Artigo
| Chemical profile and antioxidant activity of geopropolis from Melipona subnitida collected inside and outside the nest |
|
Joselena Mendonça FerreiraI; Giuseppina NegriII,*; Maria Luiza Faria SalatinoII; Dejair MessageIII; Antonio SalatinoII
I. Departamento de Biociências, Universidade Federal Rural do Semi-árido, 59625-900 Mossoró - RN, Brasil Recebido em: 13/05/2022 *e-mail: gnegri@terra.com.br Melipona subnitida ("jandaíra") is a species of stingless bee endemic to the Caatinga, a dry forest in the northeastern Brazilian semi-arid. Propolis is a product containing beeswax and plant resin. It contains compounds that can be able to prevent health problems by protecting cells against damage by oxidative agents. M. subnitida produces geopropolis, a mixture of propolis with soil. Samples of geopropolis of M. subnitida, one of inside and another outside of the nest from a hive in the municipality of Mossoró (northeastern Brazil) were analyzed by HPLC-PDA-ESI-MS/MS and their antioxidant activity evaluated by the DPPH and β-carotene/linoleic acid methods. Both samples exhibited similar chemical profile, characterized by 26 phenolic compounds, however, the compounds detected were more abundant in sample collected from inside of the nest. They were tentatively characterized as galloyl hexoses, coumaroyl-O-galloyl-hexoses, cinnamoyl-O-galloyl-hexoses, coumaroyl- and cinnamoyl- galloyl hexoses, phenylpropanoyl-O-benzoyl hexoses, flavanones and ellagitannins. Flavonoids, galloyl hexoses, coumaroyl-O-galloyl-hexosides, cinnamoyl-O-galloyl-hexosides and coumaroyl-cinnamoyl-galloyl hexosides were reported previously in this genus, however ellagitannins and phenylpropanoyl-O-benzoyl hexoses are reported for the first time. Comparing the antioxidant activity of the geopropolis samples from inside and outside of the nest, the latter was higher than the former. INTRODUCTION Stingless bees are Hymenoptera of the family Apidae, subfamily Apinae, tribe Meliponini, comprising 61 genera and over 600 species.1,2 Meliponines are ecologically important due to species diversity and wide geographical distribution in tropical zones in the Neotropics, Africa, Southwest Asia and Australia.3 In Brazil, there are 29 genus and more than 200 species of stingless bees, corresponding to nearly 20% of all Neotropical meliponines.4 Among these species, 89 are endemic.5 Melipona, Plebeia, Scaptotrigona, Trigona and Trigonisca are examples of large meliponini genera. Pollination of forest and crop species are among the benefits provided by stingless bees.5,6 The biological activities of stingless bee propolis was investigated, mainly, in propolis extracts from the American stingless bees (49%) which were predominantly originated from Brazil, followed by Asian (43%), Australian (5%), and African stingless bees (3%). The in vitro studies corresponded to 87% of the included studies, followed by in vivo studies (13%).7 Propolis is a sticky resinous substance produced by honeybees (Apis mellifera) and stingless bees (Apidae, Meliponini tribe). Plants secrete resin from buds, wounds, fruits, and flowers to defend themselves from herbivores and microorganisms, to attract pollinators and seed dispersers. Resin, a material chemically complex, can trap, immobilize, or deter predators infect wound sites, and help to guard against the proliferation of endophytic fungi. The specific chemical composition of resin varies between plant species and can even vary between individuals of the same species. Propolis contain beeswax and plant resin, used by the insects to line the entrance and seal holes in their hives.8 Propolis and geopropolis maintains the environment within the hive free of pathogens and exerts protection against infectious and parasitic organisms.8 Propolis of stingless bees and honeybees may have similar chemical profile, both products containing predominantly phenolic compounds as biologically active substances. A same plant source of resin may be used by either honeybees or stingless bees to produce propolis.9,10 The propolis productivity by stingless bees is in general lower than honeybees. A means used by several species of stingless bees to increase the mass and volume of their propolis is to aggregate relatively high proportion of soil material. This type of propolis is known as geopropolis.2 Reviews about the chemical composition and biological activity of propolis of stingless bee have been published.5,11,12 Species of Melipona are widespread in all Brazilian biomes. Several species of the genus are native in areas of Caatinga, a dry forest ecosystem from northeastern Brazil.13,14 A high diversity of secondary metabolites has been reported for geopropolis of Melipona, including phenolic acids, flavonoids, coumarins, hydrolyzable tannins, prenylated benzophenones, terpenes, steroids, saponins, fatty acids and acyl coumaroyl hexosides.15-22 Melipona subnitida Ducke ("jandaíra") is a stingless bee endemic to the semi-arid Brazilian northeastern. It produces geopropolis and was first registered in the state of Maranhão. Over the last 50 years it became abundant in all northeastern states, possibly due to increments of the commercial growth of hives of the species.4,13 M. subnitida is a relevant pollinator in the Caatinga and contributes to the local environmental conservation and fruit production.23 In extracts of geopropolis of M. subnitida collected in the state of Paraíba were detected acyl coumaroyl hexosides, kaempferol, quercetin, naringenin and aromadendrin derivatives.24 Other hives of the same species and locality yielded galloyl hexoses, coumaroyl-and cinnamoyl-galloyl hexosides, ellagic acid, aromadendrin derivatives and flavanones.18 Geopropolis of the same species from Mossoró (state of Rio Grande do Norte, northeastern Brazil) contains hydrolysable tannins, flavones, flavonols, xanthones and pentacyclic triterpenoids.17 Two other studies of geopropolis of the same species and locality revealed similarities and distinctions between their chemical profiles. One of the studies reported the presence of chalcones, flavones, flavonols, flavanonols, flavanones, phenylpropanoids and hydrolysable tannins.25 while the other reported chalcones, flavones, flavonols and flavanones.26 Plant resins are malleable when secreted but harden over time, so they can be shaped to build durable structures. A variety of environmental (e.g., temperature, light intensity, humidity, resource availability) and colony (e.g., population size, developmental stage) conditions influence resin foraging frequency at the colony level, and these factors exhibit different effects on stingless bees. Propolis or geopropolis from stingless bee are often incorporated resin into the nest environment in the form of deposit-resins, in structures such as the nest entrance and batumen.27 In a higher concentration of the wax than resin, the texture of the nest becomes harder. The waxy nest can furthermore be hardened by mud to make batumen. The inner nest is a convoluted maze-like structure, which are often designed to block the entrance of enemy intruders. The batumen, a wall-like structure that can contains resin is denominated as the external involucrum, that many stingless bee species build to separate the inner nest environment from the external world.27 Stingless bees also use resin to barricade the nest entrance at night, or when disturbed. When, the colony is under attack, the Melipona species use fresh resin to fasten it to the entrance to prevent invaders from breaching the nest. Over time, discarded resin balls accumulate near the internal entrances of the nest.27 Beside this, a single nest may contain multiple types of resin-rich materials and resin-based structures, each serving a different purpose.27 There is information about the chemical composition and variability of geopropolis of M. subnitida from the Caatinga. However, Meliponina species vary the architecture of their nests considerably, using different conformations for their internal and external structure. There is not data about the geopropolis collected in nest entrance and batumen. The present work aimed to compare the chemical composition and antioxidant activity of two sample of geopropolis collected from two distinct regions of the nest of a same hive in Mossoro, one of inside, and another outside of the nest.
EXPERIMENTAL Geopropolis collection Two samples of geopropolis of M. subnitida were collected in 2018, one of inside and another outside of the nest from beehive growing in a meliponary at the municipality of Mossoró, in the semiarid region of the state of Rio Grande do Norte (Brazil; 05°11'15" S and 37°20'39" W). The local climate is tropical, with mean annual average temperature 27 °C, relative humidity 50% and annual rain precipitation 500 mm. In genera Melipona, the outside of the nest are sturdy plates that surround and protect nests within a cavity, allowing the bees to adjust the cavity size to suit the needs of the colony. The top of the nest terminates with a perforated outside plate made of mud and resin.27 Thus, the outside of the nest refers to the entrance of the nests and edge of the lid. Bees often seal these parts with propolis. The internal region, in turn, corresponds to the part of the interior of the nest itself, where the bees deposit their young and food discs". For colonies managed in box hives, bees often seal cracks with a layer of propolis so thick that beekeepers must pry the lid from the hive body to access the nest. The method to stimulate propolis production was a system of wood box, sized 370 x 20 x 20 mm at the front and back of the hives between the hive and the nest, keeping a gap of 20 mm on both sides. The sample collected in internal region (inside of the nest) were collected by scraping the product accumulated in the crevices. The deposited propolis in the entrance of the nests (external region), generally discarded, were collected as the outside sample. Preparation of extracts The two samples of geopropolis (collected inside and outside of the nest) were powdered with liquid nitrogen, mortar, and pestle. Powdered material (1 g) of each sample was treated with 150 mL of ethanol in Soxhlet for 6 h. The extracts were filtered and kept overnight in dark flasks in freezer at -20 °C. The cold extract was filtered again to eliminate wax excess. HPLC/DAD and HPLC-DAD-ESI-MS/MS analysis Aliquots of 1 mL of the two geopropolis extracts were evaporated to dryness under nitrogen flow and the residue dissolved in HPLC grade methanol. The solutions were filtered through 0.45 μm polytetrafluoroethylene filters. Chromatographic conditions were optimized by HPLC-DAD by injecting aliquots of 10 μL into a HPLC HP 1260 chromatography (Agilent Technologies), using a Zorbax 5B-RP-18 column. Spectral UV data from all peaks were accumulated in the range 240 400 nm. At the same time the peaks were monitored with diode array detection at wavelengths of 360 and 270 nm. HPLC-PDA-ESI-MS/MS analyses were conducted with a DADSPD-M10AVP Shimadzu system, equipped with a SIL 20AC autoinjector (Shimadzu Corporation Kyoto, Japan), a CTO-20A column, SPD 20A photodiode array (PDA) detector and an Amazon Speed ETD (Bruker Daltonics), containing two LC-20AD Shimadzu pumps. The mass detector was a quadrupole ion trap equipped with atmospheric pressure ionization source through electrospray ionization interface, operated in the full scan MS/MS mode. All the operations, acquisition and data analysis were controlled by a CBM 20A software. The wavelength range of PDA detector was 210-500 nm. The mobile phases consisted of two solvents - eluent A (0.1% aq. formic acid) and eluent B (methanol). Constituents were separated using a reverse phase, C18, Zorbax - 5B - RP-18 (Hewlett Packard) column (4.6×250 mm, 5 μm) connected to a guard column. The elution starting with 20% B in A; and using a gradient to obtain after 10 min - 30% B in A, 20 min - 50% B in A; 30 min - 70% B in A; 40 min - 90% B in A; 45 min - 40% B in A and finally returned to the initial conditions (20% B) to re-equilibrate the column prior to another run. The flow rate was kept constant at 0.5 mL min-1 and the temperature of the column was maintained at 28 °C. The ionization conditions were adjusted as follows: electrospray ionization was performed using an ion source voltage of - 40 V and a capillary offset voltage of 4500 V. The full scan mass acquisition was performed using electrospray ionization in the negative and positive ionization modes by scanning from m/z 100 - 1200. Helium was used as the collision gas and nitrogen as the nebulizing gas. Nebulization was aided with a coaxial nitrogen sheath gas provided at a pressure of 27 psi. Desolvation was assisted using a counter current nitrogen flow set at a flux of 7.0 L min-1 and a capillary temperature of 325 °C. The data dependent MS/MS events were performed on the most intense ions detected in full scan MS. Tentative characterization of the constituents was based on UV/vis and MS spectra data in the negative and positive ionization modes and comparison with databases, such as HMDB (www.hmdb.ca), METLIN (http://metlin.scripps.edu), PubChem (https://pubchem.ncbi.nlm.nih.gov), MassBank database of North America (http://massbank.us/), as well as, previously reported studies containing UV/vis and MS data (Table 1).
Antioxidant assays DPPH method Radical scavenging capacity of the samples was evaluated by mixing 1.5 mL of 300 µM DPPH in ethanol. The ethanolic extracts of geopropolis collected inside and outside of the nest were prepared at concentrations of 15, 30, 45 and 60 μg/mL in Eppendorf reaction tubes (1.5mL), all in triplicate. Methanol solutions of quercetin at 2.5, 5.0, 7.5, 10.0, 15.0 and 30.0 μg/mL were used as reference. The reaction mixtures were vortexed, left in the dark at 25 °C for 30 min and the absorbances measured at 517 nm. Negative control solution was prepared using only DPPH and absolute ethanol. The absorbance was measured in a SynergyT; Neo2 Multi-Mode Microplate Reader. The results were expressed as efficient concentration (EC50). The ability to scavenge DPPH radical was calculated as % inhibition by the following equation: 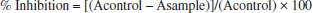 where Acontrol and Asampe are the absorbances of control and samples, respectively β-Carotene bleaching method The inhibition of oxidative loss of β-carotene in a β-carotene/linoleic acid emulsion was used to assess the antioxidant ability of the geopropolis extracts collected inside and outside of the nest. β-carotene (200 µg) dissolved in chloroform, 25 µL of linoleic acid and 200 µL of Tween 40 was solubilized in 50 mL of distilled water. The chloroform was evaporated and was added distilled water to form an emulsion. The emulsion was tested using 400 μL of the geopropolis solutions at concentrations of 40, 80 and 120 μg/mL in Eppendorf reaction tubes (1.5 mL), all in triplicate. The vials were then capped and placed at 50 °C in a water bath for 4 hours. The oxidation of β-carotene emulsion was monitored by measuring the absorbance at 470 nm, recorded immediately after the addition of the sample extracts. Ethanol (80%) was used as control. The absorbance was measured in a SynergyT; Neo2 Multi-Mode Microplate Reader and was determined every 20 min until 120 min at 470 nm. The inhibition activity to prevent β-carotene bleaching was calculated by the formula:  where Aosample, A120sample, Aocontrol and A120control are the absorbances of sample and control at t = 0 min and t = 120 min, respectively
RESULTS AND DISCUSSION HPLC-PDA-ESI-MS/MS analysis The geopropolis samples from M. subnitida collected from inside and outside of the nest exhibited similar chemical profiles, comprising 26 compounds (Table 1). The list includes gallic acid (2), galloyl hexoses (3-5, and 8), a flavone (23), a chalcone (25), caffeoyl-4-O-glucose (1), a 1-O-p-methoxybenzoyl hexose (6), flavanones (14, 24), phenylpropanoyl-O-galloyl hexoses (9, 10, 11, 12, 15, 16, 19, 22 and 26), phenylpropanoyl-O-benzoyl hexoses (18, 20, 21) and ellagitannins (7, 13 and 17). The tentative characterization of galloyl hexoses was based on their UV spectra, with absorption at 290 nm, and mass spectra with fragment ions [M - H - 170]- and [M - H - 152]-, indicating losses of gallic acid and galloyl radical, respectively.28,29 Ellagitannins were also detected and tentatively characterized. Their structural variability is high, due to different linkages of hexahydroxydiphenoyl (HHDP) groups with sugar (mainly glucose) and the possibility to form C-C and C-O linkages. Their spectra exhibit typical losses of moieties of galloyl (152 Da) and hexahydroxydiphenoyl (302 Da) radicals, as well as residues of gallic acid (170 Da), galloyl-glucose (332 Da), HHDP glucose (482 Da) and galloyl-HHDP-glucose (634 Da). Their fragmentation pattern in negative ionization mode exhibit fragment ion of m/z 301 (HHDP), m/z 331 (galloylglucose), m/z 481 (HHDP-glucose) and m/z 633 (galloyl-HHDP-glucose).30 Chalcones, flavones and flavonols exhibited characteristic UV/vis bands at 345-370 nm (band I) and 255-280 nm (band II).31 Flavanonols exhibited UV band at 280 290 nm,18,32 while phenylpropanyl hexoses at 310-320 nm.18,24 The two isomers of di-galloyl hexose (3) and (5), exhibited [M - H]- at m/z 483. Compound 3 showed loss of galloyl moiety (-152 Da) to produce base peak of m/z 331 and fragment ion of m/z 169 [M - H - galloyl - glucose]; compound 5 also showed base peak of m/z 331, however, produced an intense peak of m/z 271 (80%) [M - H - galloyl - H2O - CH2O]- or [monogalloyl glucopyranose - H - 60]- , due to the cross-ring fragmentation of the deprotonated ion.18,28,29 Tri-O-galloyl hexose (4) yielded deprotonated ion [M - H]- of m/z 635, and a fragment ion of m/z 465, corresponding to the loss of gallic acid residue [M - H - 170]-. In the positive ionization mode, compound 4 exhibited sodiated molecule [M + Na]+ of m/z 659 and fragment ions of m/z 489 (base peak) and m/z 319, corresponding to the loss of two gallic acid moieties, respectively.29 Penta-O-galloyl hexose (8) yielded deprotonated ion [M - H]- of m/z 939 and fragment ion of m/z 769 [M - H - 170]-, corresponding to the loss of gallic acid moiety.28-30 Pedunculagin I (7) exhibited [M - H]- of m/z 783, base peak of m/z 481, attributed to the loss of an HHDP group (302 Da) and fragment ion of m/z 301, corresponding to an HHDP group. 2-O-Galloylpunicalin (13) and tellimagrandin I pentose (17) exhibited [M - H]- of m/z 933 and m/z 917 and base peaks of m/z 763 and m/z 747 [M - H-170]-, respectively, indicating the loss of gallic acid moiety.29-30 The two isomers of coumaroyl-O-galloyl hexose (9) and (11) exhibited deprotonated ion [M - H]- of m/z 477. Compound 9 produced base peak at m/z 271 originated from the loss of coumaric acid (164 Da) and the successive loss of C2H2O. Compound 11 produced base peak at m/z 313, originated from the loss of coumaric acid (164 Da).28-30 The successive loss of C2H2O from the ion of m/ z 313, produced ions of m/z 271 (80%). Caffeoyl-4-O-glucose (1) exhibited sodiated [M + Na]+ and [M - H]- deprotonated molecules of m/z 365 and m/z 341 and base peak at m/z 203 and m/z 179 corresponding to the loss of hexose, respectively. The presence of coumaroyl groups in the phenylpropanoyl heterosides 10, 12, 18, 19 and 22 was indicated by loss of coumaric acid moieties (164 Da), while for phenylpropanoyl heterosides 15, 16, 21 and 26, the loss of fragments of 148 Da, indicate the presence of cinnamic acid in their structure.18,25,33 Compound 20 exhibited sodiated [M + Na]+ and [M + H]+ protonated ion of m/z 453 and m/z 430, respectively, and base peak of m/z 289 [M + H - 164]+, indicating the presence of coumaroyl group. Compound 20 was tentatively identified as 1-O-coumaroyl-6-O-benzoyl hexose and was not reported in this specie. 3,4',5,7-Tetrahydroxyflavan-4-one (14) exhibited deprotonated ion [M-H]- of m/z 287 and base peak at m/z 259 [M - H - CO]-, corresponding to the loss of carbonyl (C=O) group.18 The dihydroxy-methoxy-flavanone-O-coumaroyl-hexose (24) exhibited deprotonated ion of m/z 609, base peak of m/z 581 [M - H - CO]-, and fragment ion18 of m/z 591 [M - H - H2O]-. 4, 2',6'-Trihydroxy-4'-methoxy chalcone (25) exhibited deprotonated and protonated ion of m/z 285 and m/z 287 and base peak of m/z 165 and m/z 167, respectively, corresponding to the loss of 120 Da, indicating an OH group at position 4 of the chalcone ring B.9,10Compound 6 exhibited sodiated [M + Na]+ and [M + H]+ protonated ion of m/z 337 and m/z 315, respectively, and base peak of m/z 153 [M + H - 162]+, corresponding to the loss of hexose and was tentatively identified as 1-O-p-methoxybenzoyl hexose.34 The proposed structure based on MS data for some of constituents detected in this sample, 1,6-digalloyl hexose (3), 1-O-p-methoxybenzoyl hexose (6), 3,4',5,7-tetrahydroxyflavan-4-one (14), 1-O-coumaroyl-6-O-benzoyl hexose (20), 1-O-coumaroyl-6-O-cinnamoyl-2-O-galloyl-hexose (22) and 4,2',6'-trihydroxy-4'-methoxy chalcone (25) are shown in Figure 1.
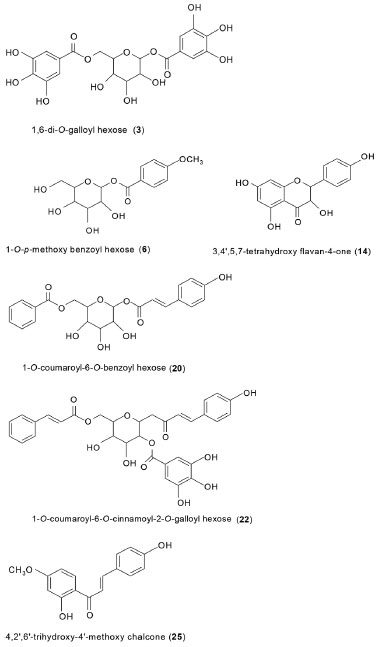 Figure 1. Proposed structures for constituents 3, 6, 14, 20, 22 and 25 detected in geopropolis from Melipona subnitida
Galloyl hexoses, phenylpropanoyl-O-galloyl hexoses and flavanones were detected in these samples of geopropolis from M. subnitida collected from inside and outside of hive from apiaries in the city of Mossoró, Rio Grande do Norte. These phenolic compounds were also found in samples of geopropolis from M. subnitida collected from apiaries at Paraíba State.18,24 and other apiaries in the city of Mossoró, Rio Grande do Norte.17,25,26 1,6-Di-O-(E)-coumaroyl-2-O-galloyl-β-D- glucopyranoside and 1-O-(E)-coumaroyl-6-O-(E)-cinnamoyl-2-O- galloyl-β-D-glucopyranoside were isolated from Melipona subnitida geopropolis.18,24 The identification of biologically active substances detected from propolis and its respective resin source and the determination of the compounds responsible for each biological activity will facilitate the development of standardized preparations, thus ensuring their higher quality and efficacy.35 The most extensive research investigating the biological activities of propolis or geopropolis from stingless bees is originated from Brazil.18,25,33,36,37 These researches showed that the antimicrobial, anti-inflammatory, and antioxidant activities of Brazilian geopropolis were similar that Asian and Australian stingless bee propolis.7 As previously mentioned, compounds similar with compounds listed in Table 1, as for example, galloyl hexoses (3-5) and cinnamoyl/coumaroyl hexoses (9-12, 15, 16 and 22) were detected in geopropolis of Melipona orbignyi33 and Melipona quadrifasciata anthidioides,38 both collected in the state of Mato Grosso do Sul, Central-West region of Brazil and Melipona subnitida collected in the state of Paraíba.18,24 In geopropolis from M. subnitida collected from different beehives at the municipality of Mossoró, state of Rio Grande do Norte, Brazil were found galloyl hexoses, hydrolysable tannins and acyl-(cinnamoyl/coumaroyl)-hexosides.17,25 However, the same classes of compounds were not found in another samples derived from the same locality.26 Stingless bees demonstrate clear preferences for some resin-producing plants, and neglect others and may target certain resin source based on the potency of its antimicrobial or repellent properties. Beside this, different resin sources may be used by distinct species of Melipona to produce propolis.18,25,27,33,38 In this study was observed that M. subnitida exhibited a specific foraging behavior, even in an environment with rich diversity as in the Northeastern Brazil semiarid region. The present study detected compounds not reported in previous studies of geopropolis from Melipona, such as compounds 6-8, 13, 17, 18, 20 and 21, which are reported for the first time as propolis constituents. An interesting aspect of the composition of propolis of M. subnitida refers to hydrolyzable tannins (compounds 7, 13, 17). Tannins are secondary metabolites common and often abundant in plants, but not in propolis. Honeybees avoid plant sources containing toxic (e.g. alkaloids, cyanogens) or unpalatable (e.g. saponins, tannins) chemicals.39,40 Tannins are not abundant in propolis of Apis mellifera, although proanthocyanidins (condensed tannins) have been detected in Brazilian green propolis.41 Hydrolyzable tannins are formed by the condensation of several residues of gallic and/or ellagic acid (composed by two molecules of gallic acid) with glucose. A marked characteristic of chemical profile of the geopropolis samples analyzed in the present work is the high abundance of constituents containing galloyl residues in their structure (Table 1). Hydrolyzable tannins seem to be common in geopropolis of Melipona species. They have been reported in geopropolis of M. fasciculata15 and M. subnitida.25 Contrary to proanthocyanidins (condensed tannins), which are virtually universal in angiosperms, hydrolyzable tannins, have restricted distribution. Ellagic acid is absent in monocotyledons and basal angiosperms and are common in wooden taxa of the core eudicots.42 Examples of plant orders with many species containing hydrolyzable tannins (gallo- and ellagitannins) are Malpighiales and Myrtales. Ellagitannins are formed through intramolecular coupling, resulting in C-C and C-O-C linkages between galloyl residues of glucogalloyls.29 Antioxidant activity The EC50 values of antioxidant activity of the two samples of geopropolis are shown in Table 2. The geopropolis extract collected from inside of the nest exhibited considerable higher activity, in comparison with the extract of the outside, irrespective of the method used for evaluation. Using the DPPH method, the EC50 of the geopropolis from inside of the nest was approximately eight times higher (17.2 ± 0.4) than the corresponding value of the outside nest (147.4 ± 0.5). A considerable higher antioxidant activity of the geopropolis from inside of the nest also prevailed in the analysis by the method of the β-carotene/linoleic acid (Table 2).
Propolis possesses high antioxidant activity determined by its phenolic compounds. Polyphenol derivatives common in propolis have been shown to exert a wealth of therapeutic benefits,43 many of them related with their antioxidant capacity. The antioxidant capacity of propolis is very important and can be considered as a clue factor for the elaboration of medicinal products, since oxidative stress causes cardiovascular, metabolic, neurodegenerative, and cancerous diseases.44 Phenolic compounds not only interrupt the chain reactions caused by free radicals but also can inhibit their formation. In addition, they have been shown to have antimicrobial and anti-inflammatory activity.45 Extracts of propolis Type 6, containing saturated hydrocarbons, methyl cinnamate, sitosterol cinnamate and ananixanthone did not show considerable antioxidant activity, which can be explained by the absence of phenolic compounds.46 Hydrolyzable tannins exhibited antioxidant activity, prevent the development of cancer and cardiovascular diseases.47 The propolis samples from inside the nest analyzed in the present work exhibited considerable antioxidant capacity (Table 2). The EC50 of samples of geopropolis of M. subnitida evaluated by other authors were of the same degree of magnitude seen in the present work: 27 mg mL-1 (DPPH method) and 35.7 mg mL-1 (β-carotene/linoleic acid method).17 However, as can be seen in Table 2 was observed a great difference in antioxidant activity between the samples from the inside and outside of the nest, although the two samples revealed similar phenolic profile. It seems out of doubt that a distinction between the two samples is the major concentration of antioxidant compounds in propolis from inside than the outside of the nest, as can be seen in Figure 2.
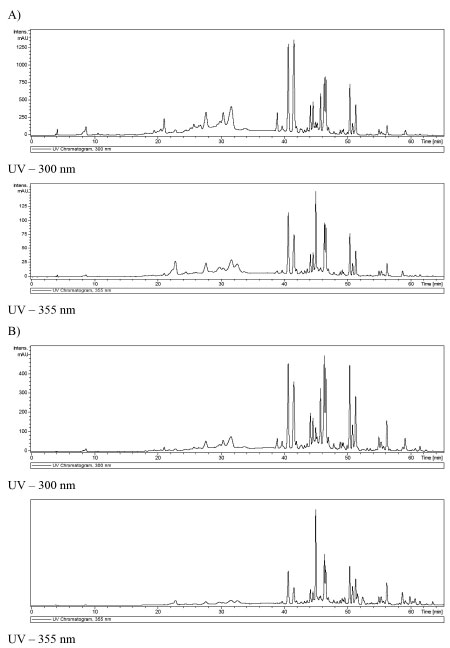 Figure 2. A) Chromatography profile of ethanolic extract from geopropolis of M. subnitida collected inside of the nest, at UV - 300 (above) and 355 nm (below); B) Chromatography profile of ethanolic extract from geopropolis of M. subnitida collected outside of the nest, at UV - 300 (above) and 355 nm (below)
Resin use remains fundamentally important for stingless bee colony function and the nest may contain multiple types of resin-rich materials serving a different purpose. Thus, the differences observed in antioxidant activity and chemical profile can be attributed to the diverse materials used in each part of nest construction (e.g., clay, resin, wax, and soil).48 Soil contributes to the volume and mass, but not to the antioxidant activity of propolis. Seasonal changes in resin collection could be related to many variables, such as resource availability, fluctuating pathogen pressure, and colony developmental stage. However, differences in resin use can occur even when many major variables (e.g., species, location, and hive structure) remain constant. In some Melipona species was observed that resin foraging activity fluctuates seasonally, but for other species (e.g., Melipona fasciculata), resin foraging is constant throughout the year.27,49 It is important mention that some abiotic factors exhibit strong effects on the bees' activity, notably temperature, atmospheric pressure, and wind speed.49 The batumen is a hard outer layer, that surrounds and protects exposed or partially exposed nests, protecting nests within a cavity, allowing the bees to adjust the cavity size to suit the needs of the colony (e.g., genera Melipona). Variation in nest architecture, including the resinous batumen surrounding the nest, were attributed to nest age, bee genetics, and micro-environment (e.g., predators, parasites, symbionts, rain, wind, and sun).50 Stingless bees use resin to build brood comb, storage pots for honey and pollen, and various protective structures within the nest.27 Thus, can be supposed that the outside of the nest contain more soil than resin. Possibly the need to seal crevices, cracks, holes, and other openings on the periphery of nest increases the pressure for higher quantity of sealing material (propolis). For stingless bees producing geopropolis, the means to satisfy this need is to increase the amount of soil added to propolis. If high volume of propolis is not required in the inner parts of the nest, it is advantageous to produce geopropolis with low proportion of soil, because the product has comparatively higher biological activity. The waxy nest can furthermore be hardened by mud to make batumen. Thus, the likely factor responsible for the distinct concentration of active compounds comparing the geopropolis from the inside and the outside of nest is the proportion of soil material. Of course, this hypothesis needs to be tested by evaluation of the relative proportion of soil in propolis from the external and internal regions of the nest, which can be done by measuring the content of ashes in one and another sample.2 Understanding the importance of resin use in stingless bee colony function is urgent as changes in beekeeping and land use practices occur, that can potentially diminish the stingless bees' ability to incorporate resin into their nest environment.
CONCLUSIONS Propolis of stingless bees is a source of biologically active constituents rarely or not known in honeybee propolis. Hydrolyzable tannins and complex galloyl-phenylpropanyl-glycosides are examples of constituents common in propolis of meliponines and rare in propolis of honeybees. The overall composition of geopropolis may render a product with relatively high antioxidant capacity. On the other hand, the high variability of chemical profile of geopropolis of Melipona species is a factor requiring strategies aiming the standardization of the product. In addition to constituents derived from plants, biologically inactive constituents (possibly soil material) represent another source of constituents contributing to increase the chemical variability of the product. The results demonstrated that geopropolis from M. subnitida collected inside of nest is rich in bioactive phenolic compounds with antioxidant activity. On the contrary, the sample collected outside of the nest, with low content of bioactive compounds, exhibited little antioxidant activity. Further studies are needed aiming to establish parameters of quality of geopropolis, without which no conditions will be met for reliable food formulation and pharmaceutical use of the product. A deeper understanding of the importance of resin for stingless bee colony function, that depend on diverse resin sources to carry out a variety of functions, could lend support to the conservation of resources that are overlooked. SUPPLEMENTARY MATERIAL Chromatography profile of ethanolic extract from geopropolis of M. subnitida collected from inside the nest and the mass spectra of constituents 1-26, in negative and positive ionization mode were provided in Figures 1S-34S.
ACKNOWLEDGMENTS This work was supported by the CNPq Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
REFERENCES 1. Felix, J. A.; Freitas, B. M.; An. Acad. Bras. Cienc. 2021, 93, e20190767. [Crossref] 2. Pereira, L. R. L.; Salatino, M. L. F.; Salatino, A.; MOJ Food Process. Technol. 2020, 8, 1. [Crossref] 3. Vale, K. A. G.; Rodrigues, A. K. de S.; Carvalho, K. S. S.; Moura, S. G.; Souza, D. C.; Britto, F. B.; Bee World 2021, 98, 1. [Crossref] 4. Pedro, S. R. M.; Sociobiology 2014, 61, 348. [Crossref] 5. Lavinas, F. C.; Macedo, E. H. B. C.; Sá, G. B. L.; Amaral, A. C. F.; Silva, J. R. A.; Vieira, B. A.; Domingos, T. F. S.; Vermelho, A. B.; Carneiro, C. S.; Rodrigues, I. A.; Braz. J. Pharmacogn. 2019, 29, 389. [Crossref] 6. Slaa, E. J.; Sánchez, C. L. A.; Malagodi-Braga, K. S.; Hofstede, F. E.; Apidologie 2006, 293. [Crossref] 7. Zulhendri, F.; Perera, C. O.; Chandrasekaran, K.; Ghosh, A.; Tandean, S.; Abdulah, R.; Herman H.; Lesmana, R.; J. Funct. Foods 2022, 88, 104902. 8. Simone-Finstrom, M.; Borba, R. S.; Wilson, M.; Spivak, M.; Insects 2017, 8, 46. [Crossref] 9. Ferreira, J. M.; Fernandes-Silva, C. C.; Salatino, A.; Message, D.; Negri, G.; eCAM 2017, Article ID 4024721. [Crossref]. 10. Ferreira, J. M.; Fernandes-Silva, C. C.; Salatino, A.; Negri, G.; Message, D.; J. Sci. Food Agric. 2017, 97, 3552. [Crossref] 11. Popova, M.; Trusheva, B.; Bankova, V.; Phytomedicine 2019, 86, 153098. [Crossref] 12. Salatino, A.; Pereira, L. R. L.; Salatino, M. L. F.; MOJ Food Process. Technol. 2019, 7, 27. 13. da Silva, G. R.; de Mello Pereira, F.; de Almeida Souza, B.; do Rego Lopes, M. T.; Campelo, J. E. G.; Mendonça Diniz, F. M. Arq. Inst. Biol. 2014, 81, 299. [Crossref] 14. Souza, U. P.; Cabrera, S. P.; da Silva, T. M. G.; da Silva, E. M. S.; Camara, C. A.; Silva, T. M. S.; Braz. J. Pharmacogn. 2019, 29, 278. [Crossref] 15. Dutra, R. P.; Abreu, B. V. B.; Cunha, M. S.; Batista, M. C. A.; Torres, L. M. B.; Nascimento; F. R. F.; Ribeiro, M. N. S.; Guerra, R. N. M.; J. Agric. Food Chem. 2014, 62, 2549. [Crossref] 16. Silva, E. C. C.; Muniz, M. P.; Nunomura, R. C. S.; Nunomura, S. M.; Zilse, G. A. C.; Quim. Nova 2013, 36, 628. [Crossref] 17. de Sousa, D. M. N.; Olinda, R. G.; Martins, C. G.; Abrantes, M. R.; Coelho, W. A. C.; Alves da Silva, J. B.; de Morais, S. M.; Batista, J. S.; Acta Veterinaria Brasilica 2015, 9, 134. 18. de Souza, S. A.; da Silva, T. M. G.; da Silva, S. E. M.; Camara, C. A.; Silva, T. M. S.; Phytochem. Anal. 2018; 29, 549. [Crossref] 19. Araújo, M. J. A. M.; Búfalo, M. C.; Conti, B. J.; Fernandes Jr, A.; Trusheva, B.; Bankova, V.; Sforcin, J. M.; J. Mol. Pathophysiol. 2015, 4, 12. 20. Batista, M. C. A.; Abreu, B. V. B.; Dutra, R. P.; Cunha, M. S.; do Amaral, F. M. M.; Torres, L. M. B.; Ribeiro, M. N. S.; Acta Amazonica 2016, 46, 315. [Crossref] 21. Cunha, M. G.; Rosalen, P. L.; Franchin, M.; de Alencar, S. M.; Ikegaki, M.; Ransom, T.; Beutler, J. A. Planta Med. 2015, 82, 190. [Crossref] 22. Cunha, M. G.; Franchin, M.; Paula-Eduardo, L. F.; Freires, I. A.; Beutler, J. A.; de Alencar, S. M.; Ikegaki, M.; Tabchoury, C. O. M.; Cunha, T. M.; Rosalen, P. L.; J. Funct. Food 2016, 26, 27. [Crossref] 23. Lima, C. B., Nunes, L. A., Ribeiro, M. F., Carvalho, C. A.; Sociobiology 2014, 61, 478. [Crossref] 24. de Souza, A. S.; Camara, C. A.; da Silva, S. E. M.; Silva, T. M. S.; eCAM 2013, Article ID 801383. [Crossref] 25. de Sousa-Fontoura, D. M. N.; Olinda, R. G.; Viana, G. A.; Costa, K. M. F. M.; Batista, J. S.; Serrano, M. O. T.; Silva, O. M. D.; Camara, C. A.; Silva, T. M. S.; Braz. J. Pharmacogn. 2020, 30, 367. [Crossref] 26. Batista, J. S.; Salatino, A.; Negri, G.; Jara, C. E. P.; Paiva, K. A. R.; Santos, W. L. A.; Teófilo, T. S.; Félix, N. S.; Silva, F. H. A.; Rodrigues, V. H. V.; Res. Soc. Dev. 2021, 10, e11210212305. [Crossref]. 27. Shanahan, M.; Spivak, M.; Insects 2021, 12, 719. [Crossref] 28. Gomes, R. B. A.; de Souza, E. S.; Barraqui, N. S. G.; Tosta, C. L.; Nunes, A. P. F.; Schuenck, R. P.; Ruas, F. G.; Ventura, J. A.; Filgueiras, P. R.; Kuster, R. M.; Ind. Crops Prod. 2020, 143, 111430. [Crossref] 29. Chang, Z.; Zhang, Q.; Liang, W.; Zhou, K.; Jian, P.; She, G.; Zhang, L.; eCAM 2019, Article ID 8623909. [Crossref] 30. Lachowicz, S.; Oszmianski, J.; Rapak, A.; Ochmian, I.; Pharmaceuticals 2020, 13, 191. [Crossref] 31. Markham, K. R.; Techniques of Flavonoid Identification, Academic Press: London, 1982, 113 p. 32. Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Baruca, A.; Peeters, K.; Miklavčič Visnjevec, A.; Jenko Praznikar, Z.; Metabolites 2020, 10, 403. [Crossref] 33. Santos, H. F. D.; Campos, J. F.; Santos, C. M. D.; Balestieri, J. B. P.; Silva, D. B.; Carollo, C. A.; Souza, K. P.; Estevinho, L. M.; dos Santos, E. L.; Int. J. Mol. Sci. 2017, 18, 953. [Crossref] 34. Brito, A.; Ramirez, J. E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M. J.; Molecules 2014, 19, 17400. [Crossref] 35. Wieczorek, P. P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I.; Molecules 2022, 27, 1600. [Crossref] 36. da Cunha, M. G.; de Cássia Orlandi Sardi, J.; Freires, I. A.; Franchin, M.; Rosalen, P. L.; Microb. Pathog. 2020, 139, 103855. [Crossref] 37. Da Silva, P. R.; Da Silva, T. M. G.; Camara, C. A.; Da Silva, E. M. S.; Dos Santos, F. D. R.; Silva T. M. S.; Rev. Caatinga 2020, 33, 246. [Crossref] 38. dos Santos, C. M.; Campos, J. F.; dos Santos, H. F. D.; Balestieri, J. B. P.; Silva, D. B.; Souza, K. P.; Carollo, C. A.; Estevinho, L. M.; dos Santos, E. L.; Oxid. Med. Cell. Longevity 2017, Article ID 8320804. [Crossref] 39. Detzel, A.; Wink, M.; Chemoecology 1993, 4, 8. [Crossref] 40. Salatino, A.; Salatino, M. L. F.; Negri, G.; Apidologie 2021, 52, 1075. [Crossref] 41. Mayworm, M. A. S.; Lima, C. A.; Tomba, A. C. B.; Fernandes-Silva, C. C.; Salatino, M. L. F.; Salatino, A.; eCAM 2014, Article ID 613647. [Crossref] 42. Soltis, D.; Soltis, P.; Endress, P.; Chase, M.; Manchester, S.; Judd, W.; Majure, L.; Mavrodiev, E.; Phylogeny and Evolution of the Angiosperms, The University of Chicago Press: Chicago, 2018, 579 p. 43. Chen, Z.; Luo, W.; Suna, D.; Bi, X.; Zeng, X.; Xiao, G.; Xu, A.; Chen, W.; Jiang, J.; Li, X.; Cao, L.; Phytomed. Plus 2021, 1, 100006. [Crossref] 44. Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otreba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A.; Plants 2022, 11, 1203. [Crossref] 45. Al-Hatamleh, M. A. I.; Boer, J. C.; Wilson, K. L.; Plebanski, M.; Mohamud, R.; Mustafa, M. Z. Biomolecules 2020, 10, 923. [Crossref] 46. dos Santos, D. C.; David, J. M.; David, J. P.; Quim. Nova 2017, 40, 171. [Crossref] 47. Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M. A.; Foods 2021, 10, 137. [Crossref] 48. de Sousa, L. P.; PLoS One 2021, 16, e0252933. 49. Oliveira, R. C.; Contrera, F. A. L.; Arruda, H.; Jaffé, R.; Costa, L.; Pessin, G.; Venturieri, G. C.; de Souza, P.; Imperatriz-Fonseca, V. L.; Frontiers in Ecology and Evolution 2021, 9, 708178. [Crossref] 50. Roubik, D. W.; Apidologie 2006, 37, 124. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access