Educação
| Identifying conceptions of the covalent bond: an analysis from a systematic review |
|
Ricardo S. Baltieri; Marco A. Cebim; Amadeu M. Bego*
Departamento de Química Analítica, Físico-Química e Inorgânica, Instituto de Química, Universidade Estadual Paulista, 14800-900 Araraquara - SP, Brasil Recebido em: 12/04/2022 *e-mail: amadeu.bego@unesp.br The covalent bond is a classical topic in science education because it is fundamental to a considerable number of other concepts and by its ability to interpret several chemical phenomena. However, the process of learning this concept presents some difficulties discussed here. This article brings together the conceptions related to the covalent bond, identified, and reported from other studies, to find similarities and to organize what is known about the topic in epistemological themes. In this paper, it was conducted a systematic review using 3 databases, reaching more than 200 reports, and classified 253 sentences of misconceptions in 7 epistemological themes. The main contribution of this article is the classification of misconceptions into themes, which enables teachers and researchers to easily identify a topic considering its complexity. This review shows different misconceptions over the same topic, how misinterpretations can appear over all the models and theories related to the covalent bond, heterogeneity of school levels with the same misconception and mixing of theoretical foundations. Finally, we claim that the knowledge of this wide sight can be especially important to further lesson/unity planning, studies, and understanding of how to make a better transition between one topic to another. INTRODUCTION The second half of the 20th century was extensively marked with developments in science education.1,2 Since then, considering what the students already know regarding a scientific matter became an important step to be considered in teaching and learning, related to the conceptual change approach.3 Particularly, in education research, some of the most common terms to refer to this process, over the decades, were preconceptions, misconceptions, or alternative conceptions, as presented by Özmen.4 They describe the formation of conceptions through the student's ideas, when they attempt to connect the information from context, culture, everyday language, etc. (preconception) to the systematized knowledge from formal spaces of learning and eventually reproduces an error according to the scientific community.5 Most of the studies concerning misconceptions took charge of its identification in the most varied topics related to, for example, chemistry, physics, mathematics, and biology.6 However, as highlighted by Vosniadou,7 in the recent decades, some investigations regarding the topic have demonstrated the need to go beyond the identification in a specific context and to understand the whole process through the subject's school history. Even though, as we discuss in this article, some research has been conducted to identify misconceptions in the most varied contexts, subjects, complexity order, and using different methods. In this article, we propose a different way to organize all these misconceptions identified in the literature by classifying them in epistemological themes. This classification allows teachers and researchers, for example, to recognize different levels of complexity in misconceptions and relate them to each topic in covalent bonds, as we hope to cover all subjects about it using epistemological themes.8 The importance of covalent bonding as a chemical concept emerged from articles that discuss advanced topics in the context of higher education,9,10 how the same misconceptions appear in different subjects, as the same misconceptions appear in different disciplines and because of the significant importance that the topic has within chemical science, as in controlled reactions (organic chemistry), energy analysis (physical-chemistry) or the very nature of chemical bonds (inorganic chemistry).11 The latter is strongly related to the authors' area and related research. Besides, an earlier investigation showed us how this topic was taught, in general, based on a crystallized tradition of following textbooks12,13 with a linear and summative approach. The details of the systematic review are presented in the Methodology section, as well as the implications in our investigation, and how we intended to discuss the data. Next, as our main goal on this article, we provide in Results and discussion section the conceptions of covalent bond (found in literature by the systematic review) organized according to epistemological themes of covalent bond scientific development presented in another article during the proposition of a conceptual profile.8 Finally, we conclude with the most important findings, limitations, and suggestions for further research.
METHODOLOGY The systematic review is a specific method of data collection that includes well-defined steps and seeks to minimize the influence of the researcher in the searches, since usually only those in accordance with the researcher's bias tend to be presented.14 Moreover, the reproducibility may be guaranteed and used as a form of validation of the method adopted, because it has parameters defined earlier and a question to be answered.15 This methodology uses some steps to achieve the publications of interest in order to exclude those in disagreement with the purpose of the research in development. The sequence of followed steps in this article is based on suggestions found in the literature:16,17 1) Definition of the research question: the research needs to be well defined regarding its purpose; therefore, all the steps can be justified in its contribution for the findings and showing precisely what has been done. In this article, we looked for the misconceptions identified by covalent bond and all its variations and closely related topics, such as chemical bond and ionic bond. To be included in the analysis for this article, the study needed to show explicitly original results, not a literature review. Thus, our question is: "What misconceptions on covalent bond, and closely related topics, have been identified and reported in the literature?" 2) Definition of the parameters (keywords, Boolean operators, and period): the same word may be related to several themes and bring much more results than necessary, leading to a waste of time. Based on an investigation with the databases used, we noticed that the terms "misconceptions" and "bond" should be used because, even though some research use "alternative conceptions," for example, the reports were related to other terms. Moreover, "bond" was more extensive than simply "covalent bond," regardless of our specific topic, it allowed us to visit some conceptions of the edge or in superposition with close themes. In addition, we used the Boolean operator "AND" to restrict the reports that address both words. Concerning the period, we searched for the time available for the databases, that is, 1900 to October 2020, but the oldest report is from 1989. 3) Organization of data: the found reports may be extremely numerous; it is essential to organize them to easily identify what we are looking for. In this article, we conducted the search in three databases, Web of Science (WoS); Education Resources Information Center (ERIC); and Scientific Electronic Library Online (SciELO) and organized all findings in a spreadsheet with title, year, journal name, and abstract. The databases were selected due to the facility to organize the found studies, wide scope of journals (WoS and SciELO) and specificity of the education area (ERIC). 4) Reading titles and abstracts: this is the first step of looking closely to the found reports and selecting them. It is necessary to evaluate, first by title and abstract, whether the report is valid for a deeper analysis or if the title and abstract are not enough to judge. It becomes clearer why the earlier steps are crucial because if we are too open or too closed for the choice of word, the quantity of report is impractical to analyze or just not enough. Moreover, it is difficult to accomplish reading if they are not well organized. In this article, considering the selected keywords are broad in meaning-i.e., the research goes beyond the necessary for answering the question-the number of findings (255) were evaluated as feasible by the authors. 5) Reading the entire article: this step is accomplished first by a floating reading -i.e., a fast reading on main topics-of the paper because the next sections to the title and abstract are enough to show us if the report comprises, or not, our purposes defined in the first step. If the floating reading was not enough, most of the cases for us, it was necessary a full reading followed by the registration of main information from the text. Steps 4 and 5 are better conducted if followed by more than one person. 6) Extracting and organizing information: based on the definitions from the first step, it is necessary to organize the information extracted from the reports, intending to answer our question. Some researchers claim to reconsider these steps several times in order to conduct the systematic review. In this article, we conducted the organization of information by classifying sentences according to the epistemological themes proposed. Next, we present our results organized in major groups called epistemological themes. These categories were proposed by an investigation8 concerning the historical development of the covalent bond concept in science history. They contemplate a set of contributions from theories or models, chronologically close to each other and are detailed along with the discussions on the conceptions. The purpose of using these themes was, firstly, to verify if the misconceptions reported in literature covers all topics closely related to covalent bond and, secondly, to organize them according to different levels of complexity for a better visualization of the research.
RESULTS AND DISCUSSION The search for the articles was conducted in WoS, and then in ERIC and SciELO databases, using the terms according to the Methodology section, from the period of 1989 to October 2020. The sequence of evaluation and selection of reports is described below in Figure 1, it shows in detail the steps 4 and 5 of systematic review. For the article to be chosen, it needed to present explicitly a sentence with some misconception concerning covalent bonds. We analyzed all 31 articles and extracted the misconceptions from them, according to step 6, finding 253 sentences classified as misconceptions. Most sentences taken from these articles were very similar or represented the same idea. All sentences, in its original form, can be found in Supplementary Material. For the review classification into epistemological themes, all sentences were rewritten in more general sentences, so we could work with a smaller number of sentences. We separated them into topics (energy, polarity, orbitals, hybridization etc.), then we looked for similar words in the sentences or the same idea written in different ways, in order to rewrite new sentences. These "new sentences" are not completely new, they can be, for example, a very similar sentence (from the articles) modified to exemplify those misconceptions.
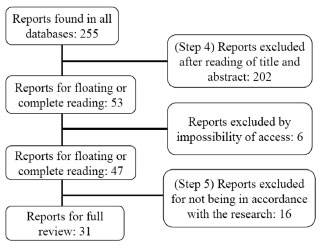 Figure 1. Steps 4 and 5 of the systematic review conducted in this article
These new sentences were classified into seven epistemological themes proposed based on secondary sources of the historical development of the concept.8 Each theme represents one or more theories or models related to the covalent bond across the scientific development of the covalent bond concept. The periods considered were those associated with the ideas of affinity in the XVIII century,18 chemical ideas from Berzelius to Lewis,19,20 and finally the period associated with the valence bond, molecular orbitals, and density functional theory.21-23 Next, we present the classification of sentences in themes. The conceptions of the covalent bond in the literature The Animism epistemological theme represents theories about affinity, from the XVIII century, and indicates an attribution of human characteristics. All misconceptions identified are shown in Table 1, including the word "want," which is related to atoms desire to establish a bond, as the theoretical model could present feelings just like human beings. Nicoll,24 for example, reported answers from an interview where the senior level students associated the bond formation as a way of atoms being happy for such achievement. Another highlighted example is Kind's25 study showing a value judgment of bonds, where the covalent is the good one because it "shares an electron" and ionic is the bad one because it "steals an electron," reported from pre-service teachers of chemistry, physics, and biology. All subsequent articles, identified as Animism, followed in the same direction presenting some human characteristics attributed to atoms or molecules, from secondary students to prospective teachers, such as in Kabapınar,26 Luxford and Bretz,27 Özgür Özcan and Temel,28 and Jenkins and Shoopman.29 Instead of attributing feelings to atoms, specialized literature points out that students should relate these phenomena to calculable and scientific parameters as energy variations, for instance.

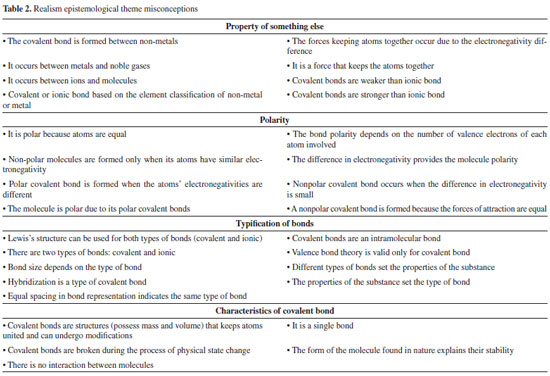
Although in that century (of affinity concept) there was not a concept as "octet," the theme refers to the bond, specifically, associated with a human feeling. According to some authors,24,25 this anthropomorphization can be the result from the first contacts with the concept of bond between atoms, when some teachers, or even textbooks, use it as an analogy for introduction to the topic. The reported problems, foreseen in misconception literature, are the difficulties of detaching from this conceptualization. Instead of understanding it as a phenomenon with its own bases (math, physics), students can extrapolate it to other situations, as atoms being "kind" because they share an electron to form a covalent bond and ionic bond being "mean" because it steals an electron.25 In the sequence, the epistemological theme named Realism was differentiated from Animism because the conceptions related to it were not linked to the idea of anthropomorphization. Besides, its main characteristic is the understanding of a bond as something secondary, a consequence of another concept. Commonly, these conceptions showed an indiscriminate use of words, without specifying how it fits in that context, such as "attraction" or "stability,"24,25 as can be seen in Table 2. The table is divided into subtopics for better comprehension and analysis. In the first group of misconceptions, all sentences refer to the covalent bond as a property controlled by atoms, ions, molecules, etc. as if all these other concepts controlled what kind of bond occurs, prior to the phenomena. Even if some relations may be usual, students and teachers should be aware of the difference between the examples of compounds with covalent bonds and the definition of the concept itself. Besides, some relations in these misconceptions' sentences are not usual, as the bond occurring between metals and noble gases, what may indicate that students simply memorize some concepts and try to establish a connection. In this latter case, the misconception is not for an example of covalent bond compound. We observed a classificatory use of words in reports from secondary students to prospective teachers, such as "metals" or "nonmetals," for explaining the bond as a property of atoms, so the covalent bond is the one formed, for instance, between nonmetal elements.28,30-32
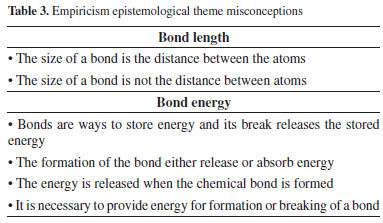
In "polarity," misconceptions continues to present the bonds as a property of another concept, but now with the polar or nonpolar behavior also as if it was defined by atoms or molecules itself. For instance, it excludes any contribution of environment or changes in the polarity of the same compound. Another common conception is the explanation of a covalent bond simply as a type of bond stronger or weaker than other bonds with no more details.33-35 All these groups of conceptions were proposed by us according to those ones shown in Table 2. They all share an absence of good explanation over bonds or covalent bond, they imply a second concept with no context at all. In addition, some ideas seem too general for a scientific concept, just like "force," that describes the bond as an abstract phenomenon, or "structure" using it as a solid that holds atoms together, including all changes at the macroscopic level as happening directly in covalent bonds. Some conceptualizations for the formation of bonds between atoms, especially the classificatory ones, can also be found in the theory of radicals and types, starting in the middle of XIX century.20,36 At that time, there was no concern in detailing or describing the bonds itself, only the classification of reactivity according to the atoms, by elements of periodic table. The result of epistemological analysis8 from that time (e.g., classification methods) and the development of the concept in the subject history shows an attempt to understand the bond as something to be classified in one or another group, covalent or ionic, metal or nonmetal, even though no parallel for this exact classification can be found in epistemological foundations. For example, it seems to have no scientific basement showing a direct connection between the periodic table labelling and the typification of bond present in the connectivity between atoms. These missing points of the identified misconceptions made us classified them as Realism because it shows the start of a conception network, but it is made with little or no foundation at all in chemical and physical sciences. The third epistemological theme, called Empiricism, is the group of misconceptions related to empirical data. For instance, the size of bonds or its energies, usually found in textbooks in table format. As we show in Table 3, some of the reported studies show the association made by secondary students and secondary science teachers, between the concept of covalent bonds and a value obtained experimentally.37,38 This concept is also common in several other articles that define the covalent bond as a range of the amount of energy or size. As expected, it can directly influence the teaching process and appears in different grades of teaching and learning.39,40 We also observed the misconception about how these tabulated data are obtained, how bond length is determinate, since students end up arbitrarily using the process of forming or breaking the covalent bond, mixing them with energy absorption or release. Both students and teachers need to be aware of how these data are obtained and what the tabulated number represents, as average numbers and the specific conditions.
The Essentialism theme was identified from some definitions of covalent bonds as exclusively composed of electrons in pairs between atoms, as a simplistic attribution of an essential characteristic: all bonds are covalent if present a pair of shared electrons, as can be seen in Erman's41 study. Besides, it is a widespread idea. From the seminal works of Lewis19,42 and Langmuir,43 it can hinder further analysis or more complex systems. For instance, Salah and Dumon's44 study shows the physical sciences higher students' conception of covalent bond as electrons shared in pairs poorly related to theories of atomic orbitals or even hybridization. Once more, both conceptions have no direct parallel in epistemological foundations. According to Table 4, other sentences include an association between the line's representation and evenly shared electrons, which is like the ones identified in Realism, but now resonating with the Lewis structure.45,46

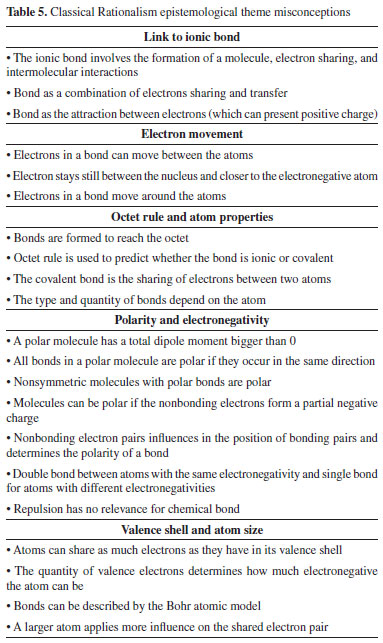
The conceptions classified as Classical Rationalism show close similarity with the previous theme because they were also influenced by Lewis and Langmuir's contributions but not limited to the covalent bond as electrons shared in pairs. In fact, the sentences reproduce the idea of a concept no longer isolated, but dependent of a closely related network of concepts.47-49 The reported conceptions encompass the covalent bond present in every system of atoms, as the only possible connection between them, instead of a connection occurring in a specific way. In the ionic bonds, for instance, as can be seen in Table 5, the covalence is a step to achieve the ionic compound, which can be related to the formation of a long-range chain, according to elementary school students.40,50,51 The process, according to elementary and undergraduate students of biology, include the formation of covalent bonds between sodium and chlorine, for instance, followed by intermolecular forces, and finally the whole progression would be called ionic.33,52 Generally, the sentences show closer connections between the conceptions and perform them more coherently. Also, we verified misconceptions concerning the movement of electrons by the molecule, the octet rule once more (as a property of each atom), the consequences of the covalent bond on the molecular polarity, and the misrelation with Bohr's atom model (of size and valence shell).46,53,54 The sentences related to electron movement show the difficulty of the students to interpret the behavior of electrons in a molecule, as these electrons could only be between the atoms or if they move in orbits, such as in Bohr's atomic model. Polarity related misconceptions show ideas attributing the behavior (of being or not polar) to the covalent bond as generic rules, and not as dependent of different properties and molecular environment. Some sentences show the bond as a property of atoms that controls it, or as a property of electrons to bind (besides equal charge repulsion) seeking to complete the octet.55-57
The first considerations on theories derived from quantum mechanics, as the valence bond theory, and its consequence on misconceptions were identified as Modern Rationalism. The sentences include concepts with more complex connections, related to a broader network of concepts, and to differentiate it from the previous epistemological theme.8 As in Table 6, some conceptions show a more detailed idea of covalent bond, using concepts of orbital and hybridization, for instance. The sentences related to orbitals present some interesting ideas for describing the concept, however they were assigned as misconceptions because the higher education students understand the space region of probability as a concrete part of the atom, the absence of abstraction represented in these concepts, or the formation of covalent bond from atomic orbitals as a description of event happening in time. Next, the sentences consider the attraction of shared electrons with the nuclei involved in the system for higher education students,44,53 or the repulsion presented by equal charges for secondary upper-level students,40 even though both still appear only as isolated conceptions happening in the molecule or any similar system. Other interesting conceptions show different ideas so far, by describing the concept of hybridization, covalent bond using orbital overlaps, mathematical instruments (e.g., probability), and representations of boxes where the electrons are supposed to fit in.10,44 Some of them, although more complex than previous conceptions, present the misuse of graphical representations as orbitals, or the quantum boxes, as concrete objects, just like some misconceptions identified in Realism, for example.
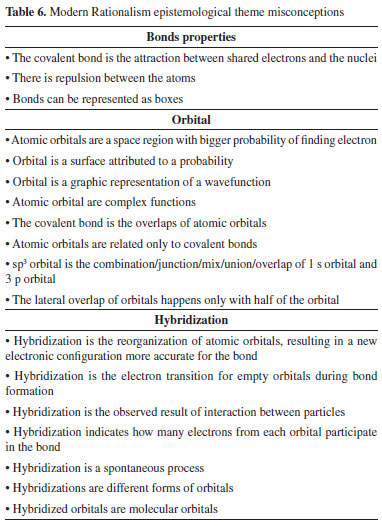
Finally, a great number of misconceptions from undergraduate students demonstrate the attempts of students to define the concept of hybridization as a process that can occur to molecules, as a mathematical method, or a subdivision of orbitals.10,58 The epistemological theme named Contemporary Rationalism was identified from theories also derived of quantum mechanics. Instead of localized orbitals overlap, it was considered the concept of a delocalized electron cloud over the molecule (or similar system) present in the molecular orbital and density functional theory. The first misconceptions are related to a different way of describing the bond from the formation of a new orbital, and no longer by overlaps in directional ways, as can be seen in Table 7. The sentences refer to the papers of Bouayad et al.10 and Stefani and Tsaparlis,9 both from undergraduate students, in which the authors identified a misinterpretation of the delocalized electron representations, or the combination of orbitals as something that happens like a concrete mixture of shapes, as mixing modeling clay.

Moreover, we verified conceptions concerning the electron cloud as if it were a hollow solid shell, where the electrons, still interpreted as particles, are running around the structure, and eventually could be found, simply frozen,10 instead of a region in space provided by probability density function. Even though the student used terms such as "electronic cloud," its use is associated with the particle-not the wave-particle dualism-nature, showing again a mixture of conceptions. Figure 2 shows a scheme with all epistemological themes and examples of misconceptions for each one from Results and discussion section for a broader view of our findings.
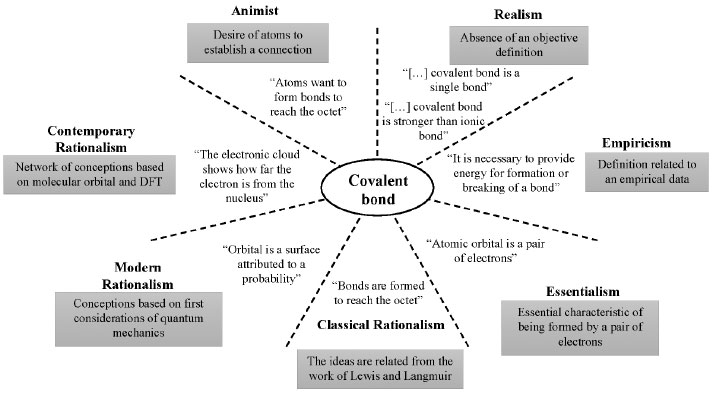 Figure 2. Scheme of epistemological themes and main conceptions regarding the covalent bond
Strengths, limitations, and future research In this paper, we presented how the concept of covalent bond has been identified and reported in the misconception literature. By gathering all these studies, we demonstrated that misconceptions concerning the covalent bond appear in different scholar levels, even for undergraduate students, and pre-service teachers, as well as these misconceptions can be classified according to different epistemological themes. Moreover, we also corroborated the literature on misconceptions showing how much a specific belief can be overly linked to a certain idea, persisting, and overlapping meanings in order to maintain their primary understanding. As it happened to Realism, for instance, when other misconceptions attributed a concrete existence to orbitals or electronic cloud. Or as the Essentialism main idea, attributing the evenly shared electrons for different theories, like if it is the only possibility. Simultaneously, this intersection of epistemological themes - and the misconceptions - was not the focus of our analysis, that is, we did not consider here that a misconception sentence could be related, or be represented, to more than one epistemological theme, although we know that may be possible. Moreover, it may not consider all possible studies on the topic by using only "misconception," even though the parameters of the research were chosen to contemplate papers looking for title, abstract, and keywords. In other words, even if the paper used another termination, "misconception" could be present in any topic. It is worth mentioning that this research depended on the findings reported in the literature, assuming phrases and references from other studies for what was considered by the authors as a misconception. It was not our purpose to analyze whether any idea could be plausible in any historical or teaching context, for example. However, taking this into account, we point out new possibilities for future research, like assessing these statements according to scientific literature. As a large number of misconceptions are brought together in the same article, it may be possible to take it to the next step and return to the classroom with much more information on this topic to plan teaching-learning sequences and better assessing students' ideas about this content. For example, the epistemological themes presented in Results and discussion section can serve as the basis for a sequence of classes on covalent bonding in higher education, even in high school, with the appropriate adaptations. Lastly, through the systematic review, we contributed showing how the research method was conducted and the implications it brought to the process, allowing other similar searches to be organized. Unlike leading an unsystematic review, here we can reach and deal with research found by a more rigorous method that seeks to decrease the authors' biases-once all the steps are clear and anyone can repeat it-allowing other research to be organized in similar ways, considering occasional future changes in the research area. In addition, the proposal of epistemological committed themes allowed us to go beyond a simple list of phrases, to a classification of sentences according to topics of different complexity levels.
CONCLUSIONS Our review of misconceptions reported in literature shows a panoramic view of the topic, how it has been conducted through the years, the similarity between different articles, recent research about the topic, and how they can be classified in epistemological themes. Nevertheless, we considered all the epistemological themes previously suggested, what indicates that much is known about the misconceptions concerning the theme. Besides, this article was about a different proposal to organize empirical data on misconceptions with a panoramic and analytic view. It was not necessary to create new epistemological themes during the process, once all the sentences were associated with some theme. According to this work, all themes present significant meanings related to some model or theory, showing that it is possible to misinterpret all of them. However, not all themes found the same quantity of papers, as for the Contemporary Rationalism, indicating possible gaps in the topics, different publics, or different levels of education. In conclusion, for the covalent bond and related topics, we discussed how a concept can be extremely complex considering all established connections, also how the themes can be organized and planned for a more effective teaching on chemical bonds. Although it is possible that some topic was excluded, the themes from Animism to Contemporary Rationalism contemplates several conceptualizations and certainly a class about the covalent bond will cover, at least, a part of what was discussed here, showing the extension of this study. Once professors or teachers recognize the problematic of using such ideas as animistic feelings, or the concrete existence of orbitals, for example, they can warn students that these conceptions are simply an analogy, or a metaphor, that may work "for now," as a scaffolding strategy, but these ideas need deeper understanding and planned actions as an example of how the information can be used. While for the general theme of misconceptions, it was possible to access how studies have reported the content. It includes, for example, different authors for different publics getting to similar statements over the same topic. For future research, our review may be used as a starting point for discussing the mistakes on these misconceptions, according to scientific reports, thinking on class context and in the conceptualization process by the students, as well as for planning and assessing teaching-learning sequences about covalent bonding. SUPPLEMENTARY MATERIAL The full material with all conceptions found by this article is available at http://quimicanova.sbq.org.br, in PDF format, with full access.
REFERENCES 1. Strike, K. A.; Posner, G. J.; In Philosophy of science, cognitive psychology, and educational theory and practice; Duschl, R. A., Hamilton, R. J., eds.; State University of New York Press: Nova York, 1992; pp. 146-176. 2. Mortimer, E.; Science & Education 1995, 4, 267. [Crossref] 3. Driver, R.; Easley, J.; Studies in Science Education 1978, 5, 61. [Crossref] 4. Özmen, H.; J. Sci. Educ. Technol. 2004, 13, 147. [Crossref] 5. Sanmartí, N.; Didáctica de las ciencias en la educación secundaria obligatoria.; 2nd ed., Sintesis: Madrid, 2009. 6. Smith III, J. P.; diSessa, A. A.; Roschelle, J.; Journal of the Learning Sciences 1994, 3, 115. [Crossref] 7. Vosniadou, S.; In Second International Handbook of Science Education; Fraser, B. J., Tobin, K. G., McRobbie, C. J., eds.; Springer: Atenas, 2012; pp. 119-130. 8. Baltieri, R. S.; Bego, A. M.; Cebim, M. A.; International Journal of Science Education 2021, 43. [Crossref] 9. Stefani, C.; Tsaparlis, G.; J. Res. Sci. Teach. 2009, 46, 520. [Crossref] 10. Bouayad, A.; Kaddari, F.; Lachkar, M.; Elachqar, A.; Procedia - Social and Behavioral Sciences 2014, 116, 4612. [Crossref] 11. Freire, M.; Talanquer, V.; Amaral, E.; International Journal of Science Education 2019, 41, 674. [Crossref] 12. Mahan, B. M.; Myers, J.; Química um curso universitário; Edgard Blücher: São Paulo, 1995; pp. 143-167. 13. Atkins, P.; Jones, L.; Princípios de química: questionando a vida moderna e o meio ambiente; Bookman: Porto Alegre, 2012; pp. 55-92. 14. Costa, A. B.; Zoltowski, A. P. C.; In Manual de Produção Científica; Koller, S. H., Couto, M. C. P. P., Hohendorff, J., eds.; Penso: Porto Alegre, 2014; pp. 55-70. 15. Juntunen, M.; Lehenkari, M.; Studies in Higher Education 2019, 1. [Crossref] 16. Castro Sotos, A. E.; Vanhoof, S.; van den Noortgate, W.; Onghena, P.; Educational Research Review 2007, 2, 98. [Crossref] 17. Ardoin, N. M.; Bowers, A. W.; Educational Research Review 2020, 31, 100353. [Crossref] 18. Wallau, W. M.; Quim. Nova 2014, 37, 1721. [Crossref] 19. Lewis, G. N.; J. Am. Chem. Soc. 1913, 35, 1448. [Crossref] 20. Clapp, L. B.; Chemistry of the Covalent Bond; W. H. Freeman and Company: Sao Francisco, 1957; pp. 360-392. 21. Pauling, L.; The nature of the chemical bond; 3rd ed., Cornell University Press: Nova York, 1960. 22. Mulliken, R. S.; Pure Appl. Chem. 1970, 24, 203. [Crossref] 23. Murrell, J. N.; Int. J. Quantum Chem. 2012, 112, 2875. [Crossref] 24. Nicoll, G.; International Journal of Science Education 2001, 23, 707. [Crossref] 25. Kind, V.; International Journal of Science Education 2014, 36, 1313. [Crossref] 26. Kabapinar, F.; Hacettepe Üniversitesi Journal of Education 2013, 28, 235. 27. Luxford, C. J.; Bretz, S. L.; J. Chem. Educ. 2014, 91, 312. [Crossref] 28. Temel, S.; Özcan, Ö.; EURASIA Journal of Mathematics, Science & Technology Education 2016, 12, 1953. [Crossref] 29. Jenkins, J. L.; Shoopman, B. T.; Science Education International 2019, 30, 152. [Crossref] 30. Kelly, J.; Krause, S.; Baker, D.; Proceedings - Frontiers in Education Conference, FIE 2010, T1G. [Crossref] 31. Prodjosantoso, A. K.; Hertina, A. M.; Irwanto; International Journal of Instruction 2019, 12, 1477. [Crossref] 32. Şen, Ş.; Varoglu, L.; Yılmaz, A.; Journal of Education and Future 2019, 65. [Crossref] 33. Peterson, R. F.; Treagust, D. F.; Journal of Chemical Education 1989, 66, 459. [Crossref] 34. Özmen, H.; Demircioǧlu, H.; Demircioǧlu, G.; Computers and Education 2009, 52, 681. [Crossref] 35. Kind, V.; Kind, P. M.; International Journal of Science Education 2011, 33, 2123. [Crossref] 36. Nogueira, H.; Porto, P.; Quim. Nova 2018, 42, 117. [Crossref] 37. Othman, J.; Treagust, D. F.; Chandrasegaran, A. L.; International Journal of Science Education 2008, 30, 1531. [Crossref] 38. Toplis, R.; Chem. Educ. Res. Pract. 2008, 9, 11. [Crossref] 39. Boo, H. K.; J. Res. Sci. Teach. 1998, 35, 569. [Crossref] 40. Zohar, A. R.; Levy, S. T.; J. Res. Sci. Teach. 2019, 56, 881. [Crossref] 41. Erman, E.; J. Res. Sci. Teach. 2017, 54, 520. [Crossref] 42. Lewis, G. N.; J. Am. Chem. Soc. 1916, 38, 762. [Crossref] 43. Langmuir, I.; J. Am. Chem. Soc. 1919, 41, 868. [Crossref] 44. Salah, H.; Dumon, A.; Chem. Educ. Res. Pract. 2014, 15, 675. [Crossref] 45. Atabek-Yigit, E.; Journal of Baltic Science Education 2015, 14, 524. [Crossref] 46. Nimmermark, A.; Öhrström, L.; Mårtensson, J.; Davidowitz, B.; Chem. Educ. Res. Pract. 2016, 17, 985. [Crossref] 47. Keil, F. C.; Concepts, Kinds, and Cognitive Development; 1st ed.; MIT Press: Cambridge, 1992. 48. Wells, G.; Cultural Studies of Science Education 2008, 3, 329. [Crossref] 49. Sepulveda, C.; Mortimer, E. F.; El-hani, C. N.; Investigações em Ensino de Ciências 2013, 18, 439. 50. Eymur, G.; Geban, Ö.; International Journal of Science and Mathematics Education 2017, 15, 853. [Crossref] 51. Tsaparlis, G.; Pappa, E. T.; Byers, B.; Chem. Educ. Res. Pract. 2018, 19, 1253. [Crossref] 52. Taagepera, M.; Arasasingham, R.; Potter, F.; Soroudi, A.; Lam, G.; Journal of Chemical Education 2002, 79, 756. [Crossref] 53. Salah, H.; Dumon, A.; Chem. Educ. Res. Pract. 2011, 12, 443. [Crossref] 54. Vrabec, M.; Proksa, M.; J. Chem. Educ. 2016, 93, 1364. [Crossref] 55. Birk, J. P.; Kurtz, M. J.; J. Chem. Educ. 1999, 76, 124. [Crossref] 56. Luxford, C. J.; Bretz, S. L.; Chem. Educ. Res. Pract. 2013, 14, 214. [Crossref] 57. Burrows, N. L.; Mooring, S. R.; Chem. Educ. Res. Pract. 2015, 16, 53. [Crossref] 58. Cil, E.; Ugras, M.; Journal of Baltic Science Education 2015, 14, 227. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access