Artigo
| Sensitive spectrophotometric determination of triprolidine hydrochloride drug in the capsules via ion pair complex formation |
|
Sameer A. M. AbdulrahmanI,II; Jalal Ali Mohammed QazzanII
I. Department of Chemistry, Faculty of Education and Sciences-Rada'a, Albaydha University, Albaydha, Yemen Recebido em 21/12/2022 *e-mail: sameeralromima@gmail.com The objective of this study was to develop two simple and selective visible spectrophotometric methods for the determination of antihistamine drug triprolidine hydrochloride (TRH) in the capsules. The methods were based on the formation of ion-pair complexes between TRH and two dyes, namely, bromocresol green (BCG) and bromophenol blue (BPB). The produced ion-pair complexes were measured at 415 and 410 nm for BCG and BPB methods, respectively. Beer's law was applicable in the concentration ranges of 2.50-15.0 µg mL-1 TRH for both methods. The molar absorptivity values were found to be 2.12 × 104 and 2.07 × 104 L mol-1 cm-1 for the BCG method and BPB method, respectively, and the Sandell's sensitivity values were 0.0149 and 0.0152 µg cm-2. The limits of detection and quantification were calculated and found to be 0.29 and 0.86 µg mL-1 for the BCG method and 0.31 and 0.95 µg mL-1 for the BPB method. The methods were applied successfully for TRH determination in bulk drug and in the capsules. INTRODUCTION Triprolidine hydrochloride (TRH), chemically known as (E)-2-(3-Pyrrolidin-1-yl-1-p-tolylprop-1-enyl)pyridine hydrochloride and its chemical structure was given in Figure 1.1 TRH is a potent histamine H1-receptor antagonist (H1-blocker) and it has a quick start and long-lasting action, up to about 12 hours.2
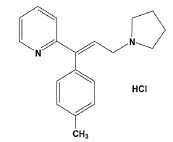 Figure 1. The chemical structure of triprolidine hydrochloride
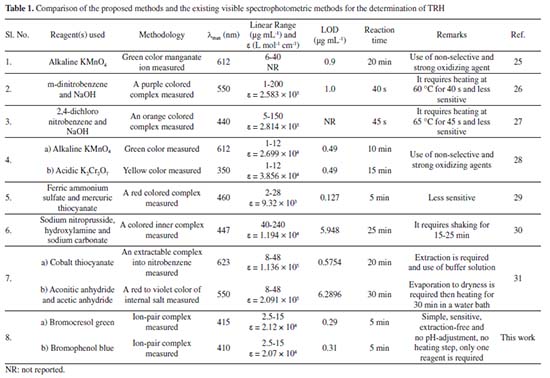
A literature survey related to the quantitative analysis of TRH revealed that several studies have been reported for the determination of TRH, either alone or in combination with other drugs, in pharmaceutical formulations. These studies include high performance liquid chromatography (HPLC),3-10 ultra high performance liquid chromatography (UHPLC),11 thin layer chromatography (TLC),12 electroanalytical methods,13-17 chemiluminescence method combined with flow injection analysis (FIA),18 capillary zone electrophoresis (CZE),19 ultraviolet (UV) spectroscopy4-6,20-24 and visible spectroscopy.25-31 Visible spectrophotometry, due to its simplicity, cost effectiveness, sensitivity, selectivity, and easy access in many quality control laboratories, has been widely used in pharmaceutical analysis. The importance of the spectrophotometric methods lies in the nature of chemical reaction(s) upon which the procedures are based rather than upon the sophistication of the instrument. Many reactions involving the formation of the color for a particular drug are quite selective or can be rendered selective through the optimization of some conditions. Although the previously developed methods can be used, most of them require expensive instruments or substances, and many laboratories in under-developing countries cannot afford to purchase and maintain these instruments. The UV spectrophotometric methods have limitations since they may suffer interference from the excipients used in pharmaceutical formulations when determining the active drug materials. Also, many of the previously developed visible spectrophotometric methods suffered from some disadvantages like the use of non-selective and strong oxidizing agents,25,28 heating step,26,27,31 less sensitivity,26,27,29 shaking for long time30 and extraction step31 as explained in Table 1.
Connors32 reported that the acid-dye technique, for the colorimetric determination of amines based on the formation of the ion-pair complex, is an important analytical application in pharmaceutical analysis. This technique can be done either based on the extraction of the formed ion-pair complex into an organic solvent and measuring its absorbance or based on extraction-free ion-pair complex formation between the drug and the acidic dye. The acidic dyes bromocresol green (BCG) and bromophenol blue (BPB) have been widely used as an ion-pairing reagent for the assay of many drugs that contain basic nitrogen atoms such as dothiepin hydrochloride33 and pentoxyverine citrate.34 The present study describes the use of BCG and BPB dyes for the first time in the spectrophotometric determination of the TRH drug. The main objective of the present work was to develop simple, selective and extraction-free visible spectrophotometric methods for the determination of TRH in pure drug and in the capsules. The methods were based on the formation of ion-pair complexes between TRH and two acidic dyes, namely, bromocresol green and bromophenol blue with maximum absorbance at 415 and 410 nm, respectively. The absorbance of the yellow colored ion-pair complexes in both methods was measured directly in dichloromethane emphasizing the simplicity of the proposed methods.
EXPERIMENTAL Instrument A visible spectrophotometer model V-1000 (AOE Instruments, Shanghai, Co. Ltd., China) provided with 1 cm matched quartz cells was used for measuring the absorbance. Chemicals and reagents All reagents and solvents used throughout this study were of analytical reagent grade. Solutions: 0.5% w/v bromocresol green (BCG) solution was prepared in dichloromethane (DCM), 0.5% w/v bromophenol blue (BPB) solution was prepared in DCM. Pharmaceutical grade TRH, which is certified to be 99.8% pure was received from the Shiba Pharma Company for Pharmaceutical Industries, Sana'a, Yemen, as a gift and was used as received. The commercial sample of the TRH drug chosen for determining TRH in capsules by the developed methods was triprolidine hydrochloride capsules-2.5 mg per capsule which is marketed by the United Laboratoriers Co. Ltd., Hong Kong, China. Standard TRH solution A standard TRH solution (200 µg mL-1) was prepared by dissolving 20 mg of pure TRH in DCM and diluting the solution to the mark in a 100 mL volumetric flask with DCM. A suitable dilution of this standard solution was done to prepare a working solution of 50 µg mL-1 TRH using DCM. Procedures Assay procedure of TRH using BCG Different volumes (0.5-3.0 mL) of a standard 50 µg mL-1 TRH solution were taken in a series of 10 mL volumetric flasks, and the total volume was adjusted to 3.0 mL by adding DCM. One milliliter of 0.5% w/v BCG solution was added to each flask. After 5 min, each flask was made up to the mark with DCM and mixed well. The absorbance of all solutions was measured at 415 nm versus a reagent blank. Assay procedure of TRH using BPB Different aliquots (0.5-3.0 mL) of a standard TRH solution (50 µg mL-1) were taken in a series of 10 mL volumetric flasks and the total volume was adjusted to 3.0 mL by adding DCM. A 1.5 mL of 0.5% w/v BPB solution was added to each flask. After 5 min, the flasks were made up to the mark with DCM and mixed well. The absorbance of these solutions was measured at 410 nm versus a reagent blank. Assay procedure for pharmaceutical formulations The content of twelve triprolidine hydrochloride capsules-2.5 mg TRH was weighed and an exactly weighed quantity equivalent to 5 mg of TRH was transferred into a 100 mL volumetric flask containing 50 mL of DCM. The content of the flask was shaken for about 15 min then the flask was diluted up to the mark with DCM. The contents were mixed well and filtered using a Whatman No. 42 filter paper. Suitable portions of the filtrate (50 µg mL-1 TRH) were used for analysis by the assay procedure using BCG and BPB methods as described earlier. Procedures for method validation The current ICH guidelines35 were used to validate the developed methods, and include linearity, limits of detection (LOD), limits of quantification (LOQ), precision, accuracy, robustness, and selectivity. Linearity was evaluated by analyzing a set of six standard solutions (0.5; 1.0; 1.5; 2.0; 2.5 and 3.0 mL of 50 µg mL-1 TRH solution) and the calibration curves were prepared as described under "assay procedure using BCG and BPB". The linearity of both methods was determined by plotting the absorbance against the concentration of TRH drug. The LOD (Equation 1) and LOQ (Equation 2) values for the developed methods were calculated using the following equations:36  where Sblank is the standard deviation of replicate blank absorbance (n = 6), and b is the slope of the calibration curve. The precision and accuracy of the developed methods were determined by preparation of solutions containing three different concentrations of TRH and analyze these solutions in seven replicates in the same day (intra-day) and five successive days (inter-day). The precision and accuracy of the developed methods were calculated in terms of percentage relative standard deviation (%RSD) and percentage relative error (%RE), respectively. The evaluation of the method robustness was accomplished by making small changes in some parameters, namely, volume of the dye (0.9, 1.0 and 1.1 mL for BCG method; 1.4, 1.5 and 1.6 mL for BPB method) and the reaction time (4, 5 and 6 min in both methods) and carrying out the analysis under the optimized experimental conditions followed by the calculation of %RSD values. To study the selectivity of the developed methods, a placebo blank was prepared by mixing known amounts of commonly employed excipients such as talc, starch, lactose, glucose, sodium citrate, acacia and gelatin and transferring this mixture into a 100 mL volumetric flask containing 50 mL of DCM. The solution was made as described under "Assay procedure for pharmaceutical formulations". A suitable aliquot of the filtrate was used for analysis by both methods following the assay procedures. To the placebo blank of the similar composition described above, 5 mg of pure TRH was added and mixed properly. A 50 µg mL-1 TRH solution of the synthetic mixture was also prepared as described under "Assay procedure for pharmaceutical formulations". The filtrate was collected in a 100 mL volumetric flask and 1.5 mL of the resulted solution (50 µg mL-1 TRH) was analyzed five times by both methods. Stoichiometric relationship To establish the stoichiometry of the formed ion-pair complexes in both methods between TRH and BCG or BPB, Job's method of continuous variations37 was used. In both methods, a 1.59 × 10-4 mol L-1 solution of TRH and 1.59 × 10-4 mol L-1 solution of BCG or BPB dyes were prepared by dissolving the calculated amount of drug and either dye in DCM. A series of solutions were prepared in different ratios of drug and BCG or BPB keeping the total volume of each mixture at 10 mL. The absorbance of all solutions was measured individually after 5 min at the respective wavelengths versus DCM. Procedures for optimization of reaction conditions The reaction conditions were optimized for the quantitative estimation of the yellow colored ion-pair complexes. These conditions included the selection of the organic solvent, the concentration of the dye and the reaction time. To select the suitable solvent for preparation of dye solutions and using the same solvent as a diluting solvent, different solvents, namely, DCM, chloroform and 1,2-dichloroethane were used with a fixed concentration of 10 µg mL-1 TRH in DCM for BCG and BPB methods. Then, the reaction of TRH with both dyes was carried out separately using these solvents followed by measuring the absorbance of each solution after 5 min at the maximum wavelength versus the corresponding blank. The effect of concentration of both dyes on the absorbance of the resulting ion-pair complexes was studied using a fixed concentration of 10 µg mL-1 TRH solution and different volumes ranged from 0.25 mL to 2.5 mL of 0.5% BCG solution and 0.5% BPB solution, separately. The optimum reaction time was determined by the addition of 1 mL of BCG solution or 1.5 mL of BPB solution to a fixed concentration of 7.5 µg mL-1 TRH solution. The reaction between the TRH and either dye was allowed to occur at different times (0; 5; 10; 15 and 20 min) followed by measuring the absorbance of the formed ion-pair complexes in a total volume of 10 mL at room temperature.
RESULTS AND DISCUSSION Absorption spectra The reaction of TRH drug with BCG or BPB dyes forms intensely yellow-colored products. The absorption spectra of the formed ion-pair complexes were recorded at 380-500 nm versus the corresponding blank solutions and the results are shown in Figure 2. The maximum absorbance of the formed ion-pair complexes was observed at 415 and 410 nm for BCG method and BPB method, respectively. All the absorbance measurements were made at these wavelengths.
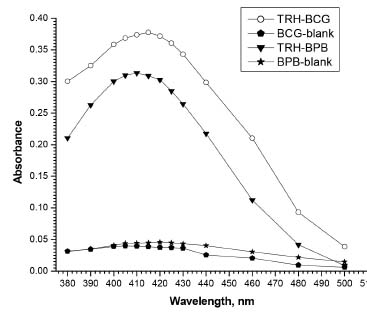 Figure 2. Absorption spectra of TRH-BCG and TRH-BPB ion-pair complexes (TRH 10 μg mL-1) against the corresponding blank
Reaction mechanism The TRH drug forms ion-pair complexes with both dyes, i.e. BCG and BPB. TRH drug contains tertiary amino group that can be protonated in the hydrochloride salt of this drug. In TRH, the protonation is very difficult in the pyridine ring (six-membered ring) because the lone pair of electrons presented on nitrogen of pyridine is involved in resonance. The nitrogen of pyrrolidine ring (five-membered ring) is considerably more basic than the pyridine nitrogen which is a part of an aromatic ring.38 Therefore, the site in TRH suitable for protonation is the nitrogen atom of the five-membered pyrrolidine ring. BCG and BPB are dyes of sulfonephthalein type and their colors are due to the opening of the lactoid ring and formation of the chromophore, i.e. quinoid group.39 The two tautomers are supposed to be present in equilibrium, but because of the strongly acidic nature of sulphonic acid group, the quinoid tautomer should predominate. At the end, the protonated TRH will forms TRH-BCG and TRH-BPB ion-pair complexes with the anions of these dyes. The possible reaction mechanism is presented in Figure 3.
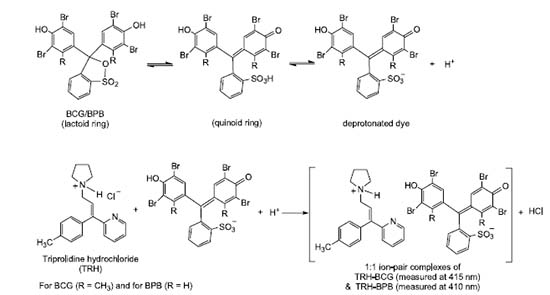 Figure 3. The possible reaction mechanism for the formation of TRH-BCG and TRH-BPB ion-pair complexes
Reaction conditions optimization Effect of organic solvents The results of this study showed that the solvent 1,2- dichloroethane could not be used for preparation of dye's solutions and as a diluting solvent since there is no significant difference between the absorbance of the sample and the blank with this solvent (Figure 4). DCM and chloroform were found appropriate solvents for preparation of dye's solutions and as a diluting solvent, but the former (DCM) was preferred because of its availability.
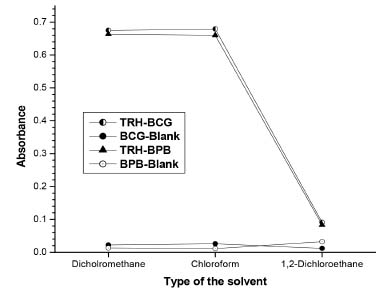 Figure 4. Effect of organic solvents on the color development (10 μg mL-1 TRH)
Effect of dye concentration The results showed that maximum color development of the ion-pair complexes was attained with 1 mL of BCG solution and 1.5 mL of BPB solution and the absorbance of the formed complexes was not affected by excess dyes (Figure 5).
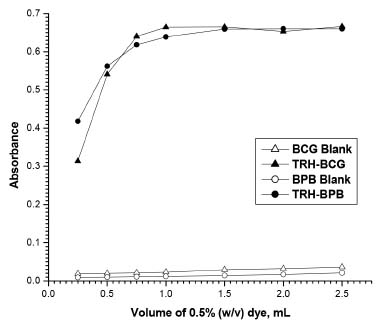 Figure 5. Effect of dyes concentrations on the color development of the formed ion-pair complexes (10 μg mL-1 TRH)
Effect of reaction time The addition of the dye's solution to TRH solution causes the development of the color immediately, and the reaction between TRH and BCG or BPB dye was found to be complete in 5 min (Figure 6). The yellow color of the formed ion-pair complexes was stable for at least 30 min with both dyes.
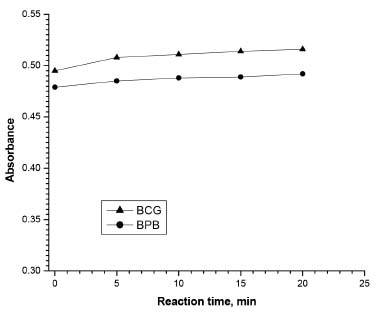 Figure 6. Effect of reaction time between TRH (7.5 μg mL-1) and BCG and BPB dyes
Composition of the formed complexes Job's continuous variations graph for the reaction between TRH and BCG or BPB shows that the interaction occurs on an equimolar basis via the formation of ion-pair complexes. The measured absorbance of each solution was plotted against the mole fraction of TRH, VTRH/(VTRH + Vdye), as shown in Figure 7. The plot reached a maximum value at a mole fraction of 0.5 which indicated that the stoichiometric ratio for each of the formed ion-pair complexes was 1:1 (TRH:BCG and TRH:BPB). The conditional stability constants (Kf) of the formed ion-pair complexes were calculated40 and found to be 1.02 × 107 and 1.87 × 107 for TRH:BCG and TRH:BPB complexes, respectively.
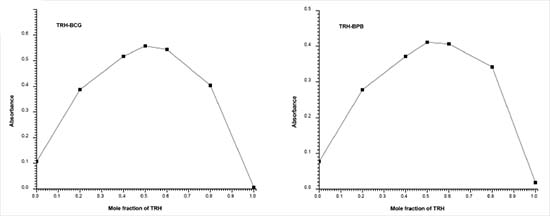 Figure 7. The Job's method of continuous variation plots for the formed (TRH-BCG & TRH-BPB) ion-pair complexes
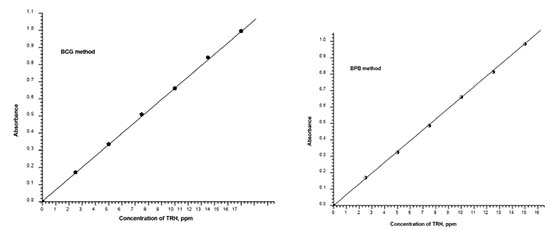
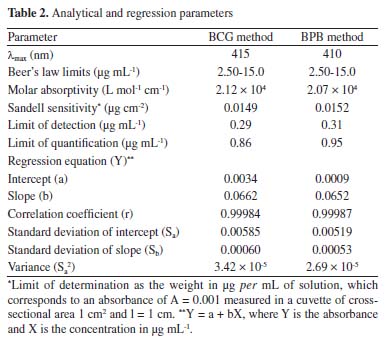
Method validation Linearity Under the optimized experimental conditions for the determination of TRH, the plots of absorbance versus concentration were constructed and found to be linear (Figure 8). Regression equations were written and Beer's law was obeyed over the concentration ranges given in Table 2. Many analytical parameters such as molar absorptivity, Sandell's sensitivity values were given in Table 2 and showed that the developed methods were sensitive.
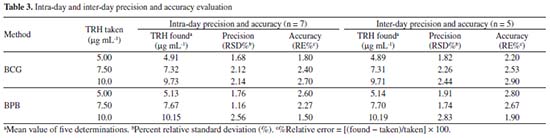
Precision and accuracy The percent relative standard deviation values (RSD%) were presented in Table 3 and found to be less than or equal to 2.56% (intra-day) and 2.83% (inter-day) which indicated the high precision of the developed methods. Also, the results of accuracy study were presented in Table 3 and showed that the accuracy of both methods was good (RE% ≤ 2.90%).
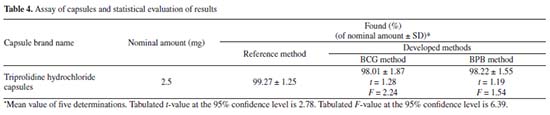
Selectivity The selectivity of the developed methods was studied using both placebo blank and synthetic mixture analyses. The solutions of both placebo blank and synthetic mixture were prepared as mentioned under the experimantal part "Procedures for method validation". The resulting extract of placebo blank was analyzed and the measured absorbance was as same as reagent blank, which concludes that the employed excipients didn't interfere with the assay using the developed methods. Upon analysis of the resulting extract of synthetic mixture, percent recoveries of TRH were found to be 98.41 ± 1.87 and 97.93 ± 2.39 (n = 5) for BCG and BPB methods, respectively. These results confirm the selectivity of developed methods. Robustness The effect of the changes made in the volume of the dye and reaction time on the absorbance of the formed ion-pair complexes in both methods were studied and found to be very small (RSD% were less than 1.86%) confirming the robustness of the developed methods. Application to the assay of capsules The developed methods were successfully applied for the determination of TRH in representative capsules i.e. triprolidine hydrochloride capsules-2.5 mg. The same capsules were also assayed by a reference method41 and we compared the results of the developed methods with those of the reference method using Student's t-test and F-test. Reference method involves a non-aqueous titration in which an accurately weighed of TRH was dissolved in a glacial acetic acid followed by the addition of mercuric acetate, then titration with 0.1 N perchloric acid with potentiometric detection of the end-point. All experimentally calculated t- and F-values were presented in Table 4 and found to be less than the tabulated ones, indicating a close similarity between the developed methods and the reference method with respect to accuracy and precision.
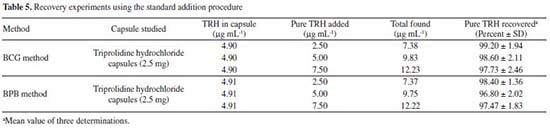
Recovery study The accuracy of the developed methods was further confirmed by performing recovery experiments using the standard addition procedure. Pre-analyzed capsule's powder (triprolidine hydrochloride capsules-2.5) was spiked with definite amounts of pure TRH at three different concentration levels (50, 100 and 150% of the quantity present in the capsule powder). The total was measured by the developed methods and the percent recovery of the added pure drug was calculated. Results of this study were presented in Table 5 and showed that no interference was observed from the various excipients present in the capsules.
CONCLUSIONS This is the first time bromocresol green and bromophenol blue have been used to determine the TRH drug spectrophotometrically. The methods presented in this study were established to be simple, sensitive, selective, cost-effective, accurate, precise, and robust; so that, they can be extensively applied for the determination of TRH in commercial capsules. Both methods depend on the addition of dye solution to the drug solution and the procedures do not involve any disadvantages like pH adjustment, heating or extraction steps. The developed methods depended on the mixing of the drug and dye solutions in dichloromethane solvent followed by measuring the absorbance of the formed ion-pair complex in both methods. In addition, these methods showed good accuracy and precision which made these methods suitable for the routine analysis of TRH in the quality control laboratories in pharmaceutical industries. Finally, no interference was observed from the excipients that might be present in the commercial capsules.
ACKNOWLEDGEMENTS The authors are thankful to the Shiba Pharma Company for Pharmaceutical Industries, Sana'a, Yemen, for gifting the pure sample of triprolidine hydrochloride. They also thank Al-Hikma University, Dhamar, Yemen for providing facilities to carry out this study.
REFERENCES 1. The British Pharmacopoeia, 2009th ed.; Stationery Office Books: London, 2009. 2. Rahman, M. M.; Sultana, R.; Khan, M. R.; Islam, M. A.; Turk. J. Pharm. Sci. 2013, 10, 49. [Link] accessed in May 2023 3. Saida, B. M.; Albajawi, S. A.; Deabas, F.; Saket, M. M.; Shareiah, R.; Abu Nameh, E. S. M.; J. Chem. Pharm. Res. 2014, 6, 327. [Link] accessed in May 2023 4. Onur, F.; Palabiyik, I. M.; Dinç, E.; Anal. Chem.: Indian. J. 2006, 2, 199. [Link] accessed in May 2023 5. Hinge, M.; Patel, K. R.; Mahida, R. J.; Pharm. Methods 2015, 6, 87. [Link] accessed in June 2023 6. Bassuoni, Y. F.; Essam, H. M.; Elzanfaly, E. S.; Zaazaa, H. E.; Kelani, K. O.; ChemistrySelect 2019, 4, 8946. [Crossref] 7. Paidipala, K.; Kamarapu, S. K.; Int. J. Pharm. Biol. Sci. 2013, 3, 172. [Link] accessed in May 2023 8. Baghel, U. S.; Sharma, H.; Chouhan, A.; Sharma, A.; Mukim, M.; Singh, D.; Res. J. Pharm. Technol. 2020, 13, 583. [Crossref] 9. Abu Reid, I. O.; Gadkariem, E. A.; J. Adv. Chem. 2017, 13, 6011. [Crossref] 10. Orsi, D.; Gagliardi, L.; Bolasco, A.; Tonelli, D.; Chromatographia 1996, 43, 496. [Crossref] 11. Mone, M. K.; Chandrasekhar, K. B.; Vyas, S.; J. Liq. Chromatogr. Relat. Technol. 2011, 34, 652. [Crossref] 12. Hayun, H.; Leswara, N. D.; Masrijal, C. D. P.; Pharm. Sci. Res. 2007, 4, 59. [Crossref] 13. Issa, Y. M.; Attia, F. M. A.; Ismail, N. S.; J. Adv. Res. 2010, 1, 79. [Crossref] 14. Zayed, S. I. M.; Habib, I. H. I.; Il Farmaco 2005, 60, 621. [Crossref] 15. Zayed, S. I. M.; Anal. Sci. 2004, 20, 1043. [Crossref] 16. Bassuoni, Y. F.; Essam, H. M.; Elzanfaly, E. S.; Zaazaa, H. E.; Kelani, K. M.; Anal. Bioanal. Electrochem. 2020, 12, 793. [Link] accessed in May 2023 17. Kanoute, G.; Guernet-Nivaud, E.; Guernet, M.; J. Pharm. Biomed. Anal. 1988, 6, 977. [Crossref] 18. Ma, D.; Du, J.; Liu, M.; Spectrosc. Lett. 2015, 48, 343. [Crossref] 19. Berardino, S. D.; Jasionowska, R.; Am. J. Anal. Chem. 2014, 5, 613. [Crossref] 20. Hayun, H.; Harianto, H.; Yenti, Y.; Pharm. Sci. Res. 2006, 3, 94. [Crossref] 21. Dönmez, O. A.; Bozdogan, A.; Kunt, G.; Div, Y.; Chem. Anal. (Warsaw, Pol.) 2007, 52, 135. [Link] accessed in May 2023 22. Sriphong, L.; Chaidedgumjorn, A.; Chaisuroj, K.; Int. J. Pharmacol. Pharm. Sci. 2009, 3, 162. [Crossref] 23. Abu Reid, I. O.; Widaa, T. A.; Int. J. Adv. Pharm. Anal. 2017, 7, 21. [Link] accessed in May 2023 24. Sinaga, S. M.; Silalahi, N. N.; Muchlisyam; Int. J. ChemTech. Res. 2017, 10, 1118. [Link] accessed in May 2023 25. Metwally, F. H.; J. Pharm. Biomed. Anal. 2001, 26, 265. [Crossref] 26. Aman, T.; Ahmad, A.; Aslam, M.; Kashmiri, M. A.; Anal. Lett. 2002, 35, 733. [Crossref] 27. Mumtaz, A.; Kazi, A. A.; Aman, T.; Sabri, M. U.; Noureen, F.; Proc. Pak. Acad. Sci. 2005, 42, 253. [Link] accessed in May 2023 28. Al-Rashidy, A. A. M.; Tikrit J. Pharm. Sci. 2009, 5, 44. [Link] accessed in May 2023 29. Ahmed, N. R.; Yaseen, H. W.; Albashar, M. N.; World J. Pharm. Pharm. Sci. 2018, 7, 151. [Link] accessed in May 2023 30. Acharyulu, M. L. N.; Rao, P. V. S. R. M.; Koti, I. S. R.; Innovare J. Sci. 2020, 8, 1. [Crossref] 31. Acharyulu, M. L. N.; Rao, P. V. S. R. M.; Koti, I. S. R.; Indian J. Adv. Chem. Sci. 2020, 8, 127. [Link] accessed in May 2023 32. Connors, K. A.; A Textbook of Pharmaceutical Analysis, 3rd ed.; Wiley India: New Delhi, 2007. 33. Abdulrahman, S. A. M.; Basavaiah, K.; Chem. Ind. Chem. Eng. Q. 2012, 18, 339. [Link] accessed in June 2023 34. Mohamed, S. H.; Issa, Y. M.; Elfeky, S. A.; Spectrochim. Acta, Part A 2019, 222, 117186. [Crossref] 35. International Conference on Harmonization (ICH) Guidelines; Validation of Analytical Procedures: Text and Methodology Q2 (R1), London, 2005. [Link] accessed in May 2023 36. Qarah, N. A. S.; Abdulrahman, S. A. M.; Algethami, F. K.; Basavaiah, K.; El-Maaiden, E.; Quim. Nova 2020, 43, 44. [Crossref] 37. Job, P.; Ann. Chim. (Cachan, Fr.) 1928, 9, 113. 38. Banyasz, J. L. In Analytical Determination of Nicotine and Related Compounds and Their Metabolites; Gorrod, J. W.; Jacob III, P., eds.; Elsevier Science: Amsterdam, 1999, ch. 5. 39. Ashour, S.; Chehna, M. F.; Bayram, R.; Int. J. Biomed. Sci. (Pomona, CA, U. S.) 2006, 2, 273. [Link] accessed in May 2023 40. Amin, A. S.; Gouda, A. A. E.; El-Sheikh, R.; Zahran, F.; Spectrochim. Acta, Part A 2007, 67, 1306. [Crossref] 41. The United States Pharmacopoeia, the National Formulary (USP 30/NF 25), 2007th ed.; The United States Pharmacopeial Convention: Maryland, 2007. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access