting funnel. The funnel was stirred for 30 s and left to separate the two phases. After separation of the phases, the denser aqueous phase was again submitted to the extraction process with 2 mL of hexane. Aliquots of almost 5 mL of the organic phase were concentrated to 500 μL using a low nitrogen flow, and the extract was stored at -10 °C in a freezer until to be used for analysis by GC-MS.25-29 Water sampling system adapted on a quadcopter UAV Due to its simplicity and wide use, the C language was employed to automatically control of all functions of the sampling system.30,31 Currently, this language is widely used to powered an open source hardware platforms such as Arduino.32 Aiming to acquire a greater autonomy with remote operation and to make in-flight decisions, some particular functions were developed such as the ability to keep the peristaltic pump (PP) OFF during 60 s while the UAV travels to the first sampling point (variable, "trip") and activate it for 5 min when it reaches the sampling location (variable, "collect"). After that, the PP would be turned OFF, and again 60 s were used for the UAV to travel to the second sample point (variable, "interval"). Then both PP and SV would be activated for 5 min to commute the sample to another sampling compartment and to pump the new sample. Following this protocol, two different samples with different geographic coordinates were acquired. To be accurate in this procedure, the C language algorithm was in situ set up while the waters were collected using a notebook and a chronometer. The C algorithm developed and the electronic circuit scheme is shown in the supplementary material section, Figure 1S, Figure 2S. Aiming to couple the sample flasks to the UAV, 3D pieces were developed. These customized pieces were created using a 3D printer from Cliever studios (Cl1 Black Edition). To produce lightweight and resistant pieces, a polylatic acid biodegradable polymer was used. First, the pieces with 28 x 10 x 30 mm (Figure 3Sa) were designed using a free 3D CAD software (OpenSCAD).33-35 The support pieces have two flaps, one on its side (1) and another on the top (2), which are used to couple the sampling flasks and to the respective UAV stems using plastic clamps, as shown in Fig 3S(b) and Figure 1. The pieces shown in Figure 3S were developed to be able to couple different types of Falcon flasks such as 15 mL or 50 mL. To couple each sampling flask to the UAV, only two support pieces and three clamps are needed.
 Figure 1. The adapted UAV with a sampling system. On the left, the flow system with the solenoid valve (SV) and peristaltic mini-pump (PP) is shown. On the right, the UAV is highlighted to show the four sampling flasks (SF) below the stems of the UAV. Also is shown a notebook used to in situ set up the parameters of the C algorithm developed. The remote control of the UAV is connected to the smartphone and powered by DJI GO 4® software. SP is the sample probe, a cylindrical copper tube employed to avoid the pendulum effect
The sampling system was adapted on a Mavic Pro®, a quadcopter UAV developed by the company DJI®. In Figure 1, the adapted drone is shown with highlights for a miniaturized peristaltic pump, a three-way solenoid valve, an electronic circuit based on a microcontroller, and the rechargeable 9 V batteries put in the upper side of the UAV. Moreover, four sampling flasks of 50 mL are shown. Only a simple double-sided scotch tape with strong fixation (3M, Brazil) was necessary to fix the flow system. The Mavic Pro® has an omnidirectional system of sensitivity to obstacles in a radius of 360 degrees. This is very important for avoiding collisions and accidents of the aircraft and allowing flights with enough safety. The weight of the sampling prototype was 300 g, and the total weight of the water samples was 200 g. The initial procedures adopted were to check the firmware update while still in the laboratory, using the software from the manufacturer DJI GO 4®, and to calibrate the compass before the first flight and during the experiment in case the UAV requested it due to interference. The procedure of calibration of the system is presented in supplementary material, figure 4S, 5S and 6S. Application of the adapted UAV as an environmental sampling tool The UAV adapted with a water sampling system was used to obtain information regarding the presence of BTEX in sea, estuary, and mangrove waters collected from the coast of Pernambuco-Brazil based on the quantification of BTEX by GC-MS, Figure 2.6,8,36 Thus, aiming to evaluate the performance of the adapted UAV as an environmental sampling tool, four samples of environmental water were collected. The first sample was collected at Candeias beach (Jaboatão dos Guararapes-PE-Brazil) with 8°12'37.58123"S and 34°55'4.61338"W coordinates (Taduademare)37 at 30 ± 1 °C being this sample water used as a control because no visible oil stains were reported in this region.8 The second sample was collected at a mangrove region of the Massangana river at Santo Agostinho-PE (8°21'37.9"S and 34°57'44.7"W (P1)) at 33 ± 1 °C. Another sample was collected in an area of the estuary (8°21'46.5"S and 34°57'41.0"W (P2)) at 32 ± 1 ºC. Another collected sample was near marine corals (8°22'05.6 "S and 34°57'43.5"W (P3)) at 31 ± 1 °C. All the samples were manually and automated collected and were sent to laboratory for further tests of pH, ion conductivity, and BTEX by GC-MS. The tide height ranged from 0.1 to 0.3 m along the sampling procedure.
 Figure 2. Images obtained by our research group during the environmental water sampling. Satellite image (adapted from Google Maps®) shows the collection points in the region close to the Port of Suape-PE-Brazil (P1, P2 and P3) (a). Sampling of water in the estuary area (P2) (b) and water in the mangrove area (P1) (c) using the adapted UAV
Because the control sample C is very far from the other (P1, P2 and P3), and, out of the visual line of sight (VLS), a secure UAV flight, according to the ANAC (National Civil Aviation Agency),38 the sequential sampling from C to P1, P2 or P3 was not performed. Thus, the sampling with sample C was performed twice, and the aliquots were together stored in the same collection flask (sample C). On the other hand, the samples P1 and P2 were sequentially collected from P1 to P2, and the procedure was repeated (n = 2). Both aliquots of each water sample were collected and together stored in its respective flasks (sample P1 and sample P2), with a total volume of 100 mL for P1 and P2. This method was called "method 1". However, to carry out the sampling of the water P3, it was necessary to use a syringe filter (glass microfiber membrane, pore size 0.45 μm, Whatman®), and change it at each sampling to avoid contamination. This filter was needed because of the suspension of sand near to marine corals, and thus, there is no commutation with samples P1 and P2. In this case, the UAV returns to home, to change the filter. Only in this case, the filtration was carried out in flow during the in-flight sampling. Aiming to ratify that the procedure aforementioned did not present cross-contamination, a "method 2" for water sampling was performed in duplicate for P1 and P2 by switching P1 and P2. Here, each aliquot of 50 mL collected of P1 or P2 was separately led to the laboratory, the LLME was carried out, and the BTEX was measured by GC-MS (table 3). Thus, the BTEX profile should be different for each sample, revealing that there was no cross-contamination for each collected aliquot. For this, the results of relative error (RE), and t-paired test (t) for 95% confidence level with n = 2 (ttabulated = 12.706) were calculated using the results of the BTEX concentration presented in table 1 for comparison purposes. This procedure to employ different BTEX concentrations to evaluate cross-contamination is recommended by the standard method for BTEX analysis.25,26
After evaluating the cross-contamination of the sampling methods using the adapted UAV, the sampling uncertainty of the automated method was carried out. For this, the results of the BTEX for GC-MS for each manual and automated sampling were obtained and they are presented in table 4. The results of the automated sampling already were demonstrated in table 2 for P1 and P2, and the results of the manual sampling are inserted in table 4 together with the data from the P3.
Analyses of pH and ion conductivity The pH analyses were measured with a pH meter HI-2221 using a combined glass electrode HI-1131 both from HANNA instruments (Brazil) calibrated with buffer solutions with pH 4.0, 7.0, and 10.0. The ion conductivity was measured using a Q-405M conductivity meter from QUIMIS (Brazil) at a temperature of 27 °C. A standard solution of 0.01 mol L-1 KCl (conductivity = 1406 μS/cm) was used for calibration. Determination of BTEX for GC-MS The LLME and GC-MS method to BTEX analysis was evaluated using recovery tests for accuracy purposes. For this, standard solutions of 10 μg L-1 and 40 μg L-1 containing each analyte were added to the water samples in which each amount of BTEX was previously measured by GC-MS. For this, analytical curves were simultaneously constructed using standard solutions mixes of 1.00 μg L-1, 10.0 μg L-1, 20.0 μg L-1, 50.0 μg L-1, and 100 μg L-1 each of benzene, toluene, ethylbenzene, and o,m,p-xylenes. A 50.0 μg L-1 concentration of benzaldehyde was used as an internal standard (IS). When necessary, hexane was used as a solvent to dilute the samples or solutions. Using the parameters of the analytical curve, the limits of detection and quantification of the method were calculated. The recovery equation used was: Recovery (%) = ((([analyte found] - [analyte added]) / [analyte added]) x 100%) + 100%. The BTEX quantification was measured using an Agilent 5975C Series MSD Quadrupole Mass Spectrometer coupled to the Agilent 7890A gas chromatography equipment with a split/splitless injector. The column DB-5ms (5% phenyl - 95% dimethylpolysiloxane) of fused silica (30 m x 0.25 mm x 0.25 μm) was used. Helium (99.999% purity) was employed as carrier gas at a flow rate of 1.0 mL min-1 with 7.0 psi of pressure. The column temperature was programmed as follows: 50 °C for 1 min, increasing to 60 °C at 2 °C min-1 and afterwards to 200 °C at 50 °C min-1 and then held at 200 °C for 2 min. The solvent cut-off time was 1 min. The injector temperature was maintained at 300 °C and 1 μL of the extracted sample was added in the glass micro-vial that is inserted into the thermal desorption (TD) system (Thermo Sep Probe, Agilent). By heating, the analytes were conducted to the capillary column of the gas chromatograph by He (g) carried flow. The TD probe is inserted into the split/splitless inlet. The split mode (5:1) was used. Because of this, the TD uses the GC inlet to efficiently introduce the volatile compounds in the system. Thus, the TD system reduces the cross-contamination once only the volatile compounds of the water sample/extract are transferred to the GC system with a fast, simple and clean process. In fact, all non-volatile are kept in the glass micro-vial and are discarded after analysis. The data were acquired and processed by the MassHunter software. The eluent from the GC column was transferred through a liner kept at 280 °C with a source of electronic impact ionization of 70 eV keep at 250 °C. The analysis was carried out in the selected ion monitoring mode (SIM) to detect the following molecule ions with m/z 78 (benzene), 92 (toluene), 106 (ethylbenzene and xylenes), and 106 (benzaldehyde), respectively. A method blank was performed to check laboratory contamination and its materials. Thus, the measure of hexane (BTEX-free solvent) was performed with all material from the sampling, filtration, LLME, and thermal desorption procedures until GC-MS analysis.25,26 The method bank was measured before each analysis.
RESULTS AND DISCUSSION Calibration and test of performance of the adapted UAV Figure 6S shows a graph of the relationship between the flow rate variation and sampling depth. This study is a simulation in a laboratory of the conditions that can happen with the UAV for in situ application, mainly regarding meteorological phenomena such as strong winds that can cause variations in the height of the tide and of the UAV sampling. As can be seen, the flow rate was lower when the relative height between the sample flasks and the water surface was greater. In fact, for high height, there is a higher resistance to pumping the fluid (Figure 1 and Figure 4S) due to the use of a longer flow line length. At a height of 1.5 m, a flow rate of 22 mL min-1 was measured, where there is a good relationship between safety to control the UAV and its performance, with an accuracy of 5%. At this flow rate, 2.3 min were enough to fill a 50 mL sample flask. The option to carry out a larger volume of 100 mL per sample allows us to perform more tests in the laboratory, such as extraction steps, repeatability, quantification and recovery tests employing GC-MS methods. The sampling system was developed to have low power-consumption. In fact, according to the diagram shown in figure 4S, all system is kept on standby for 2 min, and the PP is only kept ON during half of the time. Thus, the consumption was reduced and only 140 mA (on average) leading to an autonomy of approximately 1 h, which exceeds the UAV's flight time in the sampling conditions, which was 20 min per battery. This procedure increases the lifetime of the batteries and avoids unnecessary returns to home, leading to an increase of the sampling analytical throughput. If necessary, this time is in situ adjusted in case the UAV needs to travel to far away points. In situ application of the adapted UAV to collect environmental water sample During the experiments, a syringe filter (glass microfiber membrane, pore size 0.45 μm, Whatman®) was used for in-flight sampling, and it behaved properly with no clogging of the flow channels or the solenoid valve. Hence, there was no limitation and the samples were collected close to the waves and close to the banks, where a suspension of sand particles was observed in the water (sample P3), increasing the robustness of the adapted UAV.16,24 Due to its features, only for sampling the water P3 was needed this filter. The pieces printed in 3D were efficiently developed to fix the sampling flasks to the UAV. These pieces were developed to use different models and volumes of flasks for ease in changing them leading to an increase of the versatility of the sampling system. Thus, the sampling system was compact, lightweight and adequate to fly. The Mavic Pro® (average cost of U$ 2000 dollars), even though it is a popular UAV and not a specific one for payload transportation, it presented good performance, because the sampling system developed was quite light (300 g). This was a great result, since UAVs used for payload transportation are too expensive with costs greater than 50 thousand dollars (AeroExpo).39 Additionally, the low cost of the sampling apparatus is also very attractive, at around U$ 40 dollars. Thus, the adapted quadcopter UAV proved to be suitable performed with a low cost sampling system for environmental waters.16-24 In addition, it is the first time that an adapted UAV with these features was employed to collect sea (sample P3), estuary (sample P2) and mangrove waters (sample P1) with further BTEX analysis by GC-MS in laboratory. Analysis of some physical-chemical parameters Table 1 shows the results of the ion conductivity, pH, and temperature measurements of the collected samples. Only the temperature was performed in situ using a conventional mercury thermometer. According to the results, the high ion conductivity values are close to each other, which indicates that all water samples collected presented a high salt content and salinity similar to the sea, the estuary, and the mangrove area of the Massangana river.40,41 The samples were obtained in a low tide condition, which may justify the similarities between the pH and conductivity measurements.37 In fact, the estuary and mangrove waters evaluated are the saline wedge type. Determination of BTEX in water samples collected using the adapted UAV by GC-MS The construction of the analytical curves for benzene, toluene, ethylbenzene, and o, m,p-xylenes were carried out using a GC-MS method employing the following concentrations of 1, 10, 20, 50, and 100 μg L-1 for each analyte; 50 μg L-1 of benzaldehyde was used as an internal standard, Figure 3. The linear regression coefficients ranged from 0.9965 to 0.9990 and showed a good linear correlation for the data. The average value of RSD% was 3.0% (n = 3). The retention time for benzene, toluene, ethylbenzene, o,m,p-xylene, and benzaldehyde were on average 2.130, 3.271, 5.350, 5.582, and 10.818 min, respectively.
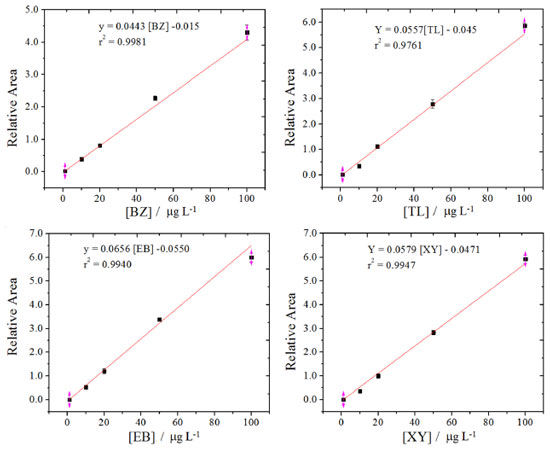 Figure 3. Analytical curves for the determination of benzene, toluene, ethylbenzene and o, m, p-xylenes by GC-MS. He gas at a flow rate of 1.0 mL / min, a column DB-5ms (30 m x 0.25 mm x 0.25 μm) and benzaldehyde as internal standard were used
The detection limits were 0.32 μg L-1, 0.32 μg L-1, 0.29 μg L-1, and 0.31 μg L-1 for benzene, toluene, ethylbenzene and m-p-xylene, respectively (n = 3). These limits are in accordance with other works reported in the literature.9-11,42-44 Moreover, the laboratory contamination yielded only traces of interferents for organic compounds and no signal of BTEX was detectable or they were lower than the detection limits for the GC-MS method. In Table 2 are presented the concentrations of benzene, toluene, ethylbenzene, and xylenes found in the obtained environmental water samples. The percentage of recovered concentration levels of 10 μg L-1 and 40 μg L-1 with their respective average and standard deviations (n = 3) are presented. As can be seen, the samples collected showed concentrations of benzene that ranged from 0.19 to 1.21 μg L-1, toluene from 1.48 to 4.83 μg L-1, ethylbenzene from 0.38 to 1.60 μg L-1, and o,m,p-xylenes from 1.11 to 3.40 μg L-1, respectively, with relative errors lower than 6.7% and recoveries percentages varied between 83.9% and 118%. Supported by these results, the analytical method presented good accuracy and precision to determine BTEX by GC-MS. Furthermore, these values found for each BTEX are lower than those recommended by CONAMA with a resolution of Nº 357/20053 for saline waters used for fishing activity or cultivation of organisms for the purpose of intensive consumption. In fact, for the Class 1 saline water, the allowed benzene concentration is lower than 51 μg L-1, not having been established for the other BTEX. For another classification of saline water, without intensive consumption activity, the allowed concentration of benzene is 700 μg L-1, 25 μg L-1 for ethylbenzene, and 215 μg L-1 for toluene. Xylene appears only in the classification for freshwater, class 1, with a concentration of 300 μg L-1. Thus, all samples collected presented concentrations of BTEX within what is appropriate for these activities. Evaluating the samples collected, it can be seen that benzene was found in greater quantity in the control sample, and toluene in this sample had one of the highest concentrations, even though oil stains were not reported in the region8. This contributes to the need to study regions like this for a long time of monitoring. In fact, these stains are made of the heaviest fraction of oil, and thus, the lightest fraction could not be presented. It is also noted that low concentrations of benzene were expected because this analyte is the most volatile among the other analytes (boiling point of 80 ºC).2 The estuary sample showed the highest concentrations of o, m, p-xylenes and ethylbenzene, being a worrying result. Among the collected samples, the mangrove samples showed, in general, the lowest amounts of BTEX. However, the impact to marine life is worrisome and thus, a continuous monitoring of these and other pollutants on this sensitive region is needed, and the method developed here could be a useful environmental tools.8 To evaluate the performance of the automated water sampling system using the adapted UAV regards the possibility of cross-contamination,25-27,45,46 the results of the BTEX concentration is presented in Table 3 for a sequential sampling between P1 and P2 by comparing method 1 and method 2. The main difference between the method is regards to the mode of collecting the sample, where in method 2 each aliquot of 50 mL was individually collected, stored and measured. The BTEX profile for each method was statistically evaluated. Based on the results presented in table 3, the profiles of BTEX in P1 and P2 were different as expected and there is no cross-contamination between the collected samples. Moreover, the water sampling using methods 1 and 2 was similar with relative errors lower than 5.3% and t-paired-test values lower than the tabulated for a confidence level of 95% with n = 2. Another interesting study of the performance of the automated method is the sampling uncertainty. For this, the results of the BTEX analysis by GC-MS using the automated method were compared to those obtained using the manual method. As method 1 and method 2 presented similar results, the results of method 2 were used for this comparison purposes. According to the Table 4, the manually and automated sampling were similar for a confidence level of 95% with n = 2 for BTEX analysis by GC-MS. Moreover, the RE were lower than 6.5%. These results shows the precision and accuracy of the automated sampling method. This data presented in Table 4 shows that the polypropylene flasks used in the UAV did not compromise the analyzes, once these flasks was used only for 5 min. After this, the same glass flasks were used to store the samples and sent them to the laboratory. Moreover, the manual sampling involves accessing local or fragile ecosystems such as the mangrove and collecting water with compounds that can be hazardous to health. In addition, the automated method is more advantageous to be faster and it employs low cost apparatus.
CONCLUSIONS The apparatus developed using a UAV with adaptations to acquire water samples was a useful and adequate analytical tool. The samples were obtained in a fast, practical, efficient way, without exposing people to harmful substances or dangerous places or to sensitive places such as mangrove areas in which the presence of man also causes environmental impacts. The use of a common UAV was satisfactory, mainly due to the miniaturization carried out allowing it to be compact and lightweight with low power consumption. The low cost of the sampling apparatus is also very attractive, at around U$40 dollars. The samples were obtained at a volume of 100 mL/sample which allowed several procedures such as extraction, repetition, and development of a full analytical method. In fact, the GC-MS provides a more selective and sensitive method useful to supply accurate and precise data to qualify and quantify the concentration of BTEX in water. The apparatus developed here can be used for other purposes such as for monitoring actions for oceanography, geology, and biochemistry in environmental protection areas.
SUPPLEMENTARY MATERIAL Electronic Supplementary Material associated with this article can be found at http://quimicanova.sbq.org.br, in pdf format, with free access.
ACKNOWLEDGMENT This work was supported mainly the CNPq (grant 421147/2018-0, 465614/2014-0), NUQAAPE/FACEPE (grant APQ-0346-1.06/14), FACEPE (APQ-0413-1.06/21, APQ 0388-1.03/14, APQ-0399-1.03/17), INES 2.0, and the authors are grateful for the CNPq, CAPES and FACEPE fellowship provided. The authors thank Prof. D. Navarro, the Ms Julio, Ms Pablo to help with GC-MS method, and the professional of the Port of Suape-PE-Brazil.
REFERENCES 1. Werder, E. J.; Lawrence, E. S.; Aaron, B.; Richard, K. K.; John, M. A.; Dale, S. P.; Environ. Res. 2019, 175, 100. [Crossref] 2. https://pubchem.ncbi.nlm.nih.gov/compound/241#section=Solubility, acessada em abril 2022. 3. CONAMA, Resolução CONAMA N° 357, de 17 de março de 2005. 4. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations, acessada em abril 2022. 5. WHO; Guidelines for drinking-water quality [electronic resource]: incorporating 1st and 2nIV addenda, Vol. 1, Recommendations, - 3rd edition, World Health Organization. 6. Ferguson, A.; Gabriele, H. S.; Mena, K.; Mar. Pollut. Bull. 2020, 150, 110746. [Crossref] 7. Su, N. R; Jung-Ah, K.; Hae-Kwan, C.; Mina, H.; Young-Koo, J.; Myung-Sook, P.; Kyung-Hwa, C.; Ho, K.; Sung-Il, C.; Kyungho, C.; Domyung, P.; Environ. Pollut. 2019, 248, 286. [Crossref] 8. Magalhães, K. M.; Barros, K. V. S.; Lima, M. C. S.; Barreira, C. C. A. R.; Rosa Filho, J. S.; Soares, M. O.; Sci. Total Environ. 2021, 10, 142872. [Crossref] 9. Esteve-Turrillas, F. A.; Pastor, A.; de la Guardia, M.; Anal. Chim. Acta 2007, 593, 108. [Crossref] 10. Gonzáles, J. L.; Albert, P.; Montserrat, L.; Sci. Total Environ. 2017, 603, 109. [Crossref] 11. Akinsanya, B.; Ayanda, I. O.; Onwuka, B.; Sali, J. K.; Heliyon 2020, 6, e03272. [Crossref] 12. Eunah, H.; Hyun, P. J.; Leandro, B.; Choi, K.; Choy, E. J.; Yu, O. H.; Lee, T. W.; Park, H.; Shim W. J.; Kang, C.; Mar. Pollut. Bull. 2015, 90, 167. [Crossref] [] 13. Koparan, C.; Koc, A. B.; Privette, C. V. Sawyer, C. B.; Water 2018, 10, 264. [Crossref] 14. Filho, I. N.; Vieceli, N. C.; Cardoso, E. M.; Lovatel, E. R.; J. Braz. Chem. Soc. 2013, 24, 410. [Crossref] 15. EMBRAPA; Manual de procedimentos de coleta de amostras em áreas agrícolas para análise da qualidade ambiental: solo, água e sedimentos; Filizola, H. F., Gomes, M. A. F., de Souza, M. D., eds.; EMBRAPA Meio Ambiente: Jaguariúna, 2006. 16. Benson, J.; Hanlon, R.; Seifried, T. M.; Baloh, P.; Powers, C. W.; Grothe, H.; Schmale, D. G.; Water 2019, 11, 157. [Crossref] 17. Lally, H. T.; O'Connor, I.; Jensen, O. P.; Graham, C. T.; Sci. Total Environ. 2019, 670, 569. [Crossref] 18. Ruiz-Jimenez, J.; Zanca, N.; Lan, H.; Jussila, M.; Hartonen, K.; Riekkola, M.; J. Chromatogr. A 2019, 1597, 202. [Crossref] 19. Mckinney, K.; Wang, D.; Ye, J.; Fouchier, J.; Guimarães, P. C.; Batista, C. E.; Souza, R. A. F.; Alves, E. G.; Gu, D.; Guenther, A. B.; Martin, S. T.; Atmos. Meas. Tech. 2019, 12, 3123. [Crossref] 20. Terada, A.; Morita, Y.; Hashimoto, T.; Mori, T.; Ohba, T.; Yaguchi, M.; Kanda, W.; Earth, Planets Space 2018, 70, 64. [Crossref] 21. Ore, J‐P.; Elbaum, S.; Burgin, A.; Detweiler, C.; Journal of Field Robotics 2015, 32, 1095. [Crossref] 22. Banerjee. P. B.; Raval, S.; Maslin, T. J.; Timms, W.; Int. J. Min., Reclam. Environ. 2020, 34, 385. [Crossref] 23. Doi, H.; Akamatsu, Y.; Watanabe, Y.; Goto, M.; Inui, R.; Katano, I.; Nagano, M.; Takahara, T.; Minamoto, T.; Limnol. Oceanogr. 2017, 15, 939. [Crossref] 24. Koparan, C.; Koc, A. B.; Privette, C. V.; Sawyer, C. B.; Water 2019, 11, 604. [Crossref] 25. Volatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS): Capillary Column Technique, USEPA, Method 8260, 1992. 26. Purge and Trap for Aqueous Samples, USEPA, Method 5030, 1994. 27. Cincinelli, A.; Stortini; A. M.; Perugini, M.; Checchini, L. L.; Mar. Chem. 2001, 76, 77. [Crossref] 28. Desideri, P. G.; Lepri, L.; Checchini, L.; Microchim. Acta 1992, 107, 55. [Crossref] 29. Bruner, F.; Furlani, G.; Mangani, F.; J. Chromatogr. A 1984, 302, 167. [Crossref] 30. dos Santos, V. B.; Fava, E. L; Curi, N. S. M.; Faria, R. C.; Guerreiro, T. B.; Fatibello-Filho, O.; Anal. Methods. 2015, 7, 3105. [Crossref] 31. Lima, R. S.; dos Santos, V. B.; Guerreiro, T. B.; Araújo, M. C. U.; Gaião, E. N.; Quim. Nova 2011, 34, 135. [Crossref] 32. Souza, A. R.; Paixão, A. C.; Uzêda, D. D.; Dias, M. A.; Duarte, S.; Amorim, H. S.; Rev. Bras. Ensino Fis. 2011, 33, 17021. [Crossref] 33. da Silva, J. I. L.; dos Santos, V. B.; Neves, C. A.; Souza, J. P. I.; Chem. Pap. 2021, 75, 1055. [Crossref] 34. Nilsiam, Y.; Pearce, J. M.; Designs 2017, 1, 5. [Crossref] 35. da Silva, E. K. N.; dos Santos, V. B.; Resque, I. S.; Neves, C. A.; Moreira, S. G. C.; Franco, M. O. K.; Suarez, W. T.; Microchem. J. 2020, 157, 104986. [Crossref] 36. Michel, J.; Spill Sci. Technol. Bull. 2000, 6, 89. [Crossref] 37. https://tabuademares.com, acessada em abril 2022. 38. ANAC; RBAC 94: requisitos gerais para aeronaves não tripuladas de uso civil, Brasília, 2017. 39. https://www.aeroexpo.online/pt/fabricante-aeronautico/drone-transporte-569.html, acessada em abril 2022. 40. Santos, L. P. S.; Araújo, R. J.; Ecotoxicol. Environ. Contam. 2013, 8, 59. [Crossref] 41. Cordeiro, I. A.; Feitosa, F. A. N.; Montes, M. J. F. A.; Otsuka, Y.; Silva, A. C.; J. Mar. Biol. Assoc. U. K. 2013, 9, 291. [Crossref] 42. Frena, M.; Tonietto, A. E.; Madureira, L. A. S.; J. Braz. Chem. Soc. 2013, 24, 1530. [Crossref] 43. Gebara, S. M. S.; Poppi, R. N.; do Nascimento, A. L. C. S.; Raposo Junior, J. L.; Quim. Nova 2013, 36, 1030. [Crossref] 44. Arambarri, I.; Lasa, M.; Garcia, R.; Millán, E.; J. Chromatogr. A.2004, 1033, 193. [Crossref] 45. dos Santos, V. B.; Fava, E. L.; Curi, N. S. M.; Faria, R. C.; Fatibello-Filho, O.; Talanta 2014, 126, 82. [Crossref] 46. dos Santos, V. B.; Fava, E. L.; Pessoa-Neto, O. D.; Bianchi, S. R.; Faria, R. C.; Fatibello-Filho, O.; Anal. Methods 2014, 6, 8526. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access