Artigo
| Alginate coated MgO-NPs improves the stability of Kluyveromyces lactis β-galactosidase for biotechnological applications |
|
Shakeel Ahmed AnsariI,*; Rukhsana SatarII; Nadeem IkramIII; Muhammad AwaisIII
I. Department of Biochemistry, Batterjee Medical College, 21442 Jeddah, Saudi Arabia Recebido em 12/02/2023 *e-mail: shakeel.ansari@bmc.edu.sa The current study was undertaken to immobilize Kluveromyces lactis β-galactosidase on alginate coated magnesium oxide nanoparticles (ACMONPs). Transmission electron microscopy showed that MgO-NPs synthesized by wet chemical approach were of 27 nm size and spherical in shape. Excellent biocompatibility of sodium alginate resulted in 90% immobilization yield for β-galactosidase due to covalent attachment. Soluble and immobilized β-galactosidase exhibited its pH and temperature-optima at pH 7.0 and at 40 oC, respectively. However, the enzyme attached to ACMONPs enhanced its activity at higher and lower pH and temperature ranges, in contrast to enzyme in solution. ACMONPs bound enzyme displayed greater enzyme activity under the effect of galactose mediated product inhibition as well as in reusability experiment. Our study indicated that 88% lactose was hydrolyzed by β-galactosidase immobilized on ACMONPs at 40 oC as compared to 71% by soluble enzyme under identical temperature in controlled batch reactors. Hence, this nanomatrix can find its potential application in continuous packed-bed reactors for obtaining lactose-free dairy products. INTRODUCTION Enzyme-based biocatalysts have a wide range of industrial applications in beverages, detergent, food, pharmaceutical and textile industries.1 Microorganisms are the major sources of enzymes but natural enzymes as biocatalysts have limitations such as lower catalytic efficiency at ambient conditions, enzyme denaturation due to inability to withstand the pressure of large-scale industrial fermenters and poor productivity in native microbial cultures. Therefore, to meet their increasing demand at the industrial level, native enzymes are often engineered to work under non physiological reactions, to design innovative and efficient enzyme catalyzed pathways, and for the production of novel metabolites.2,3 Commonly employed enzyme engineering strategies include directed evolution, site-directed mutagenesis, truncation, and terminal fusion. These powerful and revolutionary techniques of enzyme engineering provide excellent opportunities for tailoring existing enzymes and constructing highly efficient novel industrial enzymes for the cost-effective production of value-added products.4 Lactase (β-galactosidase) is one such enzyme that is of extraordinary importance to food and dairy industry.5 It catalyzes lactose into glucose and galactose. Hence, β-galactosidase is utilized for producing lactose-free items for "lactose intolerant" population.6 The free form of β-galactosidase is available commercially to be utilized for this purpose. However, major limitations accompanied by their application involve their degradation due to physical/thermal damage, catalyst poisoning/deactivation, mass transfer resistance and long reaction time.7 Such drawbacks can be outstripped by immobilizing them on solid supports. Immobilization enhanced the resistant of enzyme against harsh environmental variations apart from imparting thermo-stability and reusability which makes them a perfect fit for batch and continuous operations.8,9 Kluyveromyces lactis β-galactosidase is one of the most important enzymes in the dairy industry which finds its application in lactose hydrolysis, transglycosylation and galactosylation reactions. However, the poor stability of this enzyme against high temperature and inhibitors limits its use in the synthesis of galacto-oligosaccharides (GOS) and hydrolysis of lactose in batch and continuous reactors.10 Several investigators have therefore exploited various methods of enzyme immobilization to improve its catalytic property in these applications.11-13 Enzyme immobilization technology has also developed in parallel as a means of increasing enzyme performance and reducing process costs.14 New approaches are continuously formulated for immobilizing enzymes on solid carriers. However, nanoparticles serve as more convenient and promising candidates for such purposes as they increase surface area and reduce diffusion limitations required to increase the enzyme loading.15 Their physical properties like particle mobility and enhanced diffusion can further impact inherent catalytic efficiency of immobilized enzymes.16 Irradiation resistance, increased thermal stability and excellent reusability are added benefits as compared to conventional matrix that favors their utilization in various sectors.17 Recently, there has been a lot of interest in the study, design, synthesis and characterization of metallic NPs for various biotechnological and biomedical applications.14 Excellent durability, lower toxicity, higher stability and stronger heat resistance are other properties of metallic NPs that makes them important even for the treatment and targeting of many diseases.18,19 They further minimizes the possibility for microbial contamination apart from imparting above mentioned advantages required for enzyme immobilization. Magnesium oxide nanoparticles (MgO-NPs) are one such nanostructured material that is gaining attention in this regard due to its low cost, negligible toxicity and excellent biocompatibility.20,21 Various types of natural and synthetic ligands have been tried earlier for modifying the surface of nanoparticles in terms of their biocompatibility, biodegradability, low toxicity and high level of protein entrapment.22 In this regard, sodium alginate has gained considerable attention due to its excellent stability in biological media, biodegradable nature, low toxicity, muco-adhesive properties, and enhanced penetration capacity across biological barriers and good compatibility with protein molecules.23 It contains guluronic and mannuronic acids as the main components. It represents a water-swollen and crosslinked polymeric hydrogel that is obtained from these monomers.24,25 Owing to its mild gelation property and low cost, it is used as an emulsifier/stabilizer in various biomedical and biotechnological applications.26,27 Hence, MgO-NPs were synthesized in the present study via wet chemical approach followed by their surface modification by sodium alginate. The resulted nanomatrix was employed for Kluyveromyces lactis β-galactosidase immobilization owing to its biotechnological importance10 and subsequently exploited for hydrolyzing lactose from solution in controlled batch reactors.
EXPERIMENTAL Materials ONPG and β-galactosidase from Kluyveromyces lactis were obtained from Sigma Aldrich, USA. Magnesium chloride dihydrate (MgCl2.2H2O), sodium alginate, calcium chloride and oxalic acid were obtained from SRL, India. Preparation of MgO-NPs by wet chemical process Wet chemical approach was used for synthesizing MgO-NPs from soluble salt of magnesium (MgCl2.2H2O) and oxalic acid (H2C2O4.2H2O) as the precursor compounds. Initially, MgCl2 (1.0 mol L-1) was dissolved in water followed by the gradual dropping of oxalic acid in a beaker (250 mL) on a hot plate: 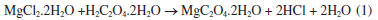 The resulting mixture was stirred for 15 min at 400 rpm at room temperature. This step yield powder which was later on filtered by washing with water and re-filtration. The product was obtained subsequently by drying the powder for 12 h at 100 ºC. Finally, calcination was implemented for 4 h at 700 ºC with the heating and cooling rate optimized at 180 ºC h-1. TEM characterization Morphology of the synthesized MgO-NPs was elucidated by placing a small drop of suspended nanoparticles on a carbon-covered copper network and enabling the dissipation of water into a vacuum dryer. TEM (Thermo Scientific™ Talos L120C TEM, USA) images were scanned on the grid containing MgO-NPs. Biocatalyst preparation In order to prepare 0.5% sodium alginate (SA) solution, SA powder (5 g L-1) was stirred at 50 ºC until the powder was completely dissolved. Calcium chloride (1.0%) was prepared to introduce the cross-linking reaction. CaCl2 (10 g L-1) was dissolved in distilled water at room temperature until the mixture became clear.23 To prepare the 0.5% alginate-coated MgO-NPs, 5 g of ZnO-NP powder was slowly added into the above-prepared alginate solution under magnetic stirring at ambient temperature to form a uniform suspension. Finally, β-galactosidase (2000 U) was mixed overnight with 10 mL of ACMONPs obtained above with slow stirring at room temperature. Enzyme conjugated to the modified nanosupport was obtained by centrifugation at 2000 rpm (Manufacturer, QJJML) for 20 min. β-galactosidase assay The reaction mixture consists of 0.1 mol L-1 potassium phosphate buffer, pH 7.0 (1.79 mL), 2.0 U suitably diluted β-galactosidase (0.01 mL) and 2.0 mmol L-1 ONPG (0.2 mL). Lactase activity assay was performed for calculating the hydrolysis of β-galactosidase by shaking this reaction mixture at 40 oC for 15 min. 1.0 mol L-1 sodium carbonate solution (2.0 mL) was used for terminating the reaction and product formed was measured spectrophotometrically at 405 nm.9 Enzyme activity at varying pH and temperature ranges Soluble β-galactosidase and enzyme bound to ACMONPs (2.0 U equivalent to 0.01 mL) were assayed in various pH (5.0-9.0) buffers of 0.1 mol L-1. The buffers used were sodium acetate (pH 5.0), potassium phosphate (6.0, 7.0) and Tris-HCl (pH 8.0, 9.0). The activity at various pH ranges were calculated by comparing the activity at pH 7.0 which was considered as 100% (control).9 Similarly, temperature-activity profiles were determined for soluble β-galactosidase and enzyme bound to ACMONPs (2.0 U equivalent to 0.01 mL) at various temperatures (20-70 oC) in potassium phosphate buffer (0.1 mol L-1, pH 7.0). The activity at various temperature ranges were calculated by comparing the activity at 50 oC which was considered as 100% (control).9 Galactose mediated product inhibition The activity of soluble β-galactosidase and enzyme bound to ACMONPs (20 µL) was calculated with galactose (1-5%, w/v) in potassium phosphate buffer (0.1 mol L-1, pH 7.0) at 40 oC for 1 h. The activity of enzyme without added galactose was considered as control (100%) for calculating the remaining percent activity.9 Reusability study β-galactosidase bound to ACMONPs (100 µL) was analyzed for its repeated use for six successive days. After every run, the enzyme was washed with an assay buffer and centrifuged (2000 rpm for 10 min) to obtain the precipitate. The activity obtained on first day was considered as 100% (control) to calculate the activity at different days.9 Batch conversion of lactose Soluble β-galactosidase and enzyme bound to ACMONPs (250 U) was incubated in lactose solution (250 mL, 0.1 mol L-1). The process was stirred continuously at 40 oC and 50 oC in water bath for 10 h, and the aliquots were drawn after 1 h time interval for assaying the formation of glucose by glucose oxidase-peroxidase assay kit.9 Statistical analysis Each value represents the mean for three independent experiments performed in triplicates, with average standard deviations < 5%. Sigma Plot-9 was used for expressing the data. Data was analyzed by one-way ANOVA. P-values < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION Synthesis of MgO-NPs Nikam et al. have critically evaluated chemical methods that were exploited earlier for scalable production of metal oxide nanoparticles.28 The main advantages associated with these methods are that it allowed the synthesis of nanoparticles with desirable shapes and sizes, phases and selective surface structures. It further allows the adjustment of reaction conditions (pH, temperature, additives and concentration of substrate) to afford the desirable properties in nanoparticles.29 Figure 1 depicts the flowchart of wet chemical approach that was used for obtaining MgO-NPs in the current study. The purpose of exploiting this approach was the quick synthesis of nanoparticles and that too without the requirement of complex instruments or techniques.
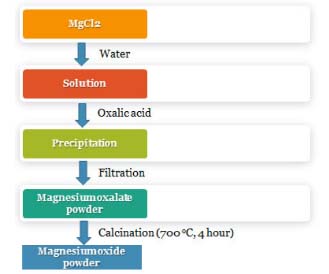 Figure 1. Depicts the flowchart for the synthesis of MgO-NPs from the precursor compounds, MgCl2.2H2O and oxalic acid
Herein, brown colored MgO-NPs were obtained which were of 27 nm as depicted by transmission electron micrographs (Figure 2).
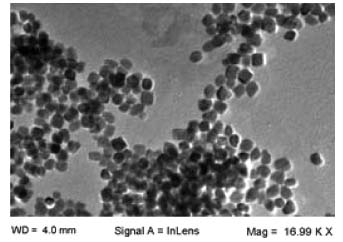 Figure 2. Transmission electron microscopy of MgO-NPs
The size of synthesized MgO-NPs was estimated by L120C transmission electron microscope by dropping the nanoparticle solution on carbon-coated copper grids at a 25 kV voltage under normal atmospheric conditions. Importance of surface modification of MgO-NPs by sodium alginate Recent study witnessed the stabilization, emulsification, biocompatibility and crosslinking properties of sodium alginate.30 Furthermore, it has been shown recently that surface modification of chitosan nanoparticles with sodium alginate enhanced its muco-adhesive property and protein encapsulation efficiency.31 In the current study, coating with alginate improved the stability of MgO-NPs. Firstly, immobilization occurs via hydrophobic adsorption between the enzyme and the hydrophobic surface of the support; subsequent covalent interactions occur between the terminal amino group of the enzyme and functional groups of the support surface, which may form additional linkages with the amino groups of the enzyme. The surface modified nanoparticles exhibited excellent immobilization yield, therefore suggesting their potential application in various biomedical and biotechnological applications. Stability of soluble β-galactosidase and enzyme bound to ACMONPs under various temperature and pH ranges Enzyme stability depends on its activity which in turn depends on its conformational structure. Hence, conformational changes in its tertiary structure lead to loss of its catalytic activity.32 The pH-activity profiles for soluble β-galactosidase and enzyme bound to ACMONPs has been shown in Figure 3. Both the enzyme preparations displayed pH 7.0 as their pH-optima. However, ACMONPs bound enzyme showed broadened pH-activity profiles in comparison to the enzyme in solution. It was noticed that at pH 6.0, β-galactosidase bound to ACMONPs retained 81% activity but SβG exhibited 62% activity under identical conditions. The plausible reason could be that highly acidic and basic solutions lead to greater alteration/distortion in the tertiary structure of soluble enzyme as compared to its immobilized counterpart.33
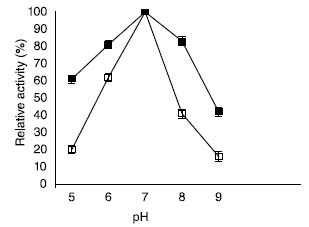 Figure 3. pH activity profiles for soluble β-galactosidase and enzyme bound to ACMONPs. Soluble β-galactosidase and enzyme bound to ACMONPs (2.0 U equivalent to 0.01 mL) was assayed in various pH (5.0-9.0) buffers of 0.1 mol L-1. The activity at various pH ranges were calculated by comparing the activity at pH 7.0 which was considered as 100% (control). Enzyme activity was determined as described in the text. The symbol (□) show soluble β-galactosidase and (■) enzyme bound to ACMONPs
Similarly, higher temperature leads to greater loss of enzyme activity which causes enzyme denaturation, thereby degrading the nanomatrix and distorting the polypeptide chain.34 Temperature-optima for both soluble β-galactosidase and enzyme attached to ACMONPs was observed at 40 oC, but ACMONPs bound enzyme displayed their activity at both lower and higher temperature ranges (Figure 4). Our study suggested that 36% activity was retained by soluble enzyme at 60 oC whereas ACMONP bound enzyme retained 84% activity under identical exposure.
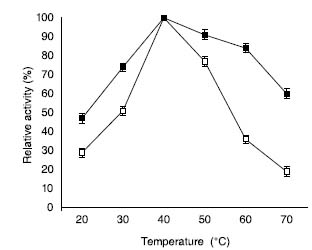 Figure 4. Temperature activity profiles for soluble β-galactosidase and enzyme bound to ACMONPs. The activity of soluble β-galactosidase and enzyme bound to ACMONPs (2.0 U equivalent to 0.01 mL) was observed at various temperatures (20-70 ºC). The activity at various temperature ranges were calculated by comparing the activity at 50 ºC which was considered as 100% (control). The symbol (□) show soluble β-galactosidase and (■) enzyme bound to ACMONPs
It is a well-known fact that galactose acts as a competitive inhibitor for β galactosidase.35,36 In the present study, it was observed that SβG retained 31% activity at 4% galactose concentration. However, ACMONP exhibited 54% enzyme activity under similar experimental conditions (Figure 5). Hence, IβG exhibited excellent resistance against galactose mediated competitive inhibition in contrast to the enzyme in solution.
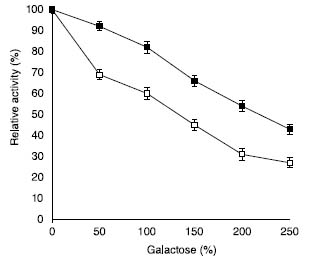 Figure 5. Effect of galactose on soluble β-galactosidase and enzyme bound to ACMONPs. The activity of soluble β-galactosidase and enzyme bound to ACMONPs (2.0 U equivalent to 0.01 mL) was measured in the presence of increasing concentrations of galactose (50-150 mmol L-1) in the assay buffer at 40 ºC. Activity of enzyme without added galactose was considered as control (100%) for the calculation of remaining percent activity at other concentrations. The symbol (□) show soluble β-galactosidase and (■) enzyme bound to ACMONPs
Reusability of ACMONPs bound β-galactosidase Enzyme reuse and recovery is important characteristic for its potential industrial application. Reusability experiment showed that even after 6th repeated use, the immobilized enzyme retained 79% activity (Figure 6). Activity loss observed could be due to the falling off of enzyme from the surface of the carrier and because of the conformational changes in the enzyme. It has been shown recently that pectinase immobilization on various biocatalyst supports improved its recyclable performance to greater extent.37,38 Earlier investigators have reported nearly 60% activity of Kluyveromyces lactis β-galactosidase when immobilized on glutaraldehyde modified support after 5th cycle of reuse.39
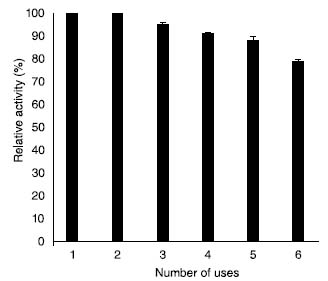 Figure 6. Reusability of enzyme bound to ACMONPs
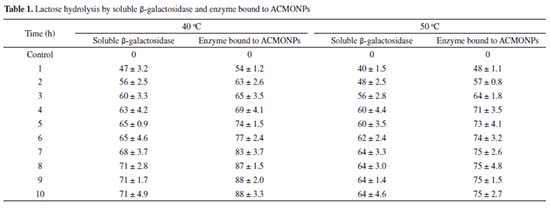
β-galactosidase bound to ACMONPs (100 µL) was taken in triplicates for six successive days and the activity determined on the first day was taken as control (100%) for calculation of remaining activity after each use. Lactose hydrolysis β-galactosidase mediated lactose hydrolysis is beneficial for environmental and biotechnology sectors. Moreover, galacto-oligosaccharides are also produced which acts as prebiotics for obtaining "healthy foods".40,41 Hence, in this article, efforts were raised to improve the stability and applicability of immobilized version of β-galactosidase in dairy industry. Table 1 showed that SβG exhibited more lactose hydrolysis at 40 oC in contrast to enzyme bound to ACMONPs initially due to its temperature optima at 40 oC (Figure 4). Greater accessibility of soluble enzyme for the substrate could be another reason for obtaining greater percent of lactose hydrolysis during initial few hours. However, continuous incubation resulted in decreased lactolysis which could be explained by product mediated inhibition for the enzyme. Our study indicated that 88% lactose was hydrolyzed by enzyme bound to ACMONPs at 40 oC as compared to 71% by SβG under identical temperature. It has been reported earlier that 50% lactose hydrolysis was achieved in 4 h by K. lactis β-galactosidase immobilized on carbon nanotubes. Moreover, glutaraldehyde modified multi-walled cabon nanotubes exhibited 86% lactose hydrolysis after 10 h at 40 oC.42,43 Hence, ACMONPs bound β-galactosidase could serve as an excellent nanomatrix for obtaining lactose-free products in continuous packed-bed reactors.
Each value represents the mean for three independent experiments performed in triplicates, with average standard deviations, < 5%.
CONCLUSION The current study successfully achieved the synthesis of MgO-NPs via wet chemical method. Surface of the synthesized nanoparticles were functionalized by sodium alginate in order to achieve greater yield of enzyme immobilization. Our study concludes that β-galactosidase immobilized on ACMONPs was significantly stabilized against harsh pH and temperature ranges as compared to the free enzyme. Enzyme immobilized on ACMONPs retained 43% activity in contrast to 27% retained by the free enzyme under 250 mmol L-1 concentration of galactose mediated competitive product inhibition. Reusability experiment revealed that 88% of enzyme activity was retained on alginate coated MgO-NPs even after 5th repeated use, thereby making the process economically attractive for obtaining lactose free products in batch and continuous reactors. The batch conversion revealed that 87% of lactose was hydrolyzed even after 8 h by β-galactosidase conjugated to ACMONPS at 40 oC as compared to its 75% conversion at 50 oC. Lactose conversion was observed more at 40 oC as it represents the temperature optima of Kluyveromyces lactis β-galactosidase. Hence, the developed nanobiocatalyst can find its application in biotechnology industry to obtain lactose-free dairy products with cost effective advantages.
ABBREVIATIONS ONPG: o-nitrophenyl β-D-galactopyranoside
ACKNOWLEDGEMENTS The authors would like to thank Mr. Jitendra Kumar for carrying out the TEM experiment.
REFERENCES 1. Zhao, M.; Rong, R.; Han, J.; Zhou, Y.; Li, C.; Wang, L.; Mao, Y.; Wang, Y.; ACS Appl. Mater. Interfaces 2019, 11, 31878. [Crossref] 2. Cordova, A.; Henriquez, P.; Nunez, H.; Rico-Rodriguez, F.; Guerrero, C.; Astudillo-Castro, C.; Illanes, A.; Catalysts 2022, 12, 1245. [Crossref] 3. Guimarães, J. R.; Carballares, D.; Rocha-Martin, J.; Tardioli, P. W.; Fernandez-Lafuente, R.; Catalysts 2022, 12, 1552. [Crossref] 4. Sengupta, S.; Das, P.; Sharma, S.; Shukla, M. K.; Kumar, R.; Kumar, R. K.; Pandey, S.; Kumar, D.; Catalysts 2023, 13, 250. [Crossref] 5. Kolev, P.; Rocha-Mendoza, D.; Ruiz-Ramirez, S.; Ortega-Anaya, J.; Jimenez-Flores, R.; Garcia-Cano, I.; JDS Communications 2022, 3, 1. [Crossref] 6. Liburdi, K.; Esti, M.; Beverages 2022, 8, 21. [Crossref] 7. Ureta, M. M.; Martins, G. N.; Figueira, O.; Pires, P. F.; Castilho, P. C.; Gomez-Zavaglia, A.; Crit. Rev. Food Sci. Nutr. 2021, 61, 721. [Crossref] 8. Ansari, S. A.; Alshanberi, A. M.; Braz. J. Chem. Eng. 2022, 39, 361. [Crossref] 9. Ansari, S. A.; Damanhoury, A. A.; Heliyon 2023, 9, e13089. [Crossref] 10. de Albuquerque, T. L.; de Sousa, M.; Gomes, E.; Silva, N. C.; Girao, N. C. A. C.; Gonçalves, L. R. B.; Fernandez-Lafuente, R.; Rocha, M. V. P.; Int. J. Biol. Macromol. 2021, 191, 881. [Crossref] 11. Videira-Quintela, D.; Guillen, F.; Prazeres, S. F.; Montalvo, G.; ChemPlusChem. 2022, 87, e202200340. [Crossref] 12. Campello, E. G. S.; Trindade, R. A.; Rego, T. V.; Burkert, J. F. M.; Burkert, C. A. V.; Int. J. Food Eng. 2012, 76, 98. [Crossref] 13. Ansari, S. A.; Husain, Q.; J. Mol. Catal. B: Enzym. 2011, 70, 119. [Crossref] 14. Federsel, H. J.; Moody, T. S.; Taylor, S. J. C.; Molecules 2021, 10, 2822. [Crossref] 15. Sulman, A. M.; Matveeva, V. G.; Bronstein, L. M.; Nanomaterials 2022, 12, 3796. [Crossref] 16. Ansari, S. A.; Husain, Q.; Biotechnol. Adv. 2012, 30, 512. [Crossref] 17. An, J.; Li, G.; Zhang, Y.; Zhang, T.; Liu, X.; Gao, F.; Peng, M.; He, Y.; Fan, H.; Catalysts 2020, 10, 338. [Crossref] 18. Naseem, T.; Durrani, T.; Environ. Chem. Ecotoxicol. 2021, 3, 59. [Crossref] 19. Yoon, Y.; Truong, P. L.; Lee, D.; Ko, S. H.; ACS Nanosci. Au 2022, 2, 64. [Crossref] 20. Ammulu, M. A.; Vinay, V. K.; Giduturi, A. K. J.; Genet. Eng. Biotechnol. 2021, 19, 21. [Crossref] 21. Hornak, J.; Int. J. Mol. Sci. 2021, 22, 12752. [Crossref] 22. de Freitas, L. A.; de Sousa, M.; Ribeiro, L. B.; de Franca, I. W. L.; Gonçalves, L. R. B.; Catalysts 2023, 13, 306. [Crossref] 23. Hammam, I.; Ali, M. A. S.; Abdellatif, A.; Coatings 2023, 13, 362. [Crossref] 24. Lee, K. Y.; Mooney, D. J.; Prog. Polym. Sci. 2012, 37, 106. [Crossref] 25. Gheorghita, P. R.; Lobiuc, A.; Dimian, M.; Covasa, M.; Polymers 2020, 20, 2417. [Crossref] 26. Mikula, K.; Skrzypczak, D.; Ligas, B. S. N.; Appl. Sci. 2019, 1, 643. [Crossref] 27. Bialik-Was, K.; Krolicka, E.; Malina, D.; Molecules 2021, 19, 2381. [Crossref] 28. Nikam, A. V.; Prasad, B. L. V.; Kulkarni, A. A.; CrystEngComm 2018, 20, 5091. [Crossref] 29. Chen, J.; Ma, Q.; Wu, X. J.; Li, L.; Liu, J.; Zhang, H.; Nano-Micro Lett. 2019, 11, 86. [Crossref] 30. Gao, Z.; Gao, C.; Jiang, W.; Xu, L.; Hu, B.; Yao, X.; Li, Y.; Wu, Y.; Food Hydrocolloids 2023, 135, 108233. [Crossref] 31. Amin, M. K.; Boateng, J. S.; Mar. Drugs 2022, 22, 156. [Crossref] 32. Riziotis, I. G.; Ribeiro, A. J. M.; Borkakoti, N.; Thornton, J. M.; J. Mol. Biol. 2022, 15, 167517. [Crossref] 33. Badieyan, S.; Wang, Q.; Zhou, X.; Li, Y.; Herron, M.; Abbott, N. L.; Chen, Z.; Marsh, E. N. G.; J. Am. Chem. Soc. 2017, 139, 2872. [Crossref] 34. Arana-Pena, S.; Rios, N. S.; Carballares, D.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L. R. B.; Fernandez-Lafuente, R.; Frontiers in Bioengineering and Biotechnology 2020, 8, 36. [Crossref] 35. Kamran, A.; Bibi, Z.; Aman, A.; Qader, S. A.; J. Food Sci. Technol. 2019, 56, 167. [Crossref] 36. Shi, X.; Wu, D.; Xu, Y.; Yu, X.; J. Dairy Sci. 2022, 105, 4772. [Crossref] 37. Qi, D.; Gao, M.; Li, X.; Lin, J.; ACS Omega 2020, 32, 20062. [Crossref] 38. Liu, C.; Zhang, L.; Tan, L.; Liu, Y.; Tian, W.; Ma, L.; Front. Chem. 2021, 9, 677868. [Crossref] 39. Gennari, A.; Mobayed, F. H.; Rafael, R. S.; Catto, A. L.; Benvenutti, E. V.; Rodrigues, R. C.; Speretto, R. A.; Volpato, G.; de Souza, C. F. V.; Braz. J. Chem Eng. 2019, 36, 1403. [Crossref] 40. Alshanberi, A. M.; Al-Shaeri, M. A.; Ansari, S. A.; Braz. Arch. Biol. Technol. 2021, 64, e21180747. [Crossref] 41. Wahba, M. I.; 3 Biotech 2023, 13, 32. [Crossref] 42. Lahiff, E.; Lynam, C.; Gilmartin, N.; O'Kennedy, R.; Diamond, D.; Anal. Bioanal. Chem. 2010, 398, 1575. [Crossref] 43. Ansari, S. A.; Satar, R.; Khan, M. J.; Chibber, S.; J. Mol. Catal. B: Enzym. 2013, 97, 258. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access