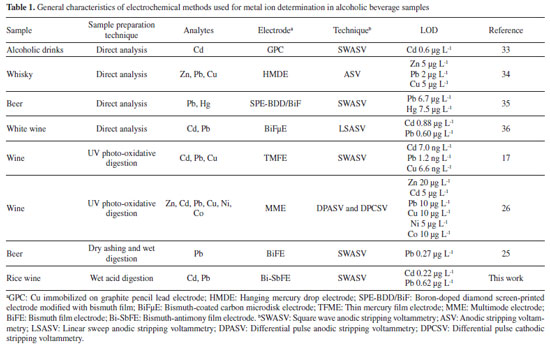
Artigo
| Bismuth-antimony film electrode for stripping voltammetric determination of trace cadmium and lead in Thai rice wine |
|
Watsaka SiriangkhawutI,*; Kraingkrai PonhongI; Piyanete ChantiratikulI; Parita MayothaII
I. Creative Chemistry and Innovation Research Unit, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahasarakham University, 44150 Maha Sarakham, Thailand Recebido em 23/11/2022 *e-mail: watsaka@hotmail.com Stripping analysis was proposed for the voltammetric determination of trace cadmium and lead in local rice wine alcoholic beverages. A bismuth-antimony film electrode (Bi-SbFE) was employed for accumulative preconcentration of trace metals by adding 4 mg L-1 of bismuth and 1 mg L-1 of antimony directly to the sample solution and simultaneously depositing bismuth, antimony, and trace cadmium and lead target metals on the glassy carbon electrode by applying a fixed potential of –1.1 V versus the Ag/AgCl reference electrode for 300 s. Pre-treatment parameters for mineralization of the sample were optimized using the wet acid digestion procedure. Under the optimum conditions, linear calibration graphs ranging 20-200 µg L-1 for Cd and Pb were obtained with detection limits of 0.22 and 0.62 µg L-1 and limits of quantitation 0.73 and 2.07 µg L-1, respectively. Reproducibility values for seven replicate analyses of 50 µg L-1 Cd and Pb were 3.6 and 3.3%, respectively. The developed method was successful for Cd and Pb analyses in Thai rice wine samples and results were validated by the flame atomic absorption spectrometric method. Most samples were not contaminated with Cd and Pb. The amount of Cd contaminants in only one of rice wine sample was still lower than the maximum permitted level of Cd in wine. INTRODUCTION Trace heavy metal contamination in daily diets has now become an important health and safety issue. Alcoholic beverages are frequently consumed but are not a staple food. Contamination of alcoholic beverages with heavy metals arises from many sources including raw materials, substances added during brewing, process type, process equipment, bottling process, aging/storage and adulteration.1 Heavy metal contents of alcoholic drinks have previously been reported.1-4 The Organisation Internationale de la Vinge et du Vin (OIV) set the maximum permitted level (MPL) of heavy metals in wine for Cd, Pb, As, Cu and Zn at 0.01, 0.15, 0.2, 1 and 5 mg L-1, respectively.5 In Thailand, the Ministry of Industry has set MPLs of heavy metals for industrial product standards in wine for As, Pb, Cu and Fe at 0.1, 0.2, 5 and 15 mg L-1, respectively. Different methods are employed for trace heavy metal analysis in beverages including flame atomic absorption spectrometry (FAAS),6,7 graphite furnace atomic absorption spectrometry (GFAAS),8-10 and inductively coupled plasma-mass spectrometry (ICP-MS).11 The stripping voltammetric method is favored for analysis of trace metals in non-alcoholic12,13 and alcoholic beverages14-20 with advantages of high sensitivity from its deposition step, selectivity, ease of operation and cost-effectiveness. Several environmentally friendly methods based on bismuth electrodes have been investigated instead of using toxic mercury electrodes for analysis of Cd and Pb.21 Stripping analysis using a bismuth-antimony film electrode (Bi-SbFE) was proposed in 2012 to determine cadmium in tap water samples. This method offers high performance in more acidic solutions with an excellent response to cadmium and lead compared to bismuth film electrode (BiFE) or antimony film electrode (SbFE) alone.22,23 To the best of our knowledge, application of a Bi-SbFE for the simultaneous determination of cadmium and lead in food and beverage samples has not been previously published elsewhere. Metals in alcoholic beverages can be either "bound" to the alcoholic matrix or "unbound" in solution.1 Direct analysis by the stripping voltammetric method only identifies the unbound fraction in the sample and the total amount of metals is not determined.1 Therefore, this method is not recommended for metal ions that bind strongly to the alcoholic matrix.24 Mineralization of samples to free the bound metal ions and destroy the organic materials interfering with voltammetric analysis, especially in complex food samples, is conducted by wet digestion,25 dry ashing25 or UV photolysis.17,26 Rice wine, obtained by fermentation of rice or glutinous rice, is traditionally consumed in southeast, east and south Asia. The fermentation broth comprises many organic compounds such as lactic acid, glucose, tartaric acid and glutamic acid.27 Previous reports have quantified the contamination by heavy metals in Thai rice,28 Asian rice and its derived food products.29 Thai rice wines, Sato and Lao U, are traditional alcoholic beverages normally home-produced using local wisdom and quality control of these products is limited. A highly efficient analytical approach with low operation cost was developed for the simultaneous determination of trace Cd and Pb by stripping voltammetry using Bi-SbFE. Wet acid digestion was optimized for rice wine sample preparation to provide reliable toxicity information.
EXPERIMENTAL Chemicals and reagents All chemicals used were of analytical reagent grade unless otherwise stated. Deionized water from Simplicity 185 (Millipore, Billerica, MA, USA) with resistivity of 18.2 MΩ cm was used throughout the experiments. Metal standard stock solutions (1000 mg L-1) of Cd and Pb for AAS were purchased from Merck (Darmstadt, Germany), while Ga for ICP was sourced from Sigma-Aldrich (Buchs, Switzerland). Working standard solutions of Cd and Pb at different concentrations were prepared by appropriately diluting the stock solution. A stock solution of 1000 mg L-1 Bi was prepared from bismuth(III) nitrate 5-hydrate (Carlo Erba, Milano, Italy) in 0.5 mol L-1 HNO3 solution. A stock solution of 1000 mg L-1 Sb was prepared from antimony(III) chloride (Sigma-Aldrich, Buchs, Switzerland) in a 2 mol L-1 HCl solution. Nitric acid (HNO3, 65%, extra pure grade, QRëC, New Zealand) and hydrogen peroxide (H2O2, 35%, extra pure grade, QRëC, New Zealand) were used to digest the samples. Hydrochloric acid (HCl, 37%, extra pure grade, QRëC, New Zealand) was used to prepare 0.01 mol L-1 HCl electrolyte solution. Acetate buffer solutions 0.1 mol L-1 at pH 4.5 were prepared from sodium acetate (Ajax Finechem, New South Wales, Australia) and acetic acid (QRëC, New Zealand). All glassware and plastic materials used were treated for 24 h in 10% v/v nitric acid and rinsed with deionized water. Instrumentation An Autolab PGSTAT 204 potentiostat (EcoChemie, Utrecht, Netherlands) interfaced to a personal computer with NOVA software, version 1.10 was employed for voltammetric measurement and data evaluation. A coated glassy-carbon (GC) disk (3 mm diameter, Metrohm, Switzerland) served as the supporting working electrode, with Ag/AgCl (3 mol L-1 NaCl) and platinum wire acting as the reference and auxiliary electrodes, respectively. Scanning electron microscope (SEM) model JSM-6460LV (JEOL, Tokyo, Japan) was used for the acquirement of SEM images. The FAAS measurements were performed with an Agilent 280FS AA atomic absorption spectrometer (Agilent Technologies, CA, USA) equipped with a deuterium background corrector. A high intensity UltrAA coded multi-element (Ag/Cd/Pb/Zn) hollow cathode lamp (Agilent Technologies, CA, USA) was employed as the radiation source. All instrumental conditions followed the manufacturer's recommendation.30 All pH measurements were conducted using a pH meter (Model 713, Metrohm, Switzerland). Sample collection and preparation Eight local rice wine (Sato and Lao U) samples were selected from provinces in northeast, Thailand. All samples were fermented from domestically cultivated Thai rice. After delivery to the laboratory, the rice wine samples were stored at 4 ºC until further analysis. All samples were analyzed in triplicate. To decompose the organic matter, 25 mL of rice wine was placed in a digestion flask. Then, 2 mL of conc. HNO3 (65%) and 5 mL of H2O2 were added, and the contents were heated on a hot plate for about 30 min until colorless. The digested solutions were evaporated to near dryness, then transferred into 25 mL volumetric flasks and brought to volume with the electrolyte solution. The solutions were then transferred into polyethylene bottles and stored at 4 ºC until further analysis. Blanks without samples were also treated in the same manner for each experiment. Stripping voltammetry by Bi-SbFE Stripping voltammetric measurement was performed with an in situ plated bismuth-antimony film and target metals in the presence of dissolved oxygen. Before use, the bare glassy carbon electrode was polished to a mirror with a 0.05 µm Al2O3 slurry, and rinsed thoroughly with deionized water, then, washed successively with deionized water, 1:1 (v/v) HNO3 aqueous solution and deionized water in an ultrasonic bath and dried in air. Three electrodes were immersed in a voltammetric cell containing 4 mg L-1 bismuth and 1 mg L-1 antimony, and 10 mL sample aliquots of 0.01 mol L-1 HCl solutions. The deposition step was carried out at –1.10 V for 300 s under stirring. After a 20 s equilibration period, the stripping voltammogram was recorded by applying a positive-going square wave anodic stripping voltammetry (SWASV) potential scan from –1.10 to 0.50 V with a frequency of 25 Hz, amplitude 25 mV, and potential step of 4 mV. Before the next measurement, the residual target metals and bismuth-antimony film were removed using 1 mol L-1 HCl as a cleaning solution, and a 60 s conditioning step at +0.5 V under stirring was then performed.
RESULTS AND DISCUSSION Optimization of electrochemical parameters In situ plating of electrode film was selected for simultaneous formation with analyte metal accumulations during the deposition step. The preliminary investigation of the operational parameters of the square wave anodic stripping voltammetry (SWASV) according to the published literature with some modifications28 were performed with BiFE, SbFE and Bi-SbFE. The satisfactory voltammograms were obtained. Therefore, these SWASV conditions were selected. Type of electrolyte and bismuth concentration The sensitivity of metal determination depends on the thickness of the metal film electrode and also on the electrolyte solution. Acetate buffer pH 4.5 and 0.01 mol L-1 HCl, normally used for BiFE and SbFE, were studied. The voltammograms obtained in different electrolyte solutions and bismuth concentrations are depicted in Figures 1S and 2S (supplementary material). As shown in Figure 1, Bi concentration was varied from 0 to 5 mg L-1 with a fixed concentration of Sb at 1 mg L-1. For acetate buffer pH 4.5, peak currents of Cd and Pb increased with increase of Bi concentration and reached the maximum at 1 mg L-1 Bi, then, peak currents of Cd and Pb decreased. For 0.01 mol L-1 HCl, peak currents of Cd and Pb increased with increase of Bi concentration and reached the maximum at 3 (for Cd) and 4 (for Pb) mg L-1 Bi. The thickness of the Bi film related to the amount of deposited Bi. At high Bi concentration, the metals had difficulty stripping out a thick Bi film, leading to lower peak current and a broader peak. Using 0.01 mol L-1 HCl as the electrolyte solution improved sensitivity for both Cd and Pb. This results concurred with Yi et al.22 who reported that pH higher than 2.0 caused hydrolysis of Sb in the solution, with lower peak currents observed at higher pH. Therefore, 0.01 mol L-1 HCl electrolyte solution and Bi concentration of 4 mg L-1 were selected for further experiments.
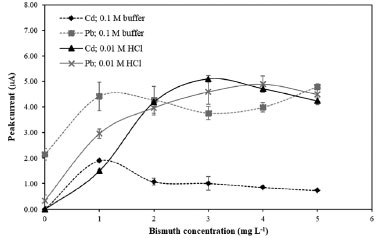 Figure 1. Effect of type of electrolyte and bismuth concentration on stripping peak currents of 100 µg L-1 Cd and Pb. Supporting electrolyte: 0.1 mol L-1 acetate buffer pH 4.5, 0.01 mol L-1 HCl; deposition potential: –1.1 V; deposition time: 120 s under stirring; equilibration time: 20 s; frequency: 25 Hz; pulse amplitude: 25 mV; potential step: 4 mV; Sb concentration: 1 mg L-1
Effect of antimony concentration The effect of Sb concentration on peak currents of Cd and Pb was studied over the concentration range 0 to 4 mg L-1 Sb and fixed concentration of Bi at 4 mg L-1. The voltammograms are depicted in Figure 3S (supplementary material). As shown in Figure 2, peak currents of both Cd and Pb increased with increase in Sb concentration from 0 to 1 mg L-1 and then decreased. At high Bi and Sb concentrations, the metal was more difficult to strip out from the thick Bi-Sb film, leading to lower peak current and a broader peak. A concentration of 1 mg L-1 Sb was chosen for further experiments.
 Figure 2. Effect of antimony concentration on stripping peak currents of 100 μg L-1 Cd and Pb. Supporting electrolyte: 0.01 mol L-1 HCl; deposition potential: -1.1 V; deposition time: 120 s under stirring; equilibration time: 20 s; frequency: 25 Hz; pulse amplitude: 25 mV; potential step: 4 mV; Bi concentration: 4 mg L-1
Different modified glassy carbon electrodes including (a) BiFE, (b) SbFE and (c) Bi-SbFE were employed for analysis using 100 µg L-1 each of Cd and Pb in 0.01 mol L-1 HCl for sensitivity comparison. Peak potential and peak shape of Cd and Pb stripping signals obtained from Bi-SbFE were similar to the signal obtained from SbFE, while BiFE exhibited the higher stripping peak current signals of Cd and Pb compared with SbFE. However, the highest stripping peak currents of Cd and Pb were obtained from Bi-SbFE (Figure 3).
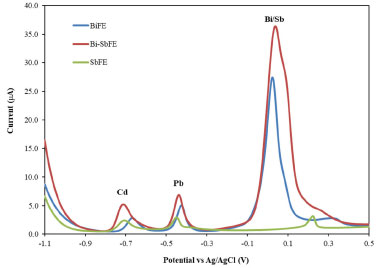 Figure 3. Voltammograms of 100 μg L-1 each of Cd and Pb in 0.01 mol L-1 HCl electrolyte solution obtained from different in-situ plating working electrodes including (a) BiFE: Bi 4 mg L-1; (b) Bi-SbFE: Bi 4 mg L-1 and Sb 1 mg L-1 and (c) SbFE: Sb 1 mg L-1. Other conditions are the same as in Figure 2
The morphological structure of the BiFE, SbFE and Bi-SbFE were evaluated through SEM analysis. To obtain a clear image, concentration of both bismuth and antimony at optimized Bi/Sb ratios had to be increased (10 times). Figure 4S (supplementary material) presents the obtained images for the working electrode surface unmodified and modified with bismuth or/and antimony film. The morphology of codeposites Bi-SbFE looks quite different from BiFE and SbFE alone, presenting larger irregular square shape which separated agglomerates of the metals. The more active sites can be responsible for an increase in the electroactive area of the electrode, providing higher stripping peak currents of Cd and Pb obtained from Bi-SbFE. Effect of deposition potential The effect of deposition potential on peak currents was investigated over the range –0.8 to –1.2 V at deposition time of 120 s. As presented in Figure 5S (supplementary material), greater sensitivity was achieved at higher negative potential. At negative potential above –1.1 V, peak currents for both Cd and Pb were stable. Thus, a deposition potential of –1.1 V was chosen for further investigations. Effect of deposition time Influence of deposition time was studied from 30 to 300 s, as shown in Figure 6S (supplementary material). Two concentrations of Cd and Pb at 50 and 100 µg L-1 were performed to verify no saturation of the metal on Bi-Sb film surface during the deposition step. Results indicated that peak current increased with increasing deposition time from 30 to 300 s. Normally, sensitivity increases with increase in deposition time. Therefore, deposition time of 300 s was chosen to determine Cd and Pb concentrations at trace levels. Optimization of digestion parameters The GFAAS8 sample preparation procedure for heavy metal analysis was selected as a guideline for the decomposition of organic matter in rice wine samples. The parameters affecting digestion efficiency are listed in Table 1S (supplementary material). Metal ions at 50 µg L-1 were spiked into the rice wine samples and percentage recoveries of the variable digestion parameters were evaluated considering the optimum conditions. Effect of oxidant The effect of oxidant was investigated by applying acid (conc. HNO3) alone and an acid-oxidant mixture (HNO3:H2O2; 1:1) to digest the rice wine samples. Results showed that H2O2 combined with HNO3 provided a clear solution, with short digestion time at higher percentage recoveries of Cd and Pb compared to acid alone, as shown in Figure 7S(a) (supplementary material). This result was consistent with published reports that oxidation of biological samples with high organic matter (e.g. protein, carbohydrates) usually gave incomplete digestion with only HNO3, while H2O2 combined with HNO3 yielded a clear solution and improved recovery.31 Volume of concentrated acid Concentrated acid at volumes of 1, 2, 3, 4 and 5 mL was studied. Low recoveries of ≤ 80% were observed using more than 3 mL of concentrated acid, as shown in Figure 7S(b) (supplementary material). Using concentrated acid to digest the organic compounds generated high peak noise and low sensitivity because pH values of the digested solution were lower than pH 1.32 Therefore, 2 mL of concentrated acid was selected as the optimum condition. After digestion, the solutions were evaporated to near dryness to remove the acid and re-dissolved in the electrolyte solution. Volume of hydrogen peroxide The volume of hydrogen peroxide (1, 2, 3, 4 and 5 mL) used did not significantly affect digestion efficiency. High percentage recoveries of ≥ 90% were observed at all volumes (1, 2, 3, 4 and 5 mL), as shown in Figure 7S(c) (supplementary material). Highest percentage recoveries with low standard deviation were obtained using 5 mL of H2O2 and this condition was chosen as the optimum. Digestion time Digestion times of 10, 20, 30, 40, 50 and 60 min were studied. A clear solution with high percentage recovery was observed after 20 min of digestion time, as shown in Figure 7S(d) (supplementary material). Therefore, optimum digestion time was selected at 30 min (≥ 90% recovery). Analytical characteristics of the proposed system The analytical characteristics of the proposed system were investigated. A standard addition method was used to estimate concentrations of Cd and Pb in the rice wine samples because this approach is effective in eliminating the problems of the matrix effect. Using the optimum conditions as described above, standard calibration ranges of 20 to 200 µg L-1 for both Cd and Pb were constructed by plotting peak currents against concentrations. Under the selected conditions, linear calibration graphs (y: peak current in µA, x: concentration in µg L-1) were obtained for Cd and Pb, as shown in Figure 8S (supplementary material), with calibration equations y = 0.0748x + 0.01; R2 = 0.9988 for Cd and y = 0.0521x + 0.02; R2 = 0.9989 for Pb. Limits of detection (LOD) (3σ/s) and quantification (LOQ) (10σ/s) (where σ is the standard deviation of the digestion blank (n = 11) and s is the slope of the calibration curve) were obtained for deposition time of 300 s at LOD 0.22 µg L-1, LOQ 0.73 µg L-1 for Cd, and LOD 0.62 µg L-1, LOQ 2.07 µg L-1 for Pb. Relative standard deviations for eleven replicate determinations of 50 µg L-1 for Cd and Pb were 3.6 and 3.3%, respectively. Table 1 compares the analytical characteristics of several methodologies employed for heavy metal determination in alcoholic beverages. The sensitivity of our proposed method was higher than hanging mercury drop electrode (HMDE) and multimode electrode (MME) techniques, with lower sensitivity compared to thin mercury film electrode (TMFE). However, Bi-SbFE is more environmentally friendly than using mercury electrodes, and the sensitivity of our proposed method was comparable to other Cu immobilized on graphite pencil lead electrode (GPC) and bismuth-based electrode. The wet acid digestion procedure also provides simple, rapid and low chemical consumption for total metal determination in complex matrix samples.
Analysis of rice wine samples The proposed stripping voltammetric method was employed to determine Cd and Pb quantities in local rice wine samples, with analysis results presented in Table 2. Representative Cd and Pb voltammograms in rice wine samples are depicted in Figure 4. To compare the results, all digested samples were also analyzed by the FAAS method.
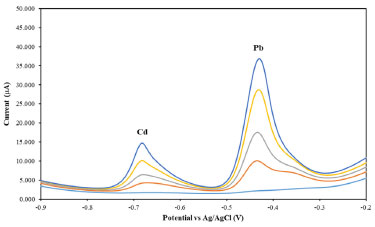 Figure 4. Voltammograms of rice wine samples obtained from the standard addition method by spiking with Cd and Pb standards. Concentration of each metal from bottom to top: 0, 50, 100, 150 and 200 μg L-1. Supporting electrolyte: 0.01 mol L-1 HCl; deposition potential: -1.1 V; deposition time: 300 s under stirring; equilibration time: 20 s; frequency: 25 Hz; pulse amplitude: 25 mV; potential step: 4 mV; Bi concentration: 4 mg L-1; Sb concentration: 1 mg L-1
Most samples were not contaminated with Cd and Pb, as presented in Table 2. Concentrations of Cd and Pb found in seven rice wine samples were below the detection limits of both methods. Satisfactory results for the studied concentration levels were obtained, with percentage recoveries of 98 to 116% (for Cd using the proposed method), 92 to 106% (for Cd using the FAAS method), 93 to 113% (for Pb using the proposed method), and 94 to 106% (for Pb using the FAAS method). Both methods showed good agreement. Maximum permitted levels (MPLs)5 of Cd and Pb in wine are 10 and 150 µg L-1, respectively. Cadmium was only found in the digested sample of Lao U (U1) and within the MPL level. Results indicated that all studied rice wine samples were safe for consumer consumption.
CONCLUSIONS A stripping voltammetric determination of trace Cd and Pb in rice wine alcoholic beverages using a Bi-SbFE eco-friendly electrode was presented, following the wet acid digestion procedure for total metal contents. The proposed method offered a convenient, sensitive, accurate and reliable determination of trace Cd and Pb contaminants in local rice wine samples without requiring preconcentration. All analyzed samples were not contaminated with Pb. Only one of rice wine sample was contaminated with Cd at lower level than the MPL. This study provides significant data on the safety and quality of rice wine consumed in Thailand. The developed method showed high potential as a good alternative for the analysis of Cd and Pb contaminants in various alcoholic beverages.
SUPPLEMENTARY MATERIAL In supplementary material, available on http://quimicanova.sbq.org.br as a PDF file, with free access, are presented the Figures 1S, 2S, 3S, 4S, 5S, 6S, 7S, 8S and Table 1S.
ACKNOWLEDGEMENTS This research was financially supported by Mahasarakham University 2017 and the National Research Council of Thailand (NRCT). Additional support was received from the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation.
REFERENCES 1. Ibanez, J. G.; Carreon-Alvarez, A.; Barcena-Soto, M.; Casillas, N.; J. Food Compos. Anal. 2008, 21, 672. [Crossref] 2. Pal, L. G.; Muhollari, T.; Bujdoso, O.; Baranyai, E.; Nagy, A.; Arnyas, E.; Adany, R.; Sandor, J.; McKee, M.; Szucs, S.; Regul. Toxicol. Pharmacol. 2020, 116, 104723. [Crossref] 3. Okareh, O. T.; Oyelakin, T. M.; Ariyo, O.; Beverages 2018, 4, 60. [Crossref] 4. Pohl, P.; Food Addit. Contam., Part A 2008, 25, 693. [Crossref] 5. International Organization of Vine and Wine; Compendium of International Methods of Wine and Must Analysis, Paris, 2016. 6. Jalbani, N.; Ahmed, F.; Gul kazi, T.; Rashid, U.; Munshi, A. B.; Kandhro, A.; Food Chem. Toxicol. 2010, 48, 2737. [Crossref] 7. Beattie, J. K.; Quoc, T. N.; Food Chem. 2000, 68, 37. [Crossref] 8. Alkis, M.; Oz, S.; Atakol, A.; Yilmaz, N.; Anli, R. E.; Atakol, O.; J. Food Compos. Anal. 2014, 33, 105. [Crossref] 9. Martinez, D.; Grindlay, G.; Gras, L.; Mora, J.; J. Food Compos. Anal. 2018, 67, 178. [Crossref] 10. Borges, S. S. O.; Beinner, M. A.; Silva, J. B. B.; Biol. Trace Elem. Res. 2015, 167, 155. [Crossref] 11. Tormen, L.; Torres, D. P.; Dittert, I. M.; Araujo, R. G. O.; Frescura, V. L. A.; Curtius, A. J.; J. Food Compos. Anal. 2011, 24, 95. [Crossref] 12. Jedryczko, D.; Pohl, P.; Welna, M.; Food Chem. 2017, 225, 220. [Crossref] 13. Lo Coco, F.; Monotti, P.; Cozzi, F.; Adami, G.; Food Control 2006, 17, 966. [Crossref] 14. Yadav, P. K.; Sharma, R. K.; The Arab Journal of Forensic Sciences & Forensic Medicine 2019, 1, 1232. [Crossref] 15. Maciel, J. V.; Souza, M. M.; Silva, L. O.; Diaz, D.; Beverages 2019, 5, 6. [Crossref] 16. Oliveira, P. R.; Lamy-Mendes, A. C.; Rezende, E. I. P.; Mangrich, A. S.; Marcolino Junior, L. H.; Bergamini, M. F.; Food Chem. 2015, 171, 426. [Crossref] 17. Illuminati, S.; Annibaldi, A.; Truzzi, C.; Finale, C.; Scarponi, G.; Electrochim. Acta 2013, 104, 148. [Crossref] 18. Blanco, C. A.; Sancho, D.; Caballero, I.; Food Res. Int. 2010, 43, 2432. [Crossref] 19. Carreon-Alvares, A.; Casillas, N.; Ibanez, J. G.; Hernandez, F.; Prado-Ramirez, R.; Barcena-Soto, M.; Gomez-Salazar, S.; Anal. Lett. 2008, 41, 469. [Crossref] 20. Barbeira, P. J. S.; Stradiotto, N. R.; Talanta 1997, 44, 185. [Crossref] 21. Svancara, I.; Prior, C.; Hoceva, S. B.; Wang, J.; Electroanalysis 2010, 22, 1405. [Crossref] 22. Yi, W. J.; Li, Y.; Ran, G.; Luo, H. Q.; Li, N. B.; Sens. Actuators, B 2012, 166-167, 544. [Crossref] 23. Ashrafi, A. M.; Vytras, K.; Int. J. Electrochem. Sci. 2013, 8, 2095. [Link] accessed in march 2023 24. Green, A. M.; Clark, A. C.; Scollary, G. R.; Fresenius' J. Anal. Chem. 1997, 358, 711. [Crossref] 25. Ghanjaoui, M. E.; Srij, M.; Hor, M.; Serdaoui, F.; El Rhazi, M.; J. Mater. Environ. Sci. 2012, 3, 85. [Link] accessed in march 2023 26. Buldini, P. L.; Cavalli, S.; Sharma, J. L.; J. Agric. Food Chem. 1999, 47, 1993. [Crossref] 27. Wei, Z. B.; Wang, J.; Cui, S. Q.; Wang, Y. W.; Anal. Methods 2016, 8, 6361. [Crossref] 28. Siriangkhawut, W.; Sittichan, P.; Ponhong, K.; Chantiratikul, P.; J. Food Compos. Anal. 2017, 59, 145. [Crossref] 29. Guo, K.; Wells, S.; Han, F. X.; Arslan, Z.; Sun, H.; Zhang, J.; Water, Air, Soil Pollut. 2017, 228, 76. [Crossref] 30. Agilent Technologies, Inc.; The Manual of Flame Atomic Absorption Spectrometry Analytical Method: Standard Conditions of Cd and Pb, 10th ed.; USA, 2012. 31. Kazi, T. G.; Jamali, M. K.; Arain, M. B.; Afridi, H. I.; Jalbani, N.; Sarfraz, R. A.; Ansari, R.; J. Hazard. Mater. 2009, 161, 1391. [Crossref] 32. Kim, H. J.; Son, D. W.; Park, J. M.; Hwang, D. Y.; Mo, C. Y.; Park, S. W.; Kim, G.; Eun, J. B.; Food Sci. Biotechnol. 2010, 19, 1211. [Crossref] 33. Ly, S. Y.; Yoo, H. S.; Chun, S. K.; Food Chem. 2013, 137, 168. [Crossref] 34. Barbeira, P. J. S.; Stradiotto, N. R.; Fresenius' J. Anal. Chem. 1998, 361, 507. [Crossref] 35. Silva, L. R. G.; Mutz, Y. S.; Stefano, J. S.; Conte-Junior, C. A.; Ferreira, R. Q.; J. Food Compos. Anal. 2022, 110, 104564. [Crossref] 36. Baldo, M. A.; Danielle, S.; Anal. Lett. 2004, 37, 995. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access