Assuntos Gerais
| What is new in non-chromatographic flavonoid purification? |
|
Leonardo de Oliveira Aguiar; Clara Ribeiro do Espírito Santo D'Onofrio; Jorge Mauricio David*
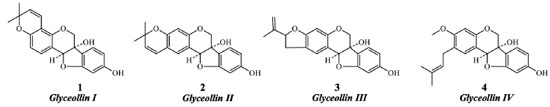
Instituto de Química, Universidade Federal da Bahia, 40170-280 Salvador - BA, Brasil Recebido em: 27/03/2022 *e-mail: jmdavid@ufba.br Flavonoids are metabolites occurring in all plant tissues and present different biological activities. They occur in a mixture of similar polarity with laborious separation and purification processes, usually furnishing low yields. Semi-preparative chromatographic methods are universally employed procedures for their isolation. This paper discusses the state-of-the-art in new non-chromatographic techniques for the separation and purification of flavonoids. In the last decades, novel methods for flavonoids purification have been developed to decrease time extraction, reduction of costs, and organic solvent. Herein, we present the state-of-the-art development of ionic liquids, eutectic solvents, chelating agents, and molecular imprinted polymers in greener extractions, enrichments, and the purification of flavonoids. As the main advantage, these proceedings permitted good yields of bioactive flavonoids and biflavonoids, and they are improved when ultrasound, microwave, and mechanical extractors are used in the extraction step. Nevertheless, most of these techniques are not specific to a single molecule's purification, except MIPs mainly used for analytical purposes. These examples indicate that the development of efficient and straightforward methods for the obtention of different bioactive flavonoids is still incipient but with potential for discovering specific and greener procedures for isolating the target compounds in good yields. INTRODUCTION Flavonoids are specialized metabolites that appear as polyphenols occurring in all plant tissues. They present ecological and specific roles in the plant survivor, such as protection against ultraviolet (UV) and visible radiation, and, to date, they draw particular attention due to different biological activities. Many flavonoid compounds are present in plants, including the fruits, vegetables, and cereals consumed daily.1-3 There are more than 6000 known flavonoids, widespread as glycosides or aglycones. They are classified into different subgroups such as flavones, chalcones, flavanones, aurones, anthocyanidins, catechins, and isoflavones with characteristic variations in their scaffold. The basic structure of flavonoids contains a benzo-pyran scaffold, with 15 carbon atoms forming two aromatic rings (A and B) and a heterocyclic ring (C). Flavonoids can also appear as monomers, dimers, or trimers.4 Studies dealing with flavonoids have indicated that oxidative stress stimulates their biosynthesis, triggered by reactive oxygen species. Other functions include plant defense against fungi, viruses, bacterial microorganisms, and insects. Additionally, flavonoids are responsible for the natural attraction of pollinator agents, controlling phytohormones, enzyme inhibition, active allelopathic agents, and antioxidants. Flavonoids are also involved in auxins' catabolism and the regulation of essential enzymes, such as indole-3-acetic acid oxidase, with implications for cell growth.5,6 Isoflavonoids, for example, are known to be phytoalexins, substances produced by plants in response to some infection caused by pathogenic agent.7 Glyceollins (1-4, Figure 1) are a group of phytoalexins, considered the most important bioactive compounds present in soybeans (Glycine max). Recently, these compounds have been investigated as an alternative methodology for the control of phytopathogens in soybean cotyledons.8,9
Apigenin (5) is considered a significant flavone with a simple structure (Figure 2) because several studies have indicated its pharmacological potential, including anti-inflammatory, antioxidant, anticancer, and cardioprotective.10 In nutritional importance, beneficial effects in dietary ingestion of apigenin, including antimicrobial functions, have also been pointed out.11 Recent studies showed apigenin influences numerous molecular targets involved in inflammation.12 Based on the in vivo, in vitro, and clinical trials, it is suggested that apigenin is a potent therapeutic agent to overcome diseases such as rheumatoid arthritis, autoimmune disorders, Parkinson's and Alzheimer's diseases, and various types of cancers.13 Although the apigenin (5) distribution in several plant species, the extraction from natural sources results in low yields. The total synthesis is achieved through a multi-step route. As a consequence, a high commercial value is attributed to apigenin. Recent strategies in organic synthesis allowed the apigenin (5) obtention by semi-synthesis from more abundant flavonoids such as the flavanone naringin (6), which occurs in good yields in Citrus spp.14
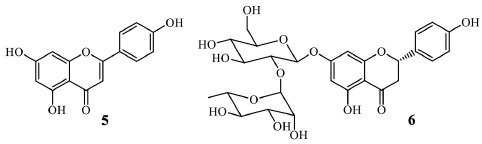 Figure 2. Apigenin (5) and naringin (6) structures
Quercetin (7) is the most abundant flavonol (Figure 3) in the diet. It is estimated that an average person consumes 10 100 mg of quercetin daily through various food sources such as apples, onions, berries, and grains. Antiviral activity, including against hepatitis B (HBV) and herpes type 1 (HSV-1), anti-inflammatory, and anticarcinogenic, are some pharmacological activities attributed to this flavonoid. Quercetin (7) is significant in improving the immunity system, and it is also used for treating conditions of the heart and blood vessels, including atherosclerosis, high cholesterol, heart disease, circulation problems, diabetes, cataracts, hay fever, peptic ulcer, inflammation, asthma, gout, viral infections.15-17 Besides, it increases endurance and improves athletic performance.18 In addition to its pharmacological importance, quercetin formulation known as Phytosome®, supplementary food for immune support, is primarily commercialized in drugstores or supermarkets due to its simple extraction and purification. Some studies described the obtention of quercetin (7) from onion (Allium cepa, Amaryllidaceae) with good yields after simple techniques such as precipitation, recrystallization, or hydrolysis of the glycosylated derivative rutin (8). Noteworthy is the fact that onion is the vegetable with the highest quercetin content, but since it is a pigment, the content changes according to the varieties, with the highest amounts in colored variations.19
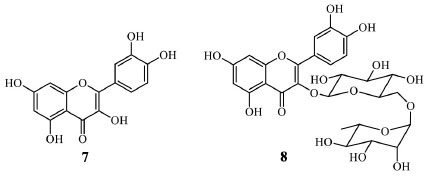 Figure 3. Quercetin (7) and rutin (8) structures
Biflavonoids are other important flavonoid subclasses. They are dimeric structures of the same and different flavonoids, and most of them are known for in vitro and in vivo effects on metabolic, cardiovascular, and cognition-related diseases, including cancer.20 They can also occur concomitantly with other flavonoids, and these compounds' separation processes are laborious. Due to the diversity of structures, biflavonoid synthesis is still a challenge. Even with the advances in modern catalysts, just a few compounds have been synthesized.21 Agathisflavone (9), amentoflavone (10), and hinokiflavone (11) are some of the most studied biflavonoids due to their pharmacological importance (Figure 4). Recently, studies described new insights and demonstrated their benefits, such as anti-inflammatory, antimicrobial, anticancer, neuroprotective, and hepatoprotective effects.22-24
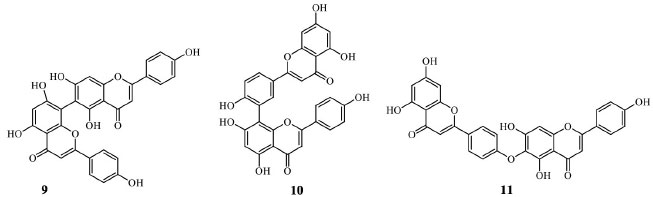 Figure 4. Agathisflavone (9), amentoflavone (10), and hinokiflavone (11) structures
Nevertheless, differently in the example mentioned earlier of quercetin (7) purification, mainly flavonoids occur in an assembled mixture with similar polarity, and for this reason, the separation and purification processes are laborious, expensively, and usually with low yields. Among the most used conventional techniques for flavonoids separation and purification, it can be considered preparative thin-layer chromatography (TLC), column chromatography, semi-preparative or preparative HPLC (high performance liquid chromatography), and gel permeation. This paper describes the state of the art of new techniques for flavonoids separation and purification. The referential materials for this paper were obtained electronically on multiple databases, such as SciFinder®. Additional information was collected from reliable and authentic databases, such as Science Direct, Web of Science, and Google Scholar. Appropriately, high-quality publications (1950 2022) were collected, and original scientific papers and patents were analyzed. "Flavonoid AND Extraction AND new method," "flavonoid AND precipitation," and "biflavonoid AND extraction AND purification AND new method" were primary keywords used. The duplicates and the chromatographic methods were removed. This paper does not intend to be a review because there is not enough material. The authors just selected 22 studies dealing with non-chromatographic methods for purification. Figure 5 summarizes all the findings, highlighting the main topic discussed herein.
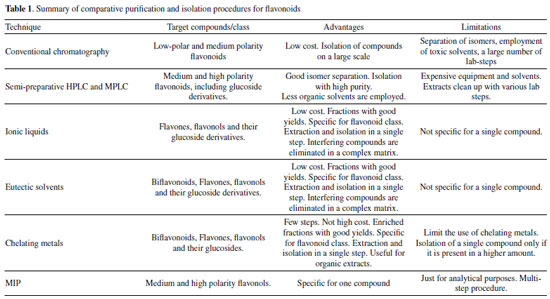
Methods for flavonoid extraction Conventional methods for the extraction of natural polyphenols, such as flavonoids, include hot extraction under reflux, decoction, maceration, infusion, and percolation (usually using methanol, ethanol, ethyl acetate, or acetone as solvents). Among the modern extraction methods, subcritical water extraction (SWE), supercritical fluid extraction (SFE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), or accelerated solvent extraction (ASE) can be highlighted. These modern methods provide faster extractions employing less solvent than usual, and the extracts are usually obtained in higher yields and similar amounts.25 On the other hand, despite these methods providing a uniformized yield of extracts, they are not specific for a class of metabolite or organic compounds. Generally, extractions with increasing organic solvent polarity are customarily carried out for flavonoids extraction. Non-polar solvents, such as hexane and petroleum ether, can be employed previously as a first step to removing grease, oils, sterols, and pigments, and then the subsequent flavonoids extraction could be improved. In the second step, solvents including chloroform, methylene dichloride, ethyl acetate, or ethyl ether can be used to extract aglycones with a few polar groups. Solvents of the higher polarity are needed for polyhydroxylated aglycone flavonoids, such as flavones, flavonols, aurones, chalcones, and glycosylated flavonoids. These compounds are accessed using n-butanol, acetone, methanol, ethanol, or water.26 To date, there are a few modern and valuable techniques for flavonoids extraction and purification. Establishing these procedures is essential and ratifies the compliance of chemical techniques with nature and human health safety. Table 1 summarizes all techniques mentioned in this paper, with some comparable advantages and limitations.
Techniques for flavonoid separation and purification Among the commonly used techniques for flavonoid separations, conventional column chromatography, radial chromatography, medium-pressure liquid chromatography (MPLC), and preparative thin-layer chromatography can be highlighted, employing as stationary phase polyamide, silica-gel, Sephadex LH-20, amberlite, or ion-exchange resins. Such techniques can be selected depending on the chemical composition of the mixture, extract amounts, and solubility. However, all of them are well-established and easily handed.27 Important techniques based on equipment for flavonoid purification include high-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography (UPLC). Flavonoids are successfully isolated employing reverse phase chromatography, and the last advances were previously reviewed.28 Different counter-current chromatography is a fast-growing separation technique based on the liquid-liquid partition principle and shows advantages compared to solid-phase chromatographic-based separations. One of the most representative procedures for flavonoid isolation, high-speed counter-current chromatography (HSCCC), was recently reviewed.29 Gas chromatography (GC) is another efficient technique for sample separation mainly used for quantitative or qualitative analytical purposes. However, the employment of GC for flavonoids is restricted to a few volatile and thermically stable aglycones. A good strategy is derivatization with silane compounds to increase the flavonoids volatility.30 The above methods are time-consuming, and the instrumental apparatus presents high costs and needs several previous steps for flavonoid purification from the organic extracts. Recrystallization using ethanol, methanol, or chloroform is common for flavonoids purification.31,32 Precipitation reactions have also been investigated for these purposes. An exciting example involves the formation of an adsorbent gel through aluminum chloride in alkaline media, which complexes with flavonoids. The desired compounds are purified after breaking the complex through washing with ethanol/water mixture and centrifugation. In other words, complexes were prepared by mixing CuCl2, FeCl2, AlCl3, and, ZnCl2, in methanol, with stoichiometric amounts of the flavonoids. The precipitates were formed immediately in all preparations, and the complex can be broken by treatment with an aqueous acid solution. However, this procedure gives poor yields, and 5-hydroxyflavonoids with a carbonyl group in C-4 produce bounds with metal that cannot be broken with acids.33 Modern methods for flavonoids extraction, separation, and purification In the last decades, few novel methods for flavonoids extraction and purification have been developed. The majority of these techniques are intended to decrease the time extraction and reduce organic solvent employment, avoiding the use of traditional chromatographic procedures. These modern techniques are also investigated to increase the extraction yields, selectivity, and compound purity. Consequently, these procedures can be considered greener and more efficient, impacting nature and human health safety. Besides, conventional chromatography and the employed solvents present high costs and are not simple procedures. For example, ionic liquid extraction assisted by ultrasound or microwave, eutectic solvent extraction, and chelation extraction are some of the selective and specific methods. Ionic liquids in flavonoid extraction and purification Ionic liquids are salts presenting melting points below 100 ºC and consisting of different cations - commonly asymmetric and organic - and organic or inorganic anions. A relatively new class of synthetic ionic liquids has been formed by combining different cations and anions, making them viable and greener for natural product extraction. They can be easily adapted to being selective to a specific compound - or group of compounds; therefore, ionic liquids are often called "designer solvents." Their utilization has been relevant in the last decades, mainly in sample preparation techniques.34 The properties of them are designed by selecting the desired cation, anion, and substituent alkyl chain length. The rapid infiltration in plant cells to dissolve the target products, low viscosity, relative hydrophobicity, and straightforward recuperation after extraction are advantages attributed to ionic liquids. For these reasons, they are being employed in the analytical determination of a range of organic compounds, including natural products.35,36 Recently, ionic liquids are being used for flavonoids extraction among natural products. For this class of metabolites, ultrasound is employed as support. In this technique, cavitation bubbles generate micro-jets in suitable pressure and temperature conditions to break down cell membranes, increasing the contact surface due to enhanced solvent penetration. In addition, some pores formed by the implosion of the bubbles allow solvent penetration, and the chemical content transport caused by that turbulence leads to a decreasing concentration gradient inside the cells with high mass transfer, and consequently, the flavonoids are extracted.37 The properties of ionic liquids are designed by selecting the desired cation, anion, and substituent alkyl chain length. This statement can be verified in the results of the extraction yield employing ionic liquids (Figure 6) of isoquercitrin (12) and astragalin (13) from the leaves, quercetin (7) from leaves, and kaempferol (14) from the flowers of Lysimachia clethroides (Primulaceae). 1-Butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4), 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM]PF6), 1-hexyl-3-methylimidazolium hexafluorophosphate ([HMIM]PF6), and 1-butyl-3-methylimidazolium bromide ([BMIM]Br) were employed as ionic liquids. 1-Butyl-3-methylimidazolium tetrafluoroborate (15) extracts more efficiently the flavonoids than the other tested ionic liquids. After the extract was centrifugated and the supernatant filtrated through a microporous membrane, an enriched extract of flavonoids was obtained. Compared to methanol extraction, the optimized method showed to be better due to multi-interactions between the target flavonoids and ionic liquid structure, including hydrogen bonds. However, the extract is a mixture of the above flavonoids. DPPH quenching was measured to prove the ionic liquid extractions' efficient advantage.38
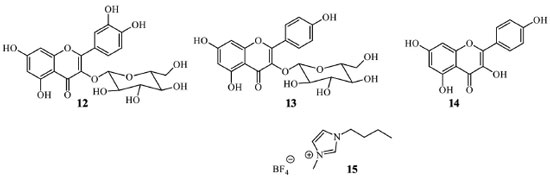 Figure 6. Flavonoids extracted by ionic liquid from the leaves and flowers of Lysimachia clethroides using [Bmim]BF4 (15)
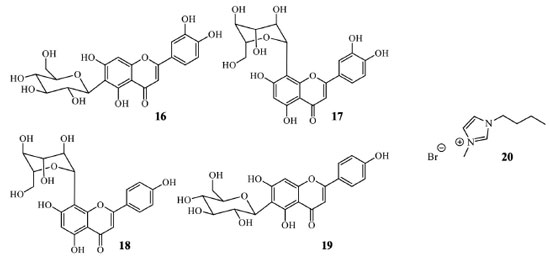
The ionic liquid 1-butyl-3-methylimidazolium bromide ([BMIM]Br, 20) when used in the ultrasound-assisted extraction of flavonoids isoorientin (16), orientin (17), vitexin (18), and isovitexin (19), also demonstrated good capacity of flavonoid extraction. This ionic liquid was selected between 15 different combinations of cations and anions employing leaves of Phyllostachys heterocycla (Figure 7). [BMIM]Br (20) reached the capacity of 4.592 mg flavonoid per gram of leaves. According to that study, the authors conclude that the anion portion interacts with flavonoid phenolic hydrogens, and the cation binds with the flavonoid nucleus. The procedure is simple; after extraction, the mixture containing flavonoids is diluted in deionized water and partitioned with n-butanol. That procedure recuperated 97.87 ± 0.25% of flavonoids and 96.52 ± 0.63% of ionic liquid from the extract.39
The mixture of [BMIM]Br (20) and lithium chloride as an addictive agent, after optimal conditions and also assisted by ultrasound, permitted to isolate vitexin (18), vitexin-4"-O-glucoside and vitexin-2"-O-rhamnoside from Phyllostachys edulis leaves. This plant is another Poaceae medicinal species mainly distributed across China, Southeast Asian countries, and Japan. The total flavonoid extracted yield was 638 μg g-1, a value superior to that obtained using the traditional extraction method and also higher when only the ionic liquid is used.40 An aqueous solution of 1-butyl-3-methyl-imidazolium dicyanamide ([C4MIM][N(CN)2]), combined with ultrasound and different experimental conditions, can be employed in the extraction and isolation of an enriched fraction of bioactive flavonoid mixture. This procedure was first employed in Apocynum venetum L. (Apocynaceae) leaves, a well-known plant in Chinese traditional medicine. A preconcentration step is necessary for this ionic liquid for a biphasic solvent partition. The addition of ammonium sulfate - (NH4)2SO4 - in the extract separated the target compounds in a superior aqueous phase, enriched with ionic liquid. Butanol-1 can be used to isolate the flavonoid mixture from the ionic liquid phase furnishing 8.67 mg by g of leaves, with a preconcentration factor of 3.78%, 93.35% of the extraction efficiency, and 83.5% of recuperation of flavonoids. However, in this study, the specific flavonoid extraction was not evaluated, just the total.41 Microwave-assisted equipment can also be employed with ionic liquids for flavonoid or biflavonoid selective extractions. When this technique was combined with different imidazolium cations (Figure 8), [HMIM][PF6] (23) permitted to obtain three biflavonoids from Selaginella doederleinii (Selaginellaceae). In optimized conditions, the yield of the biflavonoids mixture was 16.83 mg g-1.42 The ionic liquid [HMIM][BF4] was employed for amentoflavone (10) and hinokiflavone (11) (both biflavonoids) obtention from S. sinensis with respective yields of 1.96 mg g-1 and 0.79 mg g-1, higher when compared with chromatographic methods. Similar results were obtained with S. helvetica.43,44
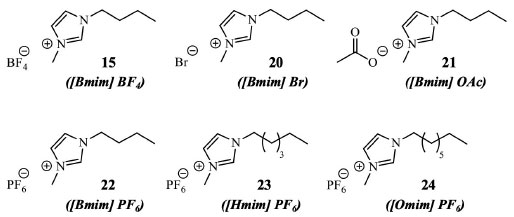 Figure 8. Ionic liquids screened for biflavonoid extractions from Selaginella doederleinii
Ionic liquids can be included in modified polymeric microsphere and employed in flavonoid isolation by multiphasic extraction (MPE) and purification. The amine derivative ionic liquid (25) was used for the obtention of flavonoids and biflavonoids (Figure 9) from Chamaecyparis obtusa (Cupressaceae). The modified polymeric microsphere can be obtained by the reaction of the epoxide groups (from glycidyl methacrylate) on dried microsphere polymer with 3-bromopropylamine hydrobromide. MPE is a sequential SPE (solid phase extraction), and it has attracted considerable interest in the context of the simultaneous extraction and separation of bioactive compounds from plants. Briefly, the powdered sample is packed in an SPE cartridge with the polymer-bonded with ionic liquid and C-18 RP. The interferents are removed with non-polar solvents, and finally, after the interfering species are out of the sorbent, the purified target compounds can be eluted with MeOH (washing and elution stage). This procedure, when applied to C. obtusa, furnished quercitrin (26) with 0.045 mg g-1, myricetin (27) with 0.018 mg g-1, and amentoflavone (10) with 0.012 mg g-1 yield, values higher than C-18 silica, C-18 without the modified microsphere polymer (Figure 9). However, this is an extractive and purification procedure the application just applied in an analytical study.45
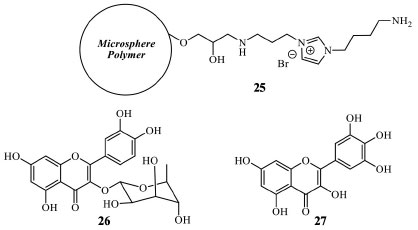 Figure 9. Polymeric microsphere modified by the ionic liquid (25), quercitrin (26), and myricetin (27) structures
Solid-solid extraction in flavonoid purification Solid extractants are a new source of materials developed, and they can be applied for flavonoids extraction. However, the ionic liquids have increased attention due to their excellent extraction performance and their greener characteristics, the solid analogs, such as oxonium salts, have been defined as substitutes for the selective extraction of flavonoids. In a recent and unique study, the bamboo-leaf flavonoids were extracted for the first time, without solvents, by pressing the material with oxonium salts, which reproduced the same intermolecular effects as ionic liquids. After chromatographic analysis, orientin (17), vitexin (18), and rutin (8) enriched the solid ion, demonstrating simplicity, low cost, environmental friendliness, and high enrichment capability.46 Eutectic solvents in flavonoid purification Eutectic solvents are mixtures containing a hydrogen donor solvent and a hydrogen receptor solvent. In terms of extraction, some eutectic solvent properties are similar to ionic liquids, with the additional advantage of being more economically accessible, ecologically friendly, and presenting a high extraction and purification efficiency. Usually, choline chloride (28) and 1,4-butanediol (29) are usually employed as hydrogen receptors and donors, respectively. An aqueous (20%) solution of 1:4 proportion of choline chloride and 1,4-butanediol was employed as eutectic solvents for extraction of 3,3',5,5',7-pentahydroflavanone (30), the main compound from Spina Gleditsiae, a Chinese natural product that consists of the dry thorns of Gleditsia sinensis (Fabaceae) (Figure 10). In the general procedure, the mixture containing flavonoids is powdered and added to a eutectic solvent, in a proportion of solid-liquid 1:30 and subjected to an ultrasound-assisted extraction under temperature and time control (55 ºC, 45 minutes, for the specific purification). After filtration, the extract is submitted to another extraction in a packed column of a macropores resin AB-8. The elution is carried out with water to remove the eutectic solvent components, and then ethanol solution 90% (v/v). The eluate was collected, combined, and concentrated to obtain the total flavonoids. The chromatographic analysis of this methodology demonstrated that the extraction efficiency using eutectic solvents is almost 60% superior to that using conventional techniques.47 In this application, a pure compound was extracted and isolated.
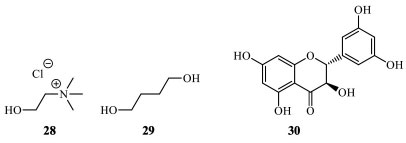 Figure 10. Choline chloride (28), 1,4-butanodiol (29), and 3,3',5,5',7-pentahydroflavanone (30) structures
An interesting study using the earlier procedure reported the isolation of a mixture of flavonoids from Chamaecyparis obtusa (Cupressaceae). This study employed a higher concentration of 1,4 butanediol about choline (5:1) in a solution with 35% water at 70 °C for 40 minutes. For best results, the procedure was optimized considering effects on diffusion, viscosity, surface tension, and solubility, resulting in the isolation of myricetin (27) and amentoflavone (10) with respective yields 3.1 x 10-2 and 51.8 x 10-2 mg g-1 in relation with plant employed.48 Tea (black and green) is a source of bioactive compounds. In a recent study of leaves of this plant employing a combination of mechanochemical extraction and deep eutectic solvent (the same as the previous examples) in different molar ratios, the extraction of flavonoids was evaluated. The efficiency for individual compounds was determined by ultrahigh performance liquid chromatography (UPLC) in a validated methodology. These procedures permitted to conclude that in all experiments, theobromine and the flavonoid epigallocatechin gallate are the compounds extracted with better yields, followed by epigallocatechin and caffeine, all the common compounds present in green tea leaves. However, the results indicated that kaempferol was extracted with a low yield (0.0043 mg g-1) using choline chloride/1,4-butanediol (1:4) compared with catechins, caffeine, and theobromine.49 The extracted amount of kaempferol significantly decreased with decreasing deep eutectic content, unlike the other target compounds. To date, there is just one example of the employment of other eutectic solvents in flavonoids extraction enrichment. The efficiency of eight eutectic solvents was evaluated from Acanthopanax senticosus (Araliaceae). A 1:1 mixture of glycerol and levulinic acid (50 ºC, for 70 minutes) under 500 W ultrasonic power was the most suitable extraction medium, showing low viscosity. The total flavonoids extracted was almost 24 mg g-1, considerably higher than conventional ultrasound-assisted extraction in ethanol.50 Chelating agents in flavonoids extraction Chelating agents for flavonoids extraction are helpful because they can reduce the oxidation of target compounds in the presence of air, increasing product purity. Additionally, they make possible longer extraction, which has positive consequences in increasing the product yields. These procedures are not new, but new insights have been developed, and some chelating metals can strongly bond with carbonyl and phenolic groups, and releasing the flavonoid is not easy. Some studies with zinc salts in the isolation of flavonoids have recently been developed. As the first example, ZnSO4·7H2O was used as a chelating agent to extract and purify dihydromyricetin (31) from dry leaves from the Vitaceae Ampelopsis grossedentata (Figure 11), a traditional Chinese medicinal plant.51 This compound is considered responsible for the activity, and it can occur in a concentration higher than 30% in young plant stem and leaves; thus, isolation from natural sources is economically advantageous.52 The procedure requires a 1:20 solid-liquid proportion between A. grossedentata powder and deionized water. The proportion between the plant powder and ZnSO4·7H2O was set at 4:1. The extraction was carried out in acidic media (pH 2.0) to avoid Zn2+ hydrolysis and oxidation of dihydromyricetin phenolic hydroxyl groups - EDTA-2Na (0,1 mol.L-1) was used to remove the target compound from the zinc complex obtained and, sequentially, hot washing and filtration led to pure dihydromyricetin crystals with 12.2% yield presenting 94.3% purity.51
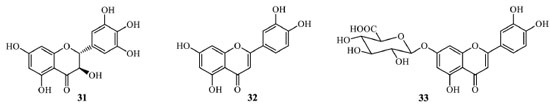 Figure 11. Structures of dihydromyricetin (31), luteolin (32), and luteolin-7-O-β-D-glucuronide (33)
Magnesium and zinc salts were employed in the development of new extraction methods to obtain glycosides and aglycones from Ixeris sonchifolia (Asteraceae), such as luteolin (32), apigenin (5), and luteolin-7-O-β-D-glucuronide (33) - the most abundant flavonoid in I. sonchifolia (Figure 11). The metallic salts were added to the methanolic extract in regulated alkaline media for this process. The precipitated complex was washed with methanol, and luteolin-7-O-β-D-glucuronide (33) was isolated from the precipitate with a 38.9% yield.53 No details with the other plant flavonoids were also present in the precipitate. Similarly, zinc salts have also been employed for flavonoid chelation from kaki fruit (Diospyros kaki, Ebenaceae). That methodology is a patent process and suggests the first step of tannin remotion by adding a protein. After acid digestion of the plant material, the flavonoids can be preconcentrated. To residual extract, a solution of zinc salt is added, the complex precipitated, and it was added to a citric acid solution to obtain the pure flavonoids when the mixture cooled.54 Employment of molecularly imprinted polymer (MIP) for the isolation of quercetin (7) Molecular imprinting is a method for generating chemically selective recognition sites to identify a specific compound in a macroporous three-dimensional polymer network. In MIPs, a specific compound acts as the template around which the interacting and cross-linking monomers are arranged and co-polymerized. The polymerization produces an imprinted nanocavity left behind to recognize new analytes after the template removal. MIPs have been extensively used for specific preconcentration of organic and inorganic compounds and employed in different areas, especially chromatography. For flavonoids, to date, there are just a few analytical studies employing this methodology; none of them employ MIP for semi-preparative or preparative purposes. The studies are concentrated on important healthy flavonoids, such as quercetin (7), and derivatives which demonstrates that MIP is more selective for extraction of the flavonoid when compared with NIP (non-imprinted polymer).55
CONCLUDING REMARKS To date, chromatographic methods are the main techniques for flavonoid purifications. Most of them employ high-cost equipment and demand a considerable number of laboratory steps. However, in the last decades, studies dealing with new techniques for the non-chromatographic, greener, and more efficient extraction and purification of flavonoids have been presented (Table 1). Some of them are old, such as the complexation with metals, but some improvements have become specific nowadays. Besides them, ionic liquid and deep eutectic solvents seem to be the more promisor procedures because they afford better yields than the conventional methods. Once they can isolate a single flavonoid or are specific for the class, and enriched fractions of these compounds. However, they are hardly employed for a single isolated flavonoid once the specificity is for all phenolics compounds or a subclass of flavonoid. Specific procedures for single compounds, like MIPs, are also employed for analytical purposes but not for preparative or semi-preparative flavonoid purifications.
ACKNOWLEDGMENTS The authors thank CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico for grants (# 406427/2018-6) and scholarships for authors.
REFERENCES 1. Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K. S.; Salem, M. L.; Cheng, K.; Curr. Med. Chem. 2020, 28, 1042. [Crossref]. 2. Wang, T.-Y.; Li, Q.; Bi, K.-S.; Asian J. Pharm. Sci. 2018, 13, 12. [Crossref]. 3. Boniface, P. K.; Ferreira, E. I.; Phyther. Res. 2019, 33, 2473. [Crossref]. 4. Dewick, P. E.; Medicinal Natural Products: A Biosynthetic Approach, 2nd ed., John Wiley & Sons Ltd: Hoboken, 2001. 5. Mierziak, J.; Kostyn, K.; Kulma, A.; Molecules 2014, 19, 16240. [Crossref]. 6. Nabavi, S. M.; Samec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; Giampieri, F.; Battino, M.; Sobarzo-Sanchez, E.; Nabavi, S. F.; Yousefi, B.; Jeandet, P.; Xu, S.; Shirooie, S.; Biotechnol. Adv. 2020, 38, 107316. [Crossref]. 7. Arruda, R. L.; Paz, A. T. S.; Bara, M. T. F.; Côrtes, M. V. de C. B.; de Filippi, M. C. C.; da Conceição, E. C.; Ciênc. Rural 2016, 46, 1206. [Crossref] 8. Bizuneh, G. K.; Adv. Tradit. Med. 2021, 21, 31. [Crossref]. 9. Mazaro, S. M.; Citadin, I.; de Gouvêa, A.; Luckmann, D.; Guimarães, S. S.; Ciênc. Rural 2008, 38, 1824. [Crossref] 10. Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y. H.; Int. J. Food Prop. 2017, 20, 1197. [Crossref]. 11. Wang, M.; Firrman, J.; Liu, L.; Yam, K.; Biomed Res. Int. 2019, 2019, 1. 12. Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E. B.; Novellino, E.; Antolak, H.; Azzini, E.; Setzer, W. N.; Martins, N.; Int. J. Mol. Sci. 2019, 20, 1305. [Crossref]. 13. Bakhoda, M.; Bahmanpour, Z.; Ilkhani, K.; Mozafarpour, P.; Pourhanifeh, M. H.; Khan, H.; Mirzaei, H.; Front. Chem. 2020, 8, 829. [Crossref]. 14. Victor, M. M.; David, J. M.; Sakukuma, M. C. K.; França, E. L.; Nunes, A. V. J.; Green Process. Synth. 2018, 7, 524. [Crossref]. 15. Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S. M.; Salem, M. A.; Merghany, R. M.; El Mahdy, N. M.; Klllç, C. S.; Sytar, O.; Sharifi-Rad, M.; Sharopov, F.; Martins, N.; Martorell, M.; Cho, W. C.; ACS Omega 2020, 5, 11849. [Crossref]. 16. Anand David, A. V.; Arulmoli, R.; Parasuraman, S.; Pharmacogn. Rev. 2016, 10, 84. [Crossref]. 17. El-Saber Batiha, G.; Beshbishy, A. M.; Ikram, M.; Mulla, Z. S.; Abd El-Hack, M. E.; Taha, A. E.; Algammal, A. M.; Ali Elewa, Y. H.; Foods 2020, 9, 374. [Crossref]. 18. Kressler, J.; Millard-Stafford, M.; Warren, G. L.; Med. Sci. Sports Exercise 2011, 43, 2396. [Crossref]. 19. Kwak, J. H.; Seo, J. M.; Kim, N. H.; Arasu, M. V.; Kim, S.; Yoon, M. K.; Kim, S. J.; Saudi J. Biol. Sci. 2017, 24, 1387. [Crossref]. 20. Kang, F.; Zhang, S.; Chen, D.; Tan, J.; Kuang, M.; Zhang, J.; Zeng, G.; Xu, K.; Zou, Z.; Tan, G.; Molecules 2021, 26, 5401. [Crossref]. 21. He, X.; Yang, F.; Huang, X.; Molecules 2021, 26, 6088. [Crossref]. 22. Goossens, J. F.; Goossens, L.; Bailly, C.; Nat. Prod. Bioprospect. 2021, 11, 365. [Crossref]. 23. Islam, M. T.; Zihad, S. M. N. K.; Rahman, M. S.; Sifat, N.; Khan, M. R.; Uddin, S. J.; Rouf, R.; IUBMB Life 2019, 71, 1192. [Crossref]. 24. Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z.; Front. Pharmacol. 2021, 12, 1. [Crossref]. 25. Chaves, J. O.; de Souza, M. C.; da Silva, L. C.; Lachos-Perez, D.; Torres-Mayanga, P. C.; Machado, A. P. da F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A. V.; Barbero, G. F.; Rostagno, M. A.; Front. Chem. 2020, 8. [Crossref]. 26. Rodríguez De Luna, S. L.; Ramírez-Garza, R. E.; Serna Saldívar, S. O.; Sci. World J. 2020, 2020, 1. [Crossref]. 27. Rijke, E. de; Out, P.; Niessen, W. M. A.; Ariese, F.; Gooijer, C.; Brinkman, U. A. T.; J. Chromatogr. A 2006, 1112, 31. [Crossref]. 28. Valls, J.; Millán, S.; Martí, M. P.; Borràs, E.; Arola, L.; J. Chromatogr. A 2009, 1216, 7143. [Crossref]. 29. Gong, Y.; Huang, X. Y.; Pei, D.; Duan, W. Da; Zhang, X.; Sun, X.; Di, D. L.; J. Chromatogr. A 2020, 1623, 461150. [Crossref]. 30. Sun, A.; Tao, X.; Sun, H.; Chen, J.; Li, L.; Wang, Y.; Int. J. Food Prop. 2014, 17, 1818. [Crossref]. 31. Shi, G. Q.; Yang, J.; Liu, J.; Liu, S. N.; Song, H. X.; Zhao, W. E.; Liu, Y. Q.; Bangladesh J. Pharmacol. 2016, 11, 18. [Crossref]. 32. Garaev, E. A.; Movsumov, I. S.; Isaev, M. I.; Chem. Nat. Compd. 2008, 44, 520. [Crossref]. 33. de Souza, R. F. V.; De Giovani, W. F.; Redox Rep. 2004, 9, 97. [Crossref]. 34. Delińska, K.; Yavir, K.; Kloskowski, A.; TrAC - Trends Anal. Chem. 2021, 143, 116396. [Crossref]. 35. Dai, J.; Sun, Y.; Xiu, Z.; Chin. J. Chem. Eng. 2021, 30, 185. [Crossref]. 36. Choi, Y. H.; Verpoorte, R.; Curr. Opin. Food Sci. 2019, 26, 87. [Crossref]. 37. Khadhraoui, B.; Ummat, V.; Tiwari, B. K.; Fabiano-Tixier, A. S.; Chemat, F.; Ultrason. Sonochem. 2021, 76, 105625. [Crossref]. 38. Wei, J. F.; Zhang, Z. J.; Cui, L. L.; Kang, W. Y.; J. Chem. 2017, 2017, [Crossref]. 39. Wang, L.; Bai, M.; Qin, Y.; Liu, B.; Wang, Y.; Zhou, Y.; Molecules 2018, 23, 2309. [Crossref]. 40. Hou, K.; Chen, F.; Zu, Y.; Yang, L.; J. Chromatogr. A 2016, 1431, 17. [Crossref]. 41. Tan, Z.; Yi, Y.; Wang, H.; Zhou, W.; Wang, C.; McPhee, D. J.; Molecules 2016, 21, 262. [Crossref]. 42. Li, D.; Qian, Y.; Tian, Y. J.; Yuan, S. M.; Wei, W.; Wang, G.; Molecules 2017, 22, 586. [Crossref]. 43. Jiang, Y.; Li, D.; Ma, X.; Jiang, F.; He, Q.; Qiu, S.; Li, Y.; Wang, G.; Molecules 2018, 23, 3284. [Crossref]. 44. Li, D.; Sun, C.; Yang, J.; Ma, X.; Jiang, Y.; Qiu, S.; Wang, G.; Molecules 2019, 24, 2507. [Crossref]. 45. Tian, M.; Bi, W.; Row, K. H.; Phytochem. Anal. 2012, 23, 576. [Crossref]. 46. Tang, J.; Cao, Y.; Wang, X.; Yan, W.; Yao, S.; J. Chromatogr. A 2021, 1658, 462595. [Crossref]. 47. Yu, J.; Zhao, L.; Sun, X.; Sun, C.; Wang, X.; Phytochem. Anal. 2021, 32, 457. [Crossref]. 48. Bi, W.; Tian, M.; Row, K. H.; J. Chromatogr. A 2013, 1285, 22. [Crossref]. 49. Wang, M.; Wang, J.; Zhou, Y.; Zhang, M.; Xia, Q.; Bi, W.; Chen, D. D. Y.; ACS Sustainable Chem. Eng. 2017, 5, 6297. [Crossref]. 50. Zhang, X.; Su, J.; Chu, X.; Wang, X.; Molecules 2022, 27, 923. [Crossref]. 51. Hu, H.; Luo, F.; Wang, M.; Fu, Z.; Shu, X.; ACS Omega 2020, 5, 13955. [Crossref]. 52. Deng, Y.; Guo, L.; Cai, H.; Chen, L.; Tan, S.; Zhang, B.; Fang, P.; Xiang, D.; Li, H.; He, G.; Yan, M.; Xenobiotica 2020, 50, 332. [Crossref]. 53. Chunfu, W.; Dongchun, L.; Jingtao, Z.; Fang, W.; Dong, W.; Jingyu, Y.; Wenying, Z.; Zhenzhen, G.; Feng, C.; Fang, L.; Yanle, Q.; Yinghui, D.; CN105726600A 2016. 54. Hongchao, H.; Xugang, S.; Huangwen, X.; CN109879845A 2019. 55. Karaman Ersoy, Ş.; Tütem, E.; Sözgen Başkan, K.; Apak, R.; Nergiz, C.; J. Chromatogr. B 2016, 1017-1018, 89. [Crossref]. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access