Revisão
| Microplastics and the amazon: from the rivers to the estuary |
|
Maria Tereza V. de SouzaI,II,*; Vanessa Sales-ShimomotoI,II; Grazyelle S. da SilvaIII; Adalberto Luis ValI,II
I. Instituto Nacional de Pesquisas da Amazônia, 69067-375 Manaus − AM, Brasil Recebido em: 20/09/2022 *e-mail: mariaterezavs17@gmail.com Plastic pollution is causing worldwide concern, especially after evidence that the various types of plastic degrade into particles of smaller sizes; known as micro- and nanoplastics. The origin of the plastics in the environment is related to human actions. The objective of this review is to describe the main routes of microplastic input in the Amazonian rivers and how local environmental characteristics can affect the transformation of plastics into microplastics until their absorption by aquatic organisms. The current situation regarding the presence of microplastic particles in freshwater is analyzed considering the environmental dynamics of the region, and focuses on rivers, estuaries and sediments, their effects on organisms, especially on fish, followed by the transportation of microplastic particles to the sea. Amapá, Amazonas, Pará and Mato-Grosso are the states of the Brazilian Amazon with scientific reports on the characterization of microplastic particles in sediment, water, and fish. These studies are local and descriptive and most of them highlight the characterization of microplastics. There is a need for field research in the various microregions of the Amazon, as well as actions to mitigate the damage, including to riverine populations, that is caused by these pollutants in the region. INTRODUCTION "Life without plastic is hard to believe",1 but its use on a global scale causes great consequences, since pollution of aquatic environments by plastic has continuously increased. Plastic pollution of aquatic environments has drawn attention worldwide, and is considered to be one of the top environmental issues of the decade.2 In 2019, during the Basel Conference in Geneva, Switzerland, around 180 governments identified plastics as hazardous waste due to their toxicity, their capacity to adsorb pollutants and their fragmentation.2 Brazil ranks fourth in world production of plastics,3 and this is due to the industrialization which has brought about a multiplication of plastic producing industries in the Brazilian scenario.4 Brazil alone produces approximately 11 million tons of plastic waste per year, which, consequently, often ends up in the soil and in aquatic environments.3 Because it has high versatility, the consumption and use of plastics have expanded significantly.5 Among the various utilities that this material provides, we can highlight its use in packaging, which allows better packaging of food; in automobiles, which makes them safer and lighter and which generates lower fuel consumption; in civil construction, which allows building costs to be reduced; in household appliances, with the use of materials that require less energy to produce; in the field of health, reducing the risk of contamination, such as in the manufacture of disposable face masks, currently used to combat the pandemic of the new coronavirus;3 in textile manufacturing, enabling the production of materials that are more resistant to moisture, and which require less effort to wash; in agriculture, and in irrigation systems, reducing losses and costs,5 among others. On the other hand, on a global scale, plastics in the environment have been reported as a threat to all living beings, whether terrestrial or aquatic.5 The Brazilian Amazon encompasses the states of Acre, Amapá, Amazonas, Pará, Rondônia, Roraima, Tocantins, Mato Grosso, and the western part of the state of Maranhão. It covers an area of 5.21 million square kilometers, what corresponds to approximately 61% of Brazilian territory.6 The Amazon basin is the largest river basin on earth and includes four out of the ten world's largest rivers, among them the Amazon River.7 Given the growing use of plastics in the Amazon, there is increasing concern about its effects on aquatic organisms, in particular on fish. This is because, over time, through the mechanical and chemical actions of the wind and water,8 associated with high temperatures and the incidence of UV rays, plastic degrades into smaller particles, the micro- and nanoplastics.9 In Brazil, specifically in the Amazon region, for example, the presence of plastic in sediments,10 rivers and aquatic biota has already been evidenced.11,12 Contamination by plastic particles has become a cause for great concern, both in terms of environmental contamination and the impact that it can have on the fish, the basis of the diet of the Amazonian people. However, we do not know how the chemical compounds present in plastic waste that enter the food chain will affect the health of populations that eat contaminated fish. When it comes to the Amazon region, it is important to remember that it concentrates the greatest diversity of freshwater fish in the world.13,14 In addition, the amount of water bodies of different types existing in the region is incomparable, with 20% of all fresh water reaching the oceans coming from the rivers of the Amazon.15 However, the first evidence of microplastic ingestion by fish was only reported in 2018, in a study conducted in the estuary of the Amazon River, and involved 189 specimens belonging to 46 species and 22 families of fish. The study showed the presence of 228 microplastic particles in the gastrointestinal tract of 30% of the sampled species.11 According to Galgani et al.,16 the accumulation of plastics in aquatic environments is often due to the erroneous way in which producers and manufacturers manage their production. Professionals directly affected by this problem, as in the case of fishers, recognize that pollution caused by plastic items is a threat to aquatic biological resources.17 Although recognized, the environmental problems caused by plastics have been growing and are likely to persist for centuries.18 In addition to the harmful mechanical effects, the ingestion of microplastics carrying various toxic compounds, such as pesticides, drugs and chemical additives poses aquatic organisms and humans in risk, as they can be bioaccumulate and cause health disturbances particularly to humans significantly dependent fish as major protein source, as occurs in the Amazon.19 Knowing this, and the socio-environmental importance, richness and endemism of the Amazon fauna and flora, the concern with the environmental management and conservation emerges significantly, particularly in relation to plastics. Therefore, it is essential to know and evaluate which are the ways and means that microplastics disperse and move in the Amazonian environment, including the contact routes with living organisms. This information is required to design strategies to prevent the consequences of microplastic pollution in the Amazon region. Despite the recent increase in the number of studies on the presence of microplastic particles in different environments, studies on the impacts of microplastics on Amazonian ecosystems are limited, and there is a need to understand the dynamics involving the transformation of microplastics in different freshwater environments, i.e., black, white, and clear water environments; transportation through water and air, and transformation of the polymers involving the unique aquatic biota of the Amazon. The main questions that still need to be answered are the following: Where are the largest plastic waste emissions to the Amazon region coming from? How are freshwater species being affected? Does the seasonal climate in the Amazon interfere with the microplastic cycle and its transformation? Are microplastics carrying and releasing contaminants along the food chain? Thus, this work aims to describe the main routes of microplastic input in the Amazonian rivers and how the characteristics of the local water system and environmental particularities can affect the transformation of microplastics until their absorption by aquatic organisms.
FROM DISCOVERY TO THE USE OF PLASTIC POLYMERS Plastic is a consumer good that has been widely used since the mastery of polymerization techniques between the years 1930-1950.5,20 It is a resistant material that comes from of the synthesis of macromolecules from smaller molecules, known as monomers.5,21 Plastics are synthetic polymers (in Latin poly means many, and mero means parts).5,21 The search for a resistant, durable and moldable material dates back to the 19th century, when John Wesley Hyatt developed a material to which he gave the name celluloid. Produced from nitrocellulose and camphor, celluloid became an alternative to the use of ivory, which was becoming scarce, in addition to involving the sacrifice of elephants.5 Celluloid at that time served its initial purpose, which was as a raw material for the manufacture of billiard balls.5 Its major problem was its two components, since nitrocellulose has explosive properties, and camphor degrades easily, thus compromising the safety and durability of the materials produced.5 In 1909, the first synthetic plastic was produced, which was a phenolic resin that became known as Bakelite.5 Bakelite is produced from the reaction between phenol and formaldehyde, forming a thermorigid composite with excellent electrical and thermal resistance, which lead it to be used in various household items, such as pot handles, telephones, radios, and toys, etc.5 Objects created from Bakelite retain their properties even after more than 100 years since their manufacture.5 Only 10 years after the creation of Bakelite, a process known as polymerization was discovered by Hermann Staudinger, thus giving rise to a new class of materials with a high molar mass and different properties from the already known materials.5 Examples of these materials are polystyrene (PS), low density polyethylene (LDPE), high density polyethylene (HDPE), polypropylene (PP), polyvinyl chloride (PVC) and polyethylene terephthalate (PET).5,20,22 Less than a century after mastering polymerization and polymer production, plastic has become a problem on a worldwide scale.23 24 25 Its production, marketing and consumption are the result of its physicochemical properties, such as high strength and durability, and it is these same properties that make it difficult to degrade in the environment.26 The pollution caused by the incorrect use and disposal of plastic materials causes losses in different instances such as fishing and tourism, reaching around US$ 8 billion per year, according to the United Nations Environment Program. It is estimated that the total production of plastic materials is approximately 250 million tons, with the Brazilian participation in the world production of plastic materials reaching approximately 6.5 million tons year-1, representing 2.7% of world production.27 An example of this is the production of packaging that represents approximately 40% of the consumption of plastic material produced on the planet.28,29 However, the environmental and economic potential wasted through inadequate plastic management is, on average, US$ 2.04 billion year-1 according to IPEA (2012).30 Nascimento and Cavalcante27 found that Brazil does not have adequate programs for the selective collection and effective treatment of municipal waste, including plastics, and that the lack of such programs causes economic, social and environmental disruptions. The increasing use of plastic materials and the evolution of their various applications are due to the greater convenience and practicality that every society receives in exchange for their use.27 However, the concern about the rampant use of plastic materials should equally involve society as a whole and not just a part of it. A large part of society that does not have access to water, sanitation, selective collection in their neighbourhoods and communities, whether near or far from urban centres, should be included and informed as part of the fight against the use and disposal of plastic materials.3 According to De Aguiar et al.,31 the Brazilian Amazon contributes to the generation of approximately 10 million tons of solid waste year-1. Urban solid waste generation, largely plastic, per capita day-1 for each state in the Brazilian Amazon is of the order of: Acre (0.99 kg); Amapá (0.64 kg); Amazonas (1.14 kg); Pará (1.26 kg); Rondônia (0.65 kg); Roraima (1.37 kg); Tocantins (0.88 kg). The difficulty involved in the disposal and degradation of plastic, its lengthy decomposition time, added to its unrestricted use and the inadequate management of its residues20,25 have caused this material to accumulate in ecosystems, where it decomposes into particles of smaller sizes, known as micro- and nanoplastics, as mentioned.26,32,33 These particles interact with the biota, and produce negative effects that are now being described by researchers.34 25 26 27 28 29 40 41 42 In the Amazon region, the impacts of micro- and nanoplastics on the biodiversity have only relatively recently begun to be the subject of research, with no information about waste plastic production and discharge in the Amazon basin, or other specific information from the Brazilian Amazon. According to Margallo et al.,43 it is generally difficult to obtain information involving waste generation and the reliability and timeliness of data cannot be guaranteed. The same authors reviewed data for average relative waste composition in Latin American and the Caribbean (LA&C) and observed that 14.94% of the total waste produced by Brazil (total waste production: 62,730,096 ton year-1 (according to the Waste Atlas) is plastic waste. Brazil is followed by Guyana (14.4%), Colombia (10.67%) Ecuador (8.05%), Peru (6.22%), Bolivia (6.15%), with no information for Venezuela and Suriname (Table 1).3,44 In the survey carried out by Valerio et al.,44 it is clear that the recycling rate for plastic waste in South American countries is very low. In Colombia it equates to ~ 19.5%, Peru ~ 13%, Bolivia ~ 4.5 % and Brazil ~ 0.8%, with no information for Ecuador, Guyana, Suriname and Venezuela. The countries that are part of the Amazon basin do not have well-established programs for waste management, much less for plastic waste. The big problem is the lack of updated information on waste disposal by the countries that form the Amazon Basin. Data on waste management is not recent and does not consider the increase in hospital plastic waste that accompanied the COVID-19 pandemic. The use of new technologies to prevent the SARS-CoV-2 virus infection, such as textile fibers impregnated with Ag and Cu nanoparticles for the manufacture of facemasks and commercial products could exacerbate plastic pollution, and there is no information in South America on the degree of contamination of synthetic nanoparticles in aquatic environments.45 To demonstrate how difficult, it is to manage the issue of plastic waste in the current scenario, we have the example of policies for the use of plastic straws. Mailes Neto et al.46 used as a proxy to examine the different points covered by the laws on the American continent related to the use of plastic straws. A total 363 regulations on straws in 22 American countries were examined. Brazil alone has 195 laws related to plastic straws and has the largest number (183) of municipal regulations involving straw consumption, concentrated primarily in São Paulo state, with municipal regulations in 65 cities, followed by Rio Grande do Sul state with 34 cities. Other countries that are part of the Amazon basin that stand out for regulations are Peru (7), Colombia (4), and Ecuador (2). Data like this involving legislation for the use of plastic straws raise questions related to regulation for the production, use and destination of everything that has plastic as its raw material. How will plastic waste be managed in the Amazon is still a question mark.46
FROM PLASTIC TO MICROPLASTIC Microplastics (MPs) have been identified as an emerging contaminant in aquatic and terrestrial environments.53 Microplastics are commonly known as water-insoluble solid polymers. With less than 5 mm in size, they result from the degradation of the plastic polymer or are broken down into a smaller size.54,55 Plastic debris can also be degraded into particles smaller than 100 nm, and these are classified as nanoplastics (NPs).56 Microplastics are categorized into primary and secondary microplastics based on their sources. Primary microplastics refer to plastic particles that are already manufactured in a small size for specific applications, such as resin beads for plastic production, and microbeads for facial washes or toothpaste.57 Secondary microplastics are formed by the fragmentation of larger plastic pieces as a consequence of physical, biological and chemical processes in the environment.58 Secondary microplastics are produced by photooxidative-, thermal-, ozone-, mechanochemical-, catalytic- and/or bio-degradation.59 The specific characteristics of plastics (chemical composition, shape, size and texture) determine how they will degrade in the environment. When exposed to UV radiation, plastics lying on the beach or floating in the water, for example, show different patterns of degradation.60 The plastic present in the environment ends up absorbing UV radiation, whose energy promotes the breakdown of the main chains of plastic polymers, initiating their degradation.61 It is the external factors such as UV, oxygen, heat and water that contribute to the degradation and aging of polymers.62 The photodegradation of plastic is highly accelerated at the surface of the waters.63 According to Muthukumar et al.,64 the discrepancy in the degradation rates (between air and floating exposures) is further exacerbated by fouling effects. Floating plastics will readily develop extensive surface fouling, rapidly covering the debris surface first with a biofilm that is then followed by an algal mat and finally a colony of invertebrates.64 It is exactly this photodegradation that entails the aging of the plastic items, which tends to modify the behavior of these particles in aquatic environments, thus contributing to the adsorption of materials that are probably harmful to the formation of biofilms on the surface of these particles and, which thus amplifies the toxicity of plastic particles. The aging process of microplastics is very slow.64 To date, it is known that the aging of these plastic particles can enhance the effect of micro- and nanoplastics due to the release of toxic additives present in the particles themselves.62
MICROPLASTICS IN FRESHWATER ENVIRONMENTS There are many studies that report the presence and effects of microplastics on the aquatic biota of marine environments, but studies carried out in freshwater environments are still recent.32 The first report of the presence of microplastics in freshwater incloved Canadian lakes and was in 2013.65 Following this discovery, many researchers have focused attention on freshwater environments and organisms, such as the ingestion of microplastics by Squalius cephalus, a freshwater fish species found in rivers around Paris,66 as well as Rutilus rutilus in the Thames River, in London.67 In South America, the presence of microplastics has been progressively reported in various aquatic environments. Pazos et al.68 identified the presence of microplastics in plankton species in the Rio De La Plata estuary, between Paraná and Uruguay, most of which come from effluents from nearby cities. Fish species from an estuary in northern Colombia were investigated and the ingestion and presence of a total of 19 microplastic particles was found inside the stomach of the individuals analyzed.69 In Argentina, the first evidence of PET, PU, PS and PP microplastics was found in the nine of the Patagonian lakes sampled.70 Cabrera et al.71 also observed the presence of microplastics in Andean glaciers and in atmospheric air near the city of Quito in Ecuador. Lebreton et al.72 proposed an annual input of 38,900 tons of plastic per year from the Amazon River into the Atlantic Ocean. It is also estimated that more than a quarter of the plastic discarded in the world's oceans originates in at least 14 of the world's rivers, including rivers in South America such as the Amazon and the Paraná Rivers.72 The status of Brazilian research on microplastics in freshwater environments was observed by Rani-Borges et al.,73 and at least 18 papers have been published throughout the country addressing the topic of microplastics. However, in the Amazon, this number is much lower, with countries such as Venezuela, Suriname and the Guianas that do not yet have research on the theme of microplastics10 11 12,49,51,69,71,74,75,76,77,78 (Table 2). In Brazil, studies have been published on the contamination of the freshwater environments in the basin of the Paraná River,79 where the authors found 704 microplastic particles per m2 in the sediments. In the floodplains of the Pantanal, microfibers accounted for 68% of the microplastics found in the water, followed by 32% of microfragments.80 In the Amazon, the presence of microplastics was identified in sediment samples from the Solimões, Negro and Amazon Rivers. A prevalence of microplastics in fiber format was observed, with sizes ranging between 0.063-5 mm, and the amounts of microplastics found in these sediments were up to 8,178 particles per kilogram of dry sediment.10 In the estuary of the Goiana River, located in the municipality of Goiana, in the state of Pernambuco, 14,724 microplastic particles were identified in a one-year collection period.81 Despite all these studies being conducted in several regions of Brazil, as well as others around the world, there is still a need for more information on the fate of microplastics in different Brazilian biomes including the Amazon basin.
DYNAMICS OF THE RIVERS OF THE AMAZON BASIN The Amazon biome has an area of 7,76 million km2, of which just over 5 million km2 are within Brazilian territory. In this region, the largest river in the world is found, the Amazon River.14 The Amazon biome presents particularities regarding the characterization of water types, as proposed by Sioli et al.,15 such as the blackwaters found, for example, in the Negro River, the whitewaters in the Solimões River and the clearwaters in the Tapajós River. The physicochemical characteristics of each type of water are distinct, with specific pH, ionic composition, transparency, and types of suspended material.82 These features are related to the geological origin of the land that they drain. The Amazon also presents a hydrological dynamism related to flood pulses that vary seasonally and temporally, and which exert a strong influence on the behavior of the biota and the biogeochemical processes in the adjacent floodplain forests.83 In addition, the flood pulse is influenced by the dry and rainy seasons.84 The floodplains include permanent lotic and lentic habitats, as well as areas that are periodically exposed to advancing and retreating floods.83 The Amazon basin is drained by the Amazon River, which discharges approximately 20% of all freshwater entering the oceans worldwide.85 The Amazon River system plays a significant role in the global hydrological cycle since its total river flow is greater than the combined flow of the ten next largest rivers. Recent research suggests that the Amazon River is a significant source of plastic pollution, with an estimated input into the Atlantic Ocean of 32,000-64,000 t per year.72 Although there are estimates regarding the disposal of plastic waste in the region, in Amazonian lakes, for example, studies with microplastics are limited, and there is not enough information that enables us to estimate the concentration of microplastics in these environments.63 de Souza-Vasconcelos (in prep)86 recently investigated the gastrointestinal tract of fish species from an Amazonian lake (Lake Janauacá) and identified the ingestion of microplastics in the form of fibers and threads with different colors and size, thus demonstrating that Amazonian lakes and their biota are exposed to plastic waste.86 In addition to the Amazon basin dynamic, which influences the distribution of plastics, the population of the region contributes to the increase in plastic pollution (Figure 1). The city of Manaus, for example, is located in the central Amazon, near the confluence of the Negro River and the upper Amazon (Solimões) River, and has an estimated population of 2.2 million people.6 There is no record in the literature about the estimated consumption of plastic by the population of the city of Manaus, and there is a lack of studies about the destination of plastics discharged in the industrial district of Manaus.87 The city of Manaus has more than 10 plastics industries, which increases the need to improve studies on the dynamics of microplastic in the region. As plastic pollution increases, so do concerns about the contamination of the environment and its aquatic biota, especially fish. In the Amazon region, the diets of riverine populations rely heavily on protein from fish, and their livelihoods depend on supplying fish to local and regional markets.88,89
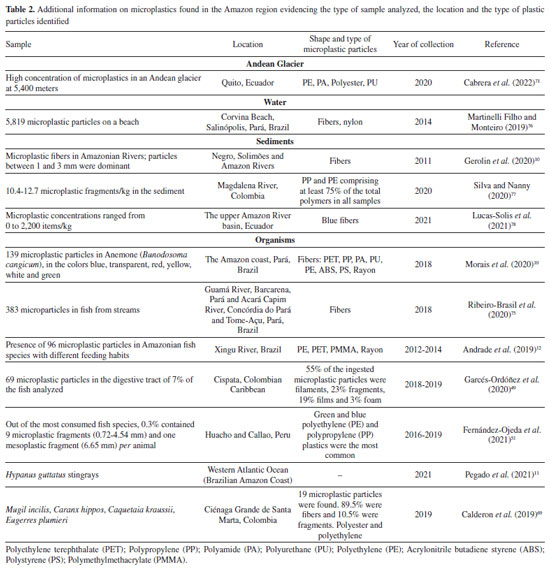
 Figure 1. Contribution of the population to the incorrect disposal of plastic and household plastic waste in the environment, and the consequent transformation of plastic into microplastic, through factors such as UV rays and the physicochemical actions of water
The colonization of the Amazon began on the banks of large rivers such as the Negro and Solimões Rivers. The migratory routes of the populations took place via the rivers and the dwellings were situated next to the water bodies of the region.90 Immense settlements constituted real cities on the banks of the Amazon River, with riverine populations living under the influence of the changes in the water levels.91 The alternation of terrestrial and aquatic phases resulting from the flood pulse, seasonally modify the landscape and led the riverine populations to develop peculiar strategies for living in the region,92 such as the so-called "marombas" that serve as suspended corrals and temporary shelters for man and animals during the flood period, and are also used as an area for the cultivation of perennial plants.93 Riverine populations, which inhabit the banks of rivers, tend to be larger when compared to more inland populations, precisely because of their proximity to water bodies, which act as transport routes, and are used for irrigation, fishing and other means of subsistence.92 With this high population density on the banks of rivers, the accumulation of products, including different types of plastic, discarded directly or indirectly in water bodies, increases. Even in shallow waters, close to large urban centers, as in the case of the cities of Manaus and Belém, microplastics have already been found.10 Microplastics have already been described being found on several Amazonian beaches; and high concentrations (417 to 8,178 microplastic particles per kilogram of dry sediment) have been found.10 The Amazon region has an aggravating factor in terms of pollution, since most of the rivers border and enter large urban centers, either in the capitals or in the cities of the interior. These urban centers end up contributing to plastic pollution due to the growing urban and industrial expansion and, consequently, incorrect waste disposal.94 It is estimated that 80% of the streams in the city of Manaus are affected by anthropogenic pollution.95 This pollution affects the aquatic biota; especially the fish that inhabit these regions and that are part of the economy and allow the subsistence of populations that depend on them.
MICROPLASTICS IN THE AMAZON: INGESTION OF MICROPLASTICS BY FISH The first record of microplastic contamination of fish in the Amazon area occurred in fish sampled in the estuary of the Amazon River. Costa and Barletta81 identified the estuary of the Amazon River as a priority area for future studies on marine plastic pollution as this area is the final collection point for the Amazon basin. Pegado et al.11 hypothesized that the amount and size of particles ingested increases with body size, weight, and vertical trophic position of fish within the estuary food web. They analyzed the digestive tract of 189 fish specimens and categorized the microplastics according to the number and size of ingested microplastic particles and the fish's body size, the fish's weight and its trophic level. A total of 228 microplastic particles were found in the digestive tract of 26 specimens. The microplastics were identified as pellets (97.4%), sheets (1.3%), fragments (0.4%), and threads (0.9%). Plastic particles were transparent, yellow, orange or blue. The predatory crevalle jack fish (Caranx hippos) was the species that ingested most microplastic particles. The findings of Pegado et al.11 indicate significant microplastic ingestion by various fish inhabiting the estuary of the Amazon. Andrade et al.12 reported the ingestion of plastic by fish in the Xingu River, after analyzing the stomach contents of fish of the family Serrasalmidae that have distinct feeding habits. The authors compared the food intake results of the fish based on their diet. The carnivorous group consisted of six species of piranhas; the herbivorous group included five species; and the omnivorous group five species. In total, 96 plastic particles were recovered from stomachs of 46 specimens (26.7%) out of the 172 specimens examined. Omnivorous fish had the highest percentage of occurrence of microplastics, and herbivores had the lowest percentage of occurrence of microplastic particles in their stomachs, though the three trophic groups did not differ significantly in the frequency or magnitude of plastic ingestion. In addition, the author reported concerns about the impact of plastic pollution on the aquatic biota of the Amazon, and the great potential to harm the health and food security of humans who depend on fisheries and other ecosystem services.12 Ribeiro-Brasil et al.75 conducted a study that evaluated the shape, size, and abundance of plastic particles in the gastrointestinal tract and gills of 14 fish species from 12 streams in the eastern Amazon. The fish specimens were collected in the Guamá River basin, in the municipality of Barcarena, and in the Acará-Capim basin, in the municipalities of Ipixuna, Concórdia, and Tomé Açu in eastern Pará, Brazil. The authors observed plastic particles in 67 (98%) out of the 68 individuals analyzed, and recorded a total of 383 plastic particles, of which 201 were located in the gastrointestinal tract and 182 in the gills. The authors concluded that fish of the streams are extremely vulnerable to pollution by microplastic particles and that urbanization is an important contributing factor to the pollution of freshwater environments with plastic waste.75 It is therefore clear that plastic pollution is affecting aquatic biota in the Amazon basin.11,12,75 More specific studies demonstrating the impacts of micro- and nanoplastics on economically and ecologically important fish species and how these plastic pollutants affect the physiology, genetics and reproduction of Amazonian fish species are urgently needed. Recently, in the work of de Souza-Vasconcelos et al. (in prep),86 the presence of microplastic particles was identified in commercial fish species, such as Semaprochilodus taeniurus, Hoplias malabaricus, Triportheus elongatus, and Cichla vazzoleri, which were collected in the Anavilhanas archipelago and Lake Janauacá, both conservation areas. In the 88 fish analyzed, a total of 183 microplastic particles were identified inside the gastrointestinal tract of 54 individuals. The microplastics found were classified as having secondary origin, from the disposal and fragmentation of larger plastics, such as nets and fishing lines, textile materials such as clothing threads, and plastic bags and bottles. The fish analyzed had different eating habits, but there was no difference between the intake of microplastic particles and the trophic level of the species (Figure 2).86
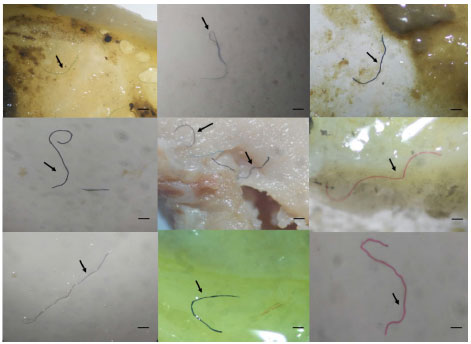 Figure 2. Types of microplastic particles found along the digestive tract of Amazonian fish species with different eating habits, from Lake Janauacá and the Anavilhanas archipelago, Amazonas, Brazil (scale bar: 1 mm) (Vasconcelos de Souza, 2022)86
DYNAMICS OF MICROPLASTICS IN THE AMAZON: AMAZON RIVERS AS SOURCES OF PLASTIC PARTICLES FOR THE OCEANS Rivers are the main entry routes for plastic into the oceans.72 It is estimated that per year about 1.15 to 2.41 million tons of plastic are carried from rivers to the oceans. Regardless of whether the plastics are floating, present in the water column or suspended, all plastics travel towards the oceans. South America has eight rivers that contribute significantly to plastic pollution in the oceans; in Central America, there are eight rivers that contribute significantly, as well as one in Europe, and one hundred and three in Asia.72 Considering the complexity and dynamics of different water bodies in the Amazon, the dynamics of micro- and nanoplastics, needs to be assessed. However, the basin is an important collector and, therefore, carrier of microplastics to the estuary and the ocean.65,89 There is a lack of information on plastic pollution in the main Amazonian rivers (Solimões, Negro and Amazon Rivers) and in the estuary. The Amazon River plume flows into the Western Tropical North Atlantic Ocean (WTNA) near the Equator and is carried north westwards along the Brazilian shelf by the North Brazil Current. This is the main source of freshwater discharge into the continental shelf of the equatorial Atlantic Ocean (Figure 3).96 97 98
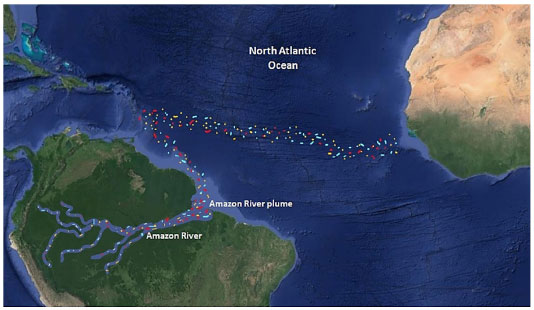 Figure 3. The Amazon River plume carrying microplastics (colored dots) to the Atlantic Ocean source
According to Junk (1997),99 the Amazon basin oscillates between the aquatic and terrestrial phases, which is called the flood pulse. It is the periods of heavy rainfall that are directly associated with the monthly inflow of plastic from rivers into the oceans.72 The rainy season is also associated with a greater amount of microplastics found in the Goiana estuary (Pernambuco), which indicates that the flow of the river is responsible for influencing the flow and speed of transport of microplastics through the estuary until they reach the ocean.81 The runoff of microplastics in the Amazon River estuary, which covers the seasonal rainy periods, is responsible for increasing the flow of rivers and, consequently, the transport of microplastics to the ocean; and this is possibly similar to what is observed in the Goiana estuary. In this way, the Amazon River is considered an important source of plastics, especially for the Tropical North Atlantic Ocean region.98
MICROPLASTICS TRANSPORTED BY AIR MASSES In the environmental dynamics of microplastics, atmospheric transport mechanisms must be also considered. Cabrera et al.71 found that large air masses and high precipitation rates have been contributing to microplastic pollution. The microplastic particles can travel long distances and even be found at high altitudes. It has been suggested that the Amazon biome may be a source of microplastic pollution for the rest of the country and even for neighboring countries in the Amazon region.71 Airborne transport of microplastic particles was first reported by Dris et al.100 when investigating contamination by MPs in urban environments in Paris, and the authors confirmed that atmospheric circulation contributes to the contamination of the freshwater environment by microplastic fibers. The use of synthetic fibers (nylon, polyester and acrylic) by the textile industry directly contributes to the release of microplastics into the atmosphere due to the washing and drying process.101 According to Browne et al.,102 a single garment can release about 1,900 fiber particles per wash. MPs can be released into the atmosphere via low-density polymeric materials that, in urban areas, can accumulate in soil and dust and are easily suspended in the atmosphere by vehicular traffic.103 The use of aerosols, wear of car tires, and modeling using 3D printers are also sources of emission of microplastics in atmosphere.104 105 106 107 Studies have detected the presence of microplastics in remote places. Brahney et al.108 found plastic microfragments of 4 and 188 µm in 98% of airborne dust obtained from protected and uninhabited areas such as the Grand Canyon National Park in the United States.108 It is worth noting that particles of sizes less than 25 µm, which corresponds to 70% of the particles found in this study, are within the size range for long-range and even global dust transportation,109,110 which explains how these microplastics reach areas far from human interference. There are still few studies that analyze possible sources and transport of micro-and nanoplastics into the atmosphere, among which we can mention the work of Wright et al.111 who reported the first evidence of airborne microplastics in London. In this study, microplastics were found in all air samples, ranging from 575 to 1,008 microplastic particles per m2, thus confirming the need to include the air environment as a source and means of transport of microplastic particles. Brahney et al.108 also concluded in their study that the main sources of re-emission of microplastics into the atmosphere are roads (84%), due to tire friction with asphalt, the ocean (11%) due to aerosols generated by marine emissions and agricultural soil (5%), with microplastic particles being transported by dust from agriculture.108 The Amazon region is simultaneously influenced by all these factors; however, analyses of the atmospheric distribution of microplastics in the region do not exist. Atmospheric microplastics have become a global environmental issue that should not be overlooked, though the current studies reveal low sampling levels and analytical consistency.112
MICROPLASTICS PRESENT IN SEDIMENTS Of all the studies with microplastics present in sediments, 33% focused on observing the presence of microplastics in sediments in Brazil.113 Sediments have been identified as one of the main means of capture and storage of microplastic particles for aquatic environments, both saltwater and freshwater.114 Yang et al.115 concluded that microplastics are ubiquitous in the sediment of rivers, lakes, and reservoirs, with the fibers predominating in freshwater environments. High-density microplastics tend to sink and accumulate in these sediments. Low-density microplastics are affected by hydrodynamic processes, such as ocean currents that modify their density, thus facilitating their accumulation in sediments.116 High concentrations of microplastics were found in sediments from different locations such as beaches along the coast of northern Europe,117 the North Sea coast,118 and in samples collected in the Mediterranean Sea and Atlantic Ocean.119 In Brazil, a study by Castro et al.120 found an average of 166.50 microplastic particles per kilo of sediment in samples of beach sediment and 20.74 microplastic particles per kilo of sediment in lower sediment samples, both in Guanabara Bay, Rio de Janeiro.120 According to Olivatto et al.,121 Guanabara Bay is among the most polluted coastal systems in the world. Baptista Neto et al.122 also found microplastics in sediment samples in Vitória Bay in Espírito Santo state. In this study, 247 microplastic particles were analyzed, with concentrations ranging from 0 to 38 particles per sediment sample. In the southern region of Brazil, in the Paranaguá estuarine complex, a recent study, developed in 2022 by Mengatto and Nagail,123 reported an abundance of microplastic particles in sediments of sandy beaches, where 389 microplastic particles per m2 were counted. The findings indicated that almost all beaches in that region, including those located within areas of environmental pollution are contaminated by microplastics.123 In the Amazon region, microplastics were found in sediments of some rivers such as the lower Solimões, lower Negro and upper Amazon rivers. In sand samples from a beach on the Amazon coast, 5,819 microplastic particles were recorded per m2.76 Sant'Anna et al.124 analyzed 30 sediment samples from 5 bathing spots near Itacoatiara, Amazonas state, and registered the presence of 202 microplastic particles per m3. Martinelli Filho and Monteiro76 were the first to evaluate the horizontal and vertical distribution of MPs in the sand of the beach at Salinópolis in the state of Pará, and the MPs occurred in all samples of all strata collected. A total of 5,819 microplastic fragments per m3 were identified, with an average of 492.5 ± 556.4 particles per m3.76
PLASTICS AND CLIMATE CHANGE According to the IPCC (Intergovernmental Panel on Climate Change), climate change is characterized as a significant variation in average climate conditions and/or in their variability, which persist for a long period (decades or more), and which can arise from internal or external natural processes, or persistent anthropogenic changes in the composition of the atmosphere or in land use.125 At the moment, concern about these changes has intensified with the frequent increase in natural phenomena, such as severe droughts and floods, for example.126 In the Amazonian context, climate change has been observed since the 1940s, and data indicate increasingly higher temperatures and reduced precipitation,127 which may generate an imbalance in the relationships between the environment and man.127 For many years, climate change and plastic pollution were discussed separately; however, recently, it has been assumed that both topics are connected.128 According to the latest IPCC report (IR06), greenhouse gas emissions were the highest in human history between 2010 and 2019, across all sectors, including the plastics industry.129 Plastics contribute to the emission of greenhouse gases from the moment they are synthesized to their degradation.128,130 In a study conducted in China, Ren et al.131 performed an analysis of the environmental impacts of the life cycle of plastic according to its different production routes (Table 3),131 and found that 1 kg of PET fiber emits up to 3.9 kg of CO2 into the atmosphere. In addition, it was found that, in 2015 alone, billions of tons of cubic meters of carbon dioxide were released into the environment via the primary production of plastic.132 The impacts of plastics on climate continue after their use and disposal. Thus, depending on how plastic waste is treated, it poses a threat to global warming.130 Recycling, for example, reduces greenhouse gas emissions, but the incineration of one ton of plastic packaging waste contributes about 2.9 tons of CO2.133
Plastic is a polymer, an organic molecule and, when discarded in the environment, decomposes aerobically, releasing greenhouse gases such as CO2 (Figure 4) that is a proxy to evaluate plastic degradation contribution to climate change.134,135 In some cases, anaerobic decomposition also releases other greenhouse gases, not only CO2, but also methane gas (CH4). This release occurs mainly from additives to plastic materials because additives contain soluble carbon compounds, which can be metabolized by methanogenic organisms or sulfate reducers.59,136
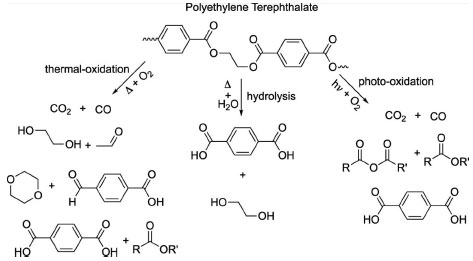 Figure 4. Environmental degradation routes of the polyethylene tetraphthalate (PET) molecule, which gives rise to smaller molecules and releases greenhouse gases. R and R' are polymer chains of variable size (ACS Sustainable Chem. Eng.)59
The rampant and increasing production of plastics, if left unchecked, on its own will contribute to 15% of global greenhouse gases by 2050.137 This is because, at all stages of the plastic's life cycle, gases such as carbon dioxide, methane and a host of other components that contribute to climate change are released.3 Royer et al.44 observed that even after disposal on the surface of oceans, plastics still release gases that are capable of increasing the greenhouse effect, which shows that from obtaining the raw material to its disposal, plastics continue to release greenhouse gases'. Even during their stay in the environment, in the period of degradation, plastics are known to release a wide range of chemicals. This means that the more plastic is produced and accumulated in the environment, the more greenhouse gases, such as methane and ethylene, accumulate in the air, negatively impacting the most diverse levels of living beings.138 However, we know little about the impacts of microplastics on the freshwater environments of the Amazon; environments with equatorial temperatures that have been affected by climate change. Extreme climate change is estimated to cause an increase of up to 6.4 ºC in average temperatures by the end of the century, with variations in the Amazon mesoregions. This rise in ambient temperatures, along with the high incidence of UV rays, are factors that are known to accelerate the degradation of plastics.60 The exposed polymers that make up plastics are routinely affected by high temperatures, UV rays and high humidity,139 and these are ubiquitous conditions in the Amazon region. The effect of high temperatures on plastic accelerates the degradation process of macroplastics into microplastics, and the degradation time may vary depending on the type the polymer.60 There are still no studies in the Amazon region that relate the presence of plastics and climate change. For this reason, there is an urgent need for studies related to this theme to be carried out, considering the particularities of the various Amazonian ecosystems. The Amazon needs to be considered as a priority area that must avoid the impacts of plastic due to the importance of fishing activity for traditional communities and due to it being unique in terms of biodiversity.76
INTERGENERATIONAL DISTRIBUTION OF MICROPLASTICS Recent studies that seek to understand how exposure to micro and nanoplastics can affect the reproductive process of organisms have shown that polystyrene-type nanoplastics can be transferred between generations. Pitt et al.,42 in an experiment with zebrafish exposed to polystyrene nanoplastic particles, observed that eggs and larvae of maternally and coparently exposed fish were contaminated by these nanoplastics. Something similar was also observed in humans by Ragusa et al.140 in which the presence of microplastics was observed in human placentas for the first time, both in maternal, fetal and amniocorial membranes. Of the 6 placentas analyzed, 12 microplastic fragments were found in 4 placentas. The intergenerational consequences of exposure to MPs are beginning to be described. Bringer et al.141 verified the intergenerational effects in oysters (Crassostrea gigas) exposed to MPs in water for a period of two months. The researchers found increased mortality in adults exposed to higher concentrations of MPs. Larvae resulting from the reproductive process of adults exposed to MPs revealed a significant increase in developmental abnormalities, including malformations during development; demonstrating that when parents are exposed to high concentrations of MPs, developmental abnormalities were more frequent in their offspring.141 Luo et al.142 demonstrated that maternal exposure of rats to polystyrene MPs in water affects the fatty acid metabolism of the F1 generation, when the relative expression of genes regulating fatty acid transport (Fabp1, Fatp2), b-oxidation (Ppar-a, Acox, Cpt1-a, Mcad) and acid synthesis (Srebp1c, Fas, Acl, Scd1) significantly decreased in descendants of the groups exposed to polystyrene MPs. It is known that from the moment a nanoparticle enters the blood, plasma proteins adsorb to its surface almost instantly.143 In general, nanoparticles have a certain affinity for proteins, which form a kind of protein crown on the surface of plastic nanoparticles.144,145 The affinity for proteins and the formation of the protein crown on the surface of these nanoparticles may explain how nanoplastics enter and accumulate in organs, as well as explain the mechanism by which the maternal transfer of these materials occurs. To date, there are no studies on intergenerational distribution of MPs and NPs among fish species in the Amazon, evidencing the need for more studies to be developed in the region so that government policies are established, and aquatic species are not further affected.
CONCLUSIONS AND PERSPECTIVES Microplastics and nanoplastics are in fact pollutants of great concern for aquatic environments; not only for the organisms that inhabit them, but also for the populations that depends on them. However, scientific attention has predominantly concentrated on the oceans, with scarce research focused on freshwater environments, especially in the Amazon region, which is an area that has the greatest diversity of freshwater fish in the world, and for which there is still a lack of information about the impacts caused by plastic waste. It is important to highlight that the Amazon region has species with unique characteristics, due to its different aquatic environments (beaches, lakes, streams, rivers, waterfalls), different types of water (black, white, clear) that have different physicochemical properties (temperature, pH, dissolved oxygen), which cause Amazonian species to have particularities and adaptations that vary according to the type of environment they inhabit. It is precisely because of these singularities that future studies should focus on exploring and understanding the behavior of micro- and nanoplastics in these different environments. Thus, it is necessary to develop specific methods for monitoring that suits each environment in the region, in order to describe in detail how exposure to micro- and nanoplastics can affect local organisms and humans. It is noteworthy that, so far, no information has been found on the current state of nanoplastics in the Amazon, thus demonstrating the precariousness of information about these nanoparticles, which are capable of enter intracellular levels and even be transferred between generations. Given the scenarios previously discussed, it is essential to carry out research on micro- and nanoplastics in the region, as well as taking preventive measures, such as environmental education, because the studies that have already been carried out have indicated which points are most vulnerable and susceptible to interaction with micro- and nanoplastics. Additionally, the following aspects should be considered: 1. The countries that are part of the Amazon basin (Brazil, Bolivia, Colombia, Guyana, French Guiana, Peru, Suriname and Venezuela) need to invest in the development of research that involves all the Amazonian microregions and deals with contamination by microand nanoplastics. Maps of the areas that consume and dispose the most plastic waste should be produced, and the most abundant types of plastic particles should be identified. The studies should seek to understand how these contaminants are being transported in the environment, how they contaminate the biota, and how they reach the ocean; 2. There is a need for integration among the Brazilian states, which are part of the Amazon, so that all of them can work simultaneously on policies and actions aimed at scientific research on microplastic particle contamination, as well as the development of actions to reduce plastic pollution; 3. We do not know the consequences of microplastic particles that are transferred through the food chain. Riverside populations that eat fish as their main source of protein may be silently affected by contamination through microplastic particles. We need to know to what extent micro- and nanoplastic particles and the substances that are part of their composition are affecting human populations; 4. Although some countries have laws for waste management, we do not know if the laws are enforced as there is no unique management policy designed specifically for plastic materials in terms of its use and disposal. The available scientific information on how plastic waste is disposed of in countries that are part of the Amazon basin is still limited. This reality must be changed. As microplastics and nanoplastics have been causing damage to fish, they possibly are causing unnoticed negative effects on the human health of the Amazonian riverine population too. In addition, given the flow of the Amazon basin to the Atlantic, which makes up about 20% of all fresh water entering the world's oceans, and the volume of plastics carried to the Atlantic Ocean, a reduction in the contribution of plastic to the rivers of northern South America that form the Amazon basin is essential, as well as studies that monitor and analyze the oceanic distribution of said contribution.
REFERENCES 1. Manzoor, S.; Naqash, N.; Rashid, G.; Singh, R.; Mater. Today: Proc. 2022, 56, 3254. [Crossref] 2. Lima, A. R. A.; Silva, M. D.; Possatto, F. E.; Ferreira, G. V. B.; Krelling, A. P. In Plastics in the Aquatic Environment - Part I: Current Status and Challenges; Stock, F.; Reifferscheid, G.; Brennholt, N.; Kostianaia, E., eds.; Springer International Publishing: Cham, 2022, p. 427. 3. Fundação Heinrich Böll; Atlas do Plástico, 2020. [Link] accessed in April 2023 4. Schlickmann, P. H.; Estudos Geográficos: Revista Eletrônica de Geografia da UNESP 2015, 13, 57. [Link] accessed in April 2023 5. Soto, J.; O Plástico no Planeta: O Uso Consciente Torna o Mundo mais Sustentável, 1ª ed.; Braskem, 2012. 6. Instituto Brasileiro de Geografia e Estatística (IBGE); Amazônia Legal, 2021. [Link] accessed in May 2023 7. Latrubesse, E. M.; Franzinelli, E.; Geomorphology 2005, 70, 372. [Crossref] 8. Bais, A. F.; Lucas, R. M.; Bornman, J. F.; Williamson, C. E.; Sulzberger, B.; Austin, A. T.; Wilson, S. R.; Andrady, A. L.; Bernhard, G.; Mckenzie, R. L.; Aucamp, P. J.; Madronich, S.; Neale, R. E.; Yazar, S.; Young, A. R.; De Gruijl, F. R.; Norval, M.; Takizawa, Y.; Barnes, P. W.; Robson, T. M.; Robinson, S. A.; Ballaré, C. L.; Flint, S. D.; Neale, P. J.; Hylander, S.; Rose, K. C.; Wängberg, S.; Häder, D. P.; Worrest, R. C.; Zepp, R. G.; Paul, N. D.; Cory, R. M.; Solomon, K. R.; Longstreth, J.; Pandey, K. K.; Redhwi, H. H.; Torikai, A.; Heikkilä, A. M.; Photochem. Photobiol. Sci. 2018, 17, 127. [Crossref] 9. Abdelhafidi, A.; Babaghayou, I. M.; Chabira, S. F.; Sebaa, M.; Procedia - Social and Behavioral Sciences 2015, 195, 2922. [Crossref] 10. Gerolin, C. R.; Pupim, F. N.; Sawakuchi, A. O.; Grohmann, C. H.; Labuto, G.; Semensatto, D.; Sci. Total Environ. 2020, 749, 141604. [Crossref] 11. Pegado, T. S. S.; Schmid, K.; Winemiller, K. O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T.; Mar. Pollut. Bull. 2018, 133, 814. [Crossref] 12. Andrade, M. C.; Winemiller, K. O.; Barbosa, P. S.; Fortunati, A.; Chelazzi, D.; Cincinelli, A.; Giarrizzo, T.; Environ. Pollut. 2019, 244, 766. [Crossref] 13. Tisseuil, C.; Cornu, J. F.; Beauchard, O.; Brosse, S.; Darwall, W.; Holland, R.; Hugueny, B.; Tedesco, P. A.; Oberdorff, T.; J. Anim. Ecol. 2013, 82, 365. [Crossref] 14. Val, A. L.; An. Acad. Bras. Cienc. 2019, 91, e20190260. [Crossref] 15. Sioli, H. In The Amazon and its Main Affluents: Hydrography, Morphology of the River Courses, and River Types; Sioli, H., ed.; Springer: Dordrecht, 1984, p. 127. 16. Galgani, F.; Frontiers in Marine Science 2015, 2, 1. [Crossref] 17. Costa, M. F.; Barletta, M.; Environmental Sciences: Processes and Impacts 2015, 11, 1868. [Crossref] 18. Barnes, D. K. A.; Galgani, F.; Thompson, R. C.; Barlaz, M.; Philos. Trans. R. Soc., B 2009, 364, 1985. [Crossref] 19. Li, J.; Liu, H.; Chen, J. P.; Water Res. 2018, 137, 362. [Crossref] 20. Geyer, R.; Jambeck, J. R.; Law, K. L.; Sci. Adv. 2017, 3, 25. [Crossref] 21. Akcelrud, L.; Fundamentos da Ciência dos Polímeros, 1ª ed.; Manole Ltda: Barueri, 2007. 22. Kik, K.; Bukowska, B.; Sicińska, P.; Environ. Pollut. 2020, 262, 114297. [Crossref] 23. Xanthos, D.; Walker, T. R.; Mar. Pollut. Bull. 2017, 118, 17. [Crossref] 24. Ritchie, H.; Roser, M.; Our World in Data 2018. [Link] accessed in May 2023 25. Zhang, K.; Hossein, A.; Tubi, A.; Fang, J. K. H.; Wu, C.; Lam, P. K. S.; Environ. Pollut. 2021, 274, 116554. [Crossref] 26. da Costa, J. P.; Santos, P. S. M.; Duarte, A. C.; Santos, T. R.; Sci. Total Environ. 2016, 566-567, 15. [Crossref] 27. do Nascimento, A. S.; Cavalcante, M. M.; Engema - Encontro Internacional sobre Gestão Empresarial e Meio Ambiente, 2015. [Link] accessed in May 2023 28. https://plasticseurope.org/knowledge-hub/plastics-the-facts-2010/, accessed in April 2023. 29. Magrini, A.; Impactos Ambientais Causados pelos Plásticos: Uma Discussão Abrangente sobre os Mitos e os Dados Científicos, 2ª ed.; E-papers: Rio de Janeiro, 2012. 30. Ipea; Relatório de Pesquisa, 2012. [Link] accessed in May 2023 31. de Aguiar, E. S.; Ribeiro, M. M.; Viana, J. H.; Pontes, A. N.; Urbe 2021, 13, 1. [Crossref] 32. Eerkes-Medrano, D.; Thompson, R. C.; Aldridge, D. C.; Water Res. 2015, 75, 63. [Crossref] 33. Larue, C.; Sarret, G.; Castillo-Michel, H.; Pradas del Real, A. E.; Small 2021, 17, 1. [Crossref] 34. Huang, J. N.; Wen, B.; Zhu, J. G.; Zhang, Y. S.; Gao, J. Z.; Chen, Z. Z.; Sci. Total Environ. 2020, 733, 138929. [Crossref] 35. Pitt, J. A.; Kozal, J. S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E. D.; Di Giulio, R. T.; Aquat. Toxicol. 2018, 194, 185. [Crossref] 36. van Pomeren, M.; Brun, N. R.; Peijnenburg, W. J. G. M.; Vijver, M. G.; Aquat. Toxicol. 2017, 190, 40. [Crossref] 37. Kashiwada, S.; Environ. Health Perspect. 2006, 114, 1697. [Crossref] 38. Huang, J. N.; Wen, B.; Xu, L.; Ma, H. C.; Li, X. X.; Gao, J. Z.; Chen, Z. Z.; J. Hazard. Mater. 2022, 421, 126830. [Crossref] 39. Huang, J. N.; Zhang, Y.; Xu, L.; He, K. X.; Wen, B.; Yang, P. W.; Ding, J. Y.; Li, J. Z.; Ma, H. C.; Gao, J. Z.; Chen, Z. Z.; J. Hazard. Mater. 2022, 424, 127751. [Crossref] 40. Abarghouei, S.; Hedayati, A.; Raeisi, M.; Hadavand, B. S.; Rezaei, H.; Abed-Elmdoust, A.; Chemosphere 2021, 276, 129977. [Crossref] 41. Manabe, M.; Tatarazako, N.; Kinoshita, M.; Aquat. Toxicol. 2011, 105, 576. [Crossref] 42. Pitt, J. A.; Trevisan, R.; Massarsky, A.; Kozal, J. S.; Levin, E. D.; Di Giulio, R. T.; Sci. Total Environ. 2018, 643, 324. [Crossref] 43. Margallo, M.; Ziegler-Rodriguez, K.; Vázquez-Rowe, I.; Aldaco, R.; Irabien, Á.; Kahhat, R.; Sci. Total Environ. 2019, 689, 1255. [Crossref] 44. Valerio, O.; Muthuraj, R.; Codou, A.; Curr. Opin. Green Sustainable Chem. 2020, 25, 100381. [Crossref] 45. Ardusso, M.; Forero-López, A. D.; Buzzi, N. S.; Spetter, C. V.; Fernández-Severini, M. D.; Sci. Total Environ. 2021, 763, 144365. [Crossref] 46. Neto, A. M.; Gomes, T. S.; Pertel, M.; Vieira, L. A. V. P.; Pacheco, E. B. A. V.; Mar. Pollut. Bull. 2021, 172, 112813. [Crossref] 47. http://www.atlas.d-waste.com/, accessed in May 2023. 48. BRASIL. Decreto nº 11.043, de 13 de abril de 2022. [Link] accessed in May 2023 49. Garcés-Ordóñez, O.; Espinosa, L. F.; Cardoso, R. P.; Cardozo, B. B. I.; dos Anjos, R. M.; Environ. Pollut. 2020, 267, 115495. [Crossref] 50. Donoso, J. M.; Rios-Touma, B.; Heliyon 2020, 6, e04302. [Crossref] 51. Fernández-Ojeda, C.; Muniz, M. C.; Cardoso, R. P.; dos Anjos, R. M.; Huaringa, E.; Nakazaki, C.; Henostroza, A.; Garcés-Ordóñez, O.; Mar. Pollut. Bull. 2021, 173, 113039. [Crossref] 52. Cañas, P. V. F.; Zavala, A. R. P.; Matheus, V. C. R.; Generación de Residuos Sólidos Domésticos en la Parroquia Coquivacoa del Municipio Maracaibo, Venezuela. [Link] accessed in May 2023 53. Kee, J.; Wong, H.; Kin, K.; Ho, K.; Tang, D.; Yap, P.; Sci. Total Environ. 2020, 719, 137512. [Crossref] 54. Azizi, N.; Nasseri, S.; Nodehi, R. N.; Jaafarzadeh, N.; Pirsaheb, M.; Mar. Pollut. Bull. 2022, 177, 113462. [Crossref] 55. Bergmann, M.; Gutow, L.; Klages, M.; Marine Anthropogenic Litter, Springer: Heidelberg, 2015. [Link] accessed in April 2023 56. Ekvall, M. T.; Lundqvist, M.; Kelpsiene, E.; Sileikis, E.; Gunnarssos, S. B.; Cedervall, T.; Nanoscale Adv. 2018, 1, 1055. [Crossref] 57. Julienne, F.; Delorme, N.; Lagarde, F.; Chemosphere 2019, 236, 124409. [Crossref] 58. Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T. S.; Mar. Pollut. Bull. 2011, 62, 2588. [Crossref] 59. Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J. H.; Abu-Omar, M.; Scott, S. L.; Suh, S.; ACS Sustainable Chem. Eng. 2020, 8, 3494. [Crossref] 60. Andrady, A. L.; Mar. Pollut. Bull. 2011, 62, 1596. [Crossref] 61. Singh, B.; Sharma, N.; Polym. Degrad. Stab. 2008, 93, 561. [Crossref] 62. Luo, H.; Liu, C.; He, D.; Xu, J.; Sun, J.; Li, J.; Pan, X.; J. Hazard. Mater. 2022, 423, 126915. [Crossref] 63. Hale, R. C.; Seeley, M. E.; La Guardia, M. J.; Mai, L.; Zeng, E. Y.; J. Geophys. Res.: Oceans 2020, 125, 1. [Crossref] 64. Muthukumar, T.; Aravinthan, A.; Lakshmi, K.; Venkatesan, R.; Inter. Biodeterior. Biodegrad. 2011, 65, 276. [Crossref] 65. Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S.; Mar. Pollut. Bull. 2013, 77, 177. [Crossref] 66. Collard, F.; Gasperi, J.; Gilbert, B.; Eppe, G.; Azimi, S.; Rocher, V.; Tassin, B.; Sci. Total Environ. 2018, 643, 1257. [Crossref] 67. Horton, A. A.; Jürgens, M. D.; Lahive, E.; van Bodegom, P. M.; Vijver, M. G.; Environ. Pollut. 2018, 236, 188. [Crossref] 68. Pazos, R. S.; Bauer, D. E.; Gómez, N.; Environ. Pollut. 2018, 243, 134. [Crossref] 69. Calderon, E. A.; Hansen, P.; Rodríguez, A.; Blettler, M. C. M.; Syberg, K.; Khan, F. R.; Water, Air, Soil Pollut. 2019, 230, 257. [Crossref] 70. Alfonso, M. B.; Scordo, F.; Seitz, C.; Mavo Manstretta, G. M.; Ronda, A. C.; Arias, A. H.; Tomba, J. P.; Silva, L. I.; Perillo, G. M. E.; Piccolo, M. C.; Sci. Total Environ. 2020, 733, 139385. [Crossref] 71. Cabrera, M.; Moulatlet, G. M.; Valencia, B. G.; Maisincho, L.; Rodríguez-Barroso, R.; Albendín, G.; Sakali, A.; Lucas-Solis, O.; Conicelli, B.; Capparelli, M. V.; Sci. Total Environ. 2022, 805, 150334. [Crossref] 72. Lebreton, L. C. M.; Van Der Zwet, J.; Damsteeg, J. W.; Slat, B.; Andrady, A.; Reisser, J.; Nat. Commun. 2017, 8, 1. [Crossref] 73. Rani-Borges, B.; Martins, T. F. G.; Pompêo, M.; Pan-American Journal of Aquatic Sciences 2021, 16, 106. [Link] accessed in May 2023 74. Morais, L. M. S.; Sarti, F.; Chelazzi, D.; Cincinelli, A.; Giarrizzo, T.; Martinelli Filho, J. E.; Environ. Pollut. 2020, 265, 114817. [Crossref] 75. Ribeiro-Brasil, D. R. G.; Torres, N. R.; Picanço, A. B.; Sousa, D. S.; Ribeiro, V. S.; Brasil, L. S.; Montag, L. F. A.; Environ. Pollut. 2020, 266, 115241. [Crossref] 76. Martinelli Filho, J. E.; Monteiro, R. C. P.; Mar. Pollut. Bull. 2019, 145, 219. [Crossref] 77. Silva, P. M.; Nanny, M. A.; Water (Switzerland) 2020, 12, 1210. [Crossref] 78. Lucas-Solis, O.; Moulatlet, G. M.; Guamangallo, J.; Yacelga, N.; Villegas, L.; Galarza, E.; Rosero, B.; Zurita, B.; Sabando, L.; Cabrera, M.; Gimiliani, G. T.; Capparelli, M. V.; Bull. Environ. Contam. Toxicol. 2021, 107, 45. [Crossref] 79. Blettler, M. C. M.; Ulla, M. A.; Rabuffetti, A. P.; Garello, N.; Environ. Monit. Assess. 2017, 189, 581. [Crossref] 80. de Faria, E.; Girard, P.; Nardes, C. S.; Moreschi, A.; Christo, S. W.; Ferreira Junior, A. L.; Costa, M. F.; Case Stud. Chem. Environ. Eng. 2021, 3, 10088. [Crossref] 81. Lima, A. R. A.; Costa, M. F.; Barletta, M.; Environ. Res. 2014, 132, 146. [Crossref] 82. Stallard, R. F.; Edmond, J. M.; J. Geophys. Res.: Oceans 1983, 88, 9671. [Crossref] 83. Junk, W. J.; Bayley, P. B.; Sparks, R. E.; Can. J. Fish. Aquat. Sci. 1989, 106, 110. [Link] accessed in May 2023 84. Junk, W. J.; Piedade, M. T. F.; Lourival, R.; Wittmann, F.; Kandus, P.; Lacerda, L. D.; Bozelli, R. L.; Esteves, F. A.; da Cunha, C. N.; Maltchik, L.; Definição e Classificação das Áreas Úmidas (AUs) Brasileiras: Base Científica para uma Nova Política de Proteção e Manejo Sustentável, edição eletrônica; EdUFMT: Cuiabá, 2014. [Link] accessed in May 2023 85. Franzinelli, E.; Potter, P. E.; The Journal of Geology 1983, 91, 23. [Crossref] 86. de Souza-Vasconcelos, M. T.: Ingestão e Efeitos Morfofisiológicos dos Microplásticos em Espécies de Peixes da Amazônia Central; Dissertação de Mestrado, Instituto Nacional de Pesquisas da Amazônia, Manaus, Brasil, 2022. [Link] accessed in June 2023 87. Oliveira, M.; Lopes, I.; Rodrigues, C.; Procedia CIRP 2016, 52, 157. [Crossref] 88. da Silva, A. L.; Begossi, A.; Environment, Development and Sustainability 2009, 11, 489. [Crossref] 89. Begossi, A.; Salivonchyk, S. V.; Hallwass, G.; Hanazaki, N.; Lopes, P. F. M.; Silvano, R. A. M.; Pittock, J.; Brazilian Journal of Biology 2018, 79, 345. [Crossref] 90. Porro, A.; O Povo das Águas: Ensaios de Etno-História Amazônica, 1ª ed.; Vozes: Petrópolis, 1996. 91. Melo Júnior, L. C. M.; Sayago, D. A. V.; Tourinho, M. M.; Fronteiras: Journal of Social, Technological and Environmental Science 2018, 7, 265. [Crossref] 92. Fraxe, T. J. P.; Homens Anfíbios: Etnografia de um Campesinato das Águas, 2ª ed.; Annablume: São Paulo, 2001. 93. Sternberg, H. O.; A Água e o Homem na Várzea do Careiro, 2ª ed.; MPEG: Belém, 1998. 94. Ferreira, S. J. F.; Miranda, S. A. F.; Marques Filho, A. O.; Silva, C. C.; Acta Amazonica 2012, 42, 533. [Crossref] 95. Couceiro, S. R. M.; Hamada, N.; Luz, S. L. B.; Forsberg, B. R.; Pimentel, T. P.; Hydrobiologia 2007, 575, 271. [Crossref] 96. Muller-Karger, F. E.; McClain, C. R.; Richardson, P. L.; Nature 1988, 333, 56. [Crossref] 97. Salisbury, J.; Vandemark, D.; Campbell, J.; Hunt, C.; Wisser, D.; Reul, N.; Chapron, B.; J. Geophys. Res.: Oceans 2011, 116, 1. [Crossref] 98. Schmidt, N.; Fauvelle, V.; Ody, A.; Castro-Jiménez, J.; Jouanno, J.; Changeux, T.; Thibaut, T.; Sempéré, R.; Environ. Sci. Technol. 2019, 53, 7513. [Crossref] 99. Junk, W. J. In General Aspects of Floodplain Ecology with Special Reference to Amazonian Floodplains; Caldwell, M. M.; Heldmaier, G.; Lange, O. L.; Mooney, H. A.; Schulze, E. D.; Soomer, U., eds.; Springer: Plon, 1997, p. 3. 100. Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B.; Mar. Pollut. Bull. 2016, 104, 290. [Crossref] 101. Cesa, F. S.; Turra, A.; Baruque-Ramos, J.; Sci. Total Environ. 2017, 598, 1116. [Crossref] 102. Browne, M. A.; Dissanayake, A.; Galloway, T. S.; Lowe, D. M.; Thompson, R. C.; Environ. Sci. Technol. 2008, 42, 5026. [Crossref] 103. Abbasi, S.; Rezaei, M.; Ahmadi, F.; Turner, A.; Chemosphere 2022, 292, 133456. [Crossref] 104. Kole, P. J.; Löhr, A. J.; Van Belleghem, F. G. A. J.; Ragas, A. M. J.; Int. J. Environ. Res. Public Health 2017, 14, 1265. [Crossref] 105. Zhang, Y.; Gao, T.; Kang, S.; Sillanpää, M.; Environ. Pollut. 2019, 254, 112953. [Crossref] 106. Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M.; Earth-Sci. Rev. 2020, 203, 103118. [Crossref] 107. Zhang, H.; Estuarine, Coastal Shelf Sci. 2017, 199, 74. [Crossref] 108. Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S.; Science 2020, 368, 1257. [Crossref] 109. Betzer, P. R.; Carder, K. L.; Duce, R. A.; Merrill, J. T.; Tindale, N. W.; Uematsu, M.; Costello, D. K.; Young, R. W.; Feely, R. A.; Breland, J. A.; Bernstein, R. E.; Greco, A. M.; Nature 1988, 336, 568. [Crossref] 110. Mahowald, N.; Albani, S.; Kok, J. F.; Engelstaeder, S.; Scanza, R.; Ward, D. S.; Flanner, M. G.; Aeolian Research 2014, 15, 53. [Crossref] 111. Wright, S. L.; Ulke, J.; Font, A.; Chan, K. L. A.; Kelly, F. J.; Environ. Int. 2020, 136, 105411. [Crossref] 112. Yuan, Z.; Li, H. X.; Lin, L.; Pan, Y. F.; Liu, S.; Hou, R.; Xu, X. R.; Gondwana Res. 2022, 108, 200. [Crossref] 113. Fernandes, A.; Bertoldi, C.; Lara, L.; Stival, J.; Alves, N.; Cabrera, P.; Grassi, M.; J. Braz. Chem. Soc. 2022, 33, 303. [Crossref] 114. Cózar, A.; Echevarría, F.; González-Gordillo, J. I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, A. T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; Fernández-de-Puelles, M. L.; Duarte, C. M.; Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 10239. [Crossref] 115. Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C.; Sci. Total Environ. 2021, 780, 146546. [Crossref] 116. Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C. R.; Mar. Environ. Res. 2015, 111, 5. [Crossref] 117. Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C. R.; Mar. Pollut. Bull. 2011, 62, 2199. [Crossref] 118. Karlsson, T. M.; Vethaak, A. D.; Almroth, B. C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H. A.; Mar. Pollut. Bull. 2017, 122, 403. [Crossref] 119. Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C. R.; Environ. Pollut. 2013, 182, 495. [Crossref] 120. Castro, R. O.; Silva, M. L.; Marques, M. R. C.; de Araújo, F. V; Mar. Pollut. Bull. 2016, 110, 555. [Crossref] 121. Olivatto, G. P.; Martins, M. C. T.; Montagner, C. C.; Henry, T. B.; Carreira, R. S.; Mar. Pollut. Bull. 2019, 139, 157. [Crossref] 122. Baptista Neto, J. A.; Gaylarde, C.; Beech, I.; Bastos, A. C.; da Silva-Quaresma, V.; de Carvalho, D. G.; Ocean & Coastal Management 2019, 169, 247. [Crossref] 123. Mengatto, M. F.; Nagai, R. H.; Mar. Pollut. Bull. 2022, 177, 113530. [Crossref] 124. Sant'Anna, B.; Oliveira, L. G.; Hattori, G. Y.; Research Square 2022, 1, 1. [Crossref] 125. Beck, S.; Mahony, M.; WIREs Climate Change 2018, 9, 1. [Crossref] 126. Nahur, A. C.; Lutes, M. W.; Ribeiro, L. P.; As Mudanças Climáticas: Riscos e Oportunidades, 2015. [Link] accessed in May 2023 127. Marengo, J. A.; Souza Junior, C.; Mudanças Climáticas: Impactos e Cenários para a Amazônia, São Paulo, 2018. [Link] accessed in May 2023 128. Ford, H. V.; Jones, N. H.; Davies, A. J.; Godley, B. J.; Jambeck, J. R.; Napper, I. E.; Suckling, C. C.; Williams, G. J.; Woodall, L. C.; Koldewey, H. J.; Sci. Total Environ. 2022, 806, 150392. [Crossref] 129. Careta, M. A.; Mukherji, A.; Arfanuzzaman, M.; Betts, R. A.; Gelfan, A.; Hirabayashi, Y.; Lissner, T. K.; Liu, J.; López-Gunn, E.; Morgan, R.; Mwanga, S.; Supratid, S.; Araos, M.; Balasubramanya, S.; Brackel, A. K. C.; Caesar, J.; Caggiano, H. B.; Cook, B.; Dockendorff, C.; Yashoda, Y. In Climate Change: Impacts, Adaptation, and Vulnerability; Pörtner, H. O.; Roberts, D. C.; Tignor, M.; Poloczanska, E. S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; Okem, A.; Rama B., eds.; Cambridge University Press: Cambridge, 2022, ch. 4. [Link] accessed in May 2023 130. Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y.; J. Cleaner Prod. 2020, 254, 120138. [Crossref] 131. Ren, Y.; Shi, L.; Bardow, A.; Geyer, R.; Suh, S.; Resour., Conserv. Recycl. 2020, 156, 104699. [Crossref] 132. Geyer, R. In Plastic Waste and Recycling; Letcher, T. M., ed.; Elsevier: Cambridge, 2020, p. 13-32. [Crossref] 133. Hamilton, L. A.; Feit, S.; Muffet, C.; Kelso, M.; Rubright, S. M.; Bernhard, C.; Schaeffer, E.; Moon, D.; Morris, J.; Labbé-Bellas, R. In Plastic & Climate: The Hidden Costs of a Plastic Planet; Kistler, A.; Muffet, C., eds.; Ciel: Washington, 2019. [Link] accessed in May 2023 134. Lahvis, M. A.; Baehr, A. L.; Water Resour. Res. 1996, 32, 2231.[Crossref] 135. Kijchavengkul, T.; Kale, G.; Auras, R.; ACS Symp. Ser. 2009, 1004, 31. [Crossref] 136. Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M.; Int. J. Mol. Sci. 2009, 10, 3824. [Crossref] 137. World Economic Forum; Ellen MacArthur Foundation, Switzerland, 2016. [Link] accessed in May 2023 138. Royer, S. J.; Ferron, S.; Wilson, S. T.; Karl, D. M.; PLoS One 2018, 13, e0200574. [Crossref] 139. Pospísil, J.; Pilař, J.; Billingham, N. C.; Marek, A.; Horák, Z.; Nespůrek, S.; Polym. Degrad. Stab. 2006, 91, 417. [Crossref] 140. Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M. C. A.; Baiocco, F.; Draghi, S.; D'Amore, E.; Rinaldo, D.; Matta, M.; Giorgini, E.; Environ. Int. 2021, 146, 106274. [Crossref] 141. Bringer, A.; Cachot, J.; Dubillot, E.; Prunier, G.; Huet, V.; Clérandeau, C.; Evin, L.; Thomas, H.; Environ. Pollut. 2022, 294, 118600. [Crossref] 142. Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y.; Environ. Pollut. 2019, 255, 113122. [Crossref] 143. Hadjidemetriou, M.; Kostarelos, K.; Nat. Nanotechnol. 2017, 12, 288. [Crossref] 144. Jiang, X.; Weise, S.; Hafner, M.; Rocker, C.; Zhang, F.; Parak, W. J.; Nienhaus, G. U.; J. R. Soc., Interface 2009, 7, S5. [Crossref] 145. Walkey, C. D.; Chan, W. C. W.; Chem. Soc. Rev. 2012, 41, 2780. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access