Educação
| Application of named reactions in polymer chemistry teaching |
|
Dawei LuI,*; Xudong ZouII; Chaofan LiIII; Zhifeng WangII; Bojun XuI
I. College of Materials Science and Engineering, Beijing University of Chemical Technology, 102299 Nankou Town, Beijing, China Recebido em 03/03/2023 *e-mail: 2020020431@buct.edu.cn Being an essential component of organic chemistry, named reactions are indispensable in organic synthesis. More and more named reactions have been devised to create unique polymer backbones, which has significantly accelerated the creation of new polymer materials. Yet, the university chemical knowledge system is deficient in relevant introductions. The purpose of this study is to incorporate the specified reactions within the teaching of polymer chemistry. These reactions are divided into six categories: transition metal-catalyzed coupling reactions, condensation reactions, pericyclic reactions, multi-component reactions, free radical reactions, and other reactions. This work methodically covers these six types of reactions, which are critical for polymer backbone synthesis, and presents the practice in teaching. It can assist students to learn polymer chemistry in conjunction with organic chemistry and provide some reference for students and teachers. INTRODUCTION Organic chemistry has evolved since the mid-eighteenth century, with thousands of organic chemical reactions identified by domestic and international chemists.1,2 Several classical organic reactions are named after the discoverers or improvers to honor the chemists who initially discovered and investigated the reaction.3 They are thus referred to as named reactions. Because of their capacity to create the appropriate covalent bonds efficiently and skillfully, named reactions play a vital role in the synthesis of small molecules and have become a major aspect of organic chemistry. Many monographs on specific reactions have been published in China and abroad such as Named Reactions comprised by Jack Li4 and famous work entitled Strategic Applications of Named Reactions in Organic Synthesis.5 Polymer synthesis, particularly the formation of main chains, is under the purview of polymer chemistry. Common organic reactions, such as free radical polymerization of unsaturated monomers, condensation polymerization of carboxylic acids and alcohols, and so on, are inseparable from organic chemistry. New types of polymers, such as conducting polymers6 and self-healing elastomers,7 have evolved in recent years, owing in great part to the advent of novel reactions, particularly named reactions. These reactions are increasingly active not only in the field of small molecule synthesis but also in the field of polymer synthesis.8-10 The mechanism and kinetic properties of the four major polymerizations, as well as the techniques of polymerization implementation, are typically studied in polymer chemistry courses. Regrettably, few polymer chemistry textbooks or educational materials have addressed the use of named reactions in polymer synthesis, and few lecturers have attempted to teach this aspect of polymer chemistry.11-13 As a result, in this paper, we will present some of the important named reactions in polymer backbone synthesis in a more systematic manner, as well as practice in teaching polymer chemistry, which may be used as a supplement to students' knowledge of polymer chemistry or as a reference for teachers.
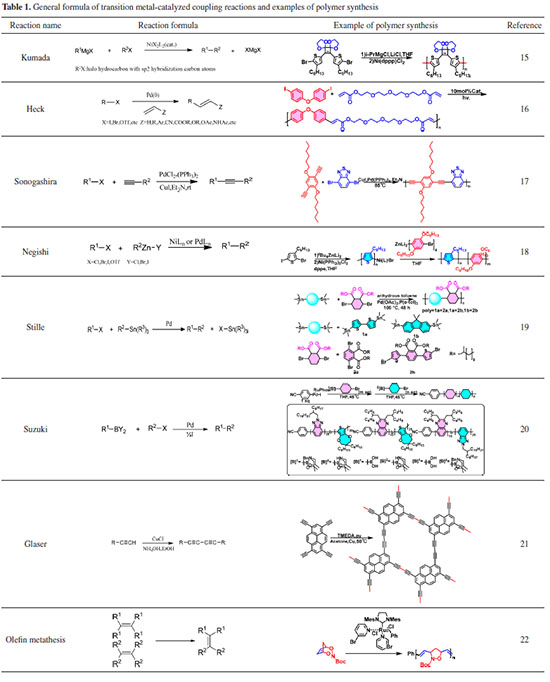
INTRODUCTION TO NAMED REACTION FOR POLYMER CONSTRUCTING Transition metal-catalyzed coupling reactions Transition metal-catalyzed coupling reactions are metal-catalyzed processes that create new carbon-carbon bonds. The primary distinction between different coupling reactions is the type of metal used in catalysis, as well as the hybridization form of the two coupled carbon atoms engaged in the reaction. The basic reactions that comprise the entire reaction process are oxidative addition, transmetallation, and reductive elimination.14 Because of its moderate and efficient conditions, this sort of reaction is crucial in the synthesis of small molecules. Such processes are classified as condensation polymerizations in polymer science, with kinetic behavior like living polymerization systems, where the active center is a metal-organic complex. These processes offer significant advantages in the synthesis of copolymers. Table 1 shows examples of how Kumada coupling, Heck coupling, Sonogashira coupling, Negishi coupling, Stille coupling, and Suzuki coupling have been effectively employed in the synthesis of conjugated polymers by the design of bi-functionalization of monomers.
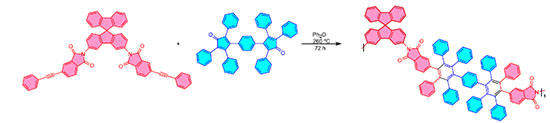
Pericyclic reactions Pericyclic reactions occur when more than two bonds in a reactant molecule travel through a ring transition state, triggering the shattering of old connections as well as the production of new bonds at the same time. This reaction is a multi-centered one-step synergistic process that includes electrocyclization, cycloaddition, σ-bond migration, and other reactions.23 The reactions are characterized by the absence of ionic and radical intermediates. In the entire process, only cyclic transition stages exist. It is also reversible under light and heat conditions. Because of these features, it is less vulnerable to solvents and catalysts. Pericyclic reactions, as the name implies, may easily form ring skeletons and hence play an important role in organic synthesis. Diels-Alder and Huisgen reactions are the two most important pericyclic processes to produce polymer skeletons. Diels-Alder reaction The reaction between dienes and olefins to form cyclohexene derivatives is called Diels-Alder cycloaddition (D-A reaction or [4π + 2π] cyclization). D-A cycloaddition has been one of the most commonly utilized synthetic techniques since its discovery. Taking advantage of the fact that temperature can control the direction in which D-A and retro D-A reactions proceed, D-A reactions, particularly with furan/maleimide derivatives, have been used to create novel polymeric materials with recyclable, repairable, and thermally responsive properties, as shown in Figure 1.
Huisgen reaction The Huisgen reaction, also known as 1,3-dipole cycloaddition, is a cycloaddition reaction between a 1,3-dipole and an olefin, an alkyne, or a corresponding derivative, and the product is a five-membered heterocyclic compound. There are numerous typical 1,3-dipole, the most well-known of which are azide compounds. The cycloaddition between various alkenes or alkynes and azides has received the most attention (also known as the "Click" reaction).25 It has high reaction yields, gentle reaction conditions, water resistance, easy operation, good reaction compatibility, and quick rates. As a result, this sort of dipole is very commonly used in polymer synthesis. Bifunctionalized azides and alkynes allow for the quick and easy creation of polymer backbones containing 1,2,3-triazoles. The resultant polymers are typically linear, soluble, and thermally stable, as illustrated in Figure 2.
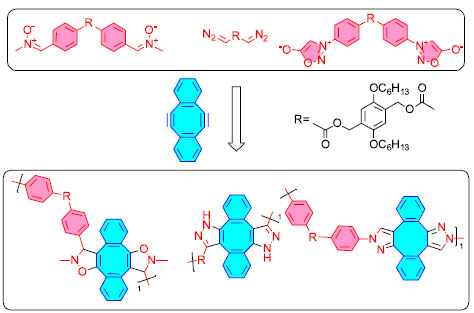 Figure 2. Application examples of Huisgen reaction in polymer backbone synthesis26
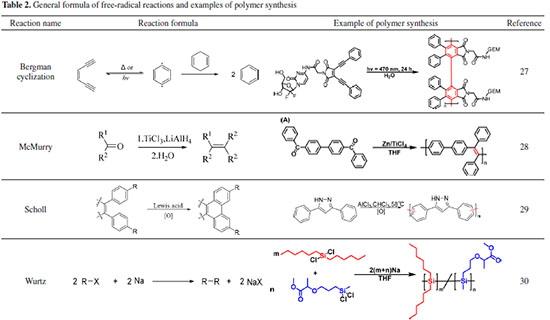
Free radical reactions These are not the typical chain radical polymerization reactions that are discussed in polymer chemistry textbooks. These reactions, such as the McMurry reactions, typically involve single-electron processes with metals. McMurry reactions, for example, have been realized using unsaturated backbones made from double aldehydes. Scholl reactions are primarily employed to expand aromatic ring conjugation systems and carbon chains, making them an effective supplement to traditional classical coupling reactions (e.g. Heck and Suzuki, etc.) in the production of polymers under specific conditions. Because the reaction is non-homogeneous and vulnerable to side reactions such as elimination and rearrangement, the synthetic significance of Wurtz coupling is somewhat limited, resulting in relatively poor yields. The reaction has very few applications in the field of carbon chain polymer synthesis, but its involvement in the synthesis of polysilanes is critical, as indicated in Table 2.
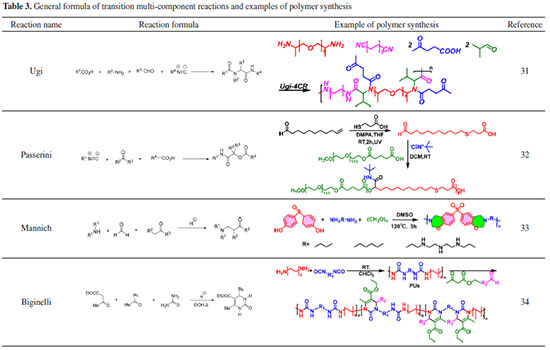
Multicomponent reactions MCRs are modular and efficient reactions that employ at least three different reactants in a single pot to produce molecules with complicated functionality. MCRs have been utilized in chemical synthesis for many years and have made significant contributions to the synthesis of small molecules, but only in the last fifteen years has their promise in polymer synthesis been recognized. When compared to traditional polymerization methods, the use of MCRs in polymer synthesis has brought a novel approach to polymer synthesis with advantages such as good functional group compatibility, high reaction yields, and ease of handling. MCRs can also introduce two or more functional groups into polymers at the same time. As a result, MCRs have emerged as novel techniques in the field of functional polymer synthesis. Then on-isocyanide-based Biginelli reaction Mannich reaction, as well as the isocyanide-based Passerini reaction and Ugi reaction, are examples of MCRs that have been successfully employed in polymer synthesis. Table 3 shows examples of how these reactions have been used.
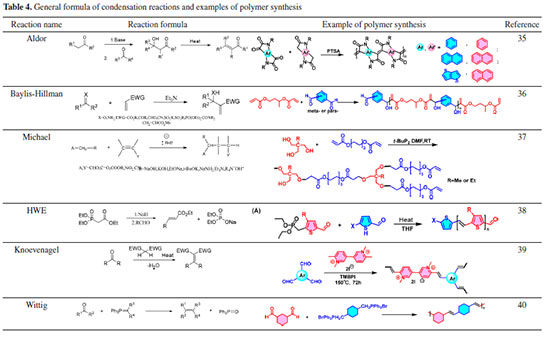
Condensation reactions The reaction that joins two carbon atoms that are not connected between molecules is collectively called a condensation reaction. A new carbon-carbon bond is created in a condensation reaction by attaching a positively charged carbon to a negatively charged carbon. Several condensation reactions have been effectively used in the synthesis of polymer backbones, including Aldol condensation, Baylis Hillman reaction, Horner Wadsworth Emmons reaction, Knoevenagel condensation, and Wittig reaction. The inherent mildness, good chemical compatibility, and low cost of these reactions are still evident in polymer synthesis, and Table 4 shows examples of specific applications of such reactions.
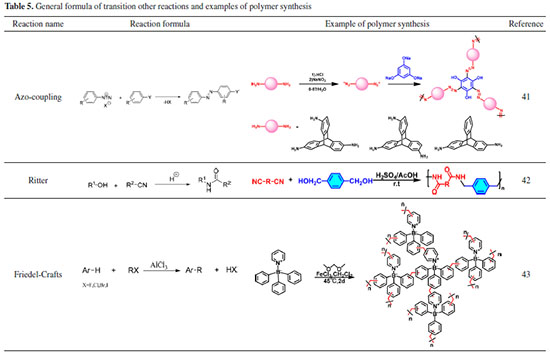
Other reactions In addition to the reactions mentioned above, there are a few noteworthy reactions that can prepare main chains that are difficult to achieve using traditional polymerization methods. The Ritter reaction enables the easy preparation of polyetheramides in high yields at room temperature; the Friedel-Crafts reaction enables the easy preparation of conjugated and porous polymers using inexpensive commercial feedstocks and catalysts; and the Azo-coupling reaction enables the easy preparation of polymers with azo backbones, as shown in Table 5 for example applications.
THE APPLICATION OF NAMED REACTIONS IN THE TEACHING OF POLYMER CHEMISTRY IN PRACTICE The named reaction is used in the introduction class of polymer chemistry The introduction class is a brief overview of the history of the subject and the course content, and it is usually not included in the final exam, which makes some teachers pay less attention to the introduction class than to the main content of the course. In fact, the introduction class is extremely important, and its content may influence the teaching of the main content of the course.44 A good start is almost halfway to success, and if the introductory class can stimulate students' interest and motivation in the course, it will be of great help to the subsequent development of the course and to achieve good teaching results. Since many students in secondary school and even in university are more exposed to organic chemistry and generally like it, and the previous course of polymer chemistry is organic chemistry,45 therefore, in the introduction class, in addition to the content of the introductory textbook, the characteristics of the course, the specific requirements for students to learn and the teaching methods and plans, etc., we can introduce some of the named reactions. For example, the Diels-Alder reaction in Figure 1, the aldol condensation reaction in Table 4, and the Friedel-Crafts acylation reaction in Table 5 are well-introduced in organic chemistry and familiar to students. The teacher can briefly discuss how the well-known reactions in the field of organic synthesis can be applied to the construction of polymer main chains and the significance of the reactions for polymer synthesis, etc.46,47 Such lectures can make the connection between polymer chemistry and organic chemistry closer and eliminate the sense of separation between disciplines and students' unfamiliarity with new knowledge to a certain extent. This will stimulate students' interest in learning, motivate them to learn, and promote self-reliance in their knowledge system.48,49 Named reactions in the polymer chemistry classroom teaching Polymer chemistry knowledge is organized into chapters. As a result, in the introduction teaching of a chapter, we can introduce certain named reactions connected to the chapter's content to increase students' interest in learning. During the teaching process, we can introduce various forms of reactions based on the content of the teaching to widen students' knowledge horizons while learning relevant knowledge. For example, during the free radical polymerization teaching process, we can introduce Bergman cyclization, McMurry reaction, Scholl coupling, Wurtz coupling, and other reactions based on the teaching content, so that students fully understand traditional free radical polymerization and feel the different methods of free radical generation, thus deepening their understanding. Typical organic chemistry condensation reactions such as Aldor condensation, Baylis-Hillman reaction, HWE reaction, Knoevenagel reaction, Michael addition, Wittig reaction, and so on can be introduced during the teaching of the condensation polymerization chapter. The typical condensation chapter of polymer chemistry normally only discusses the preparation of polyamides, which might easily lead students to believe that condensation polymerization is limited to the functional groups between single carboxylic acid derivatives. Properly introducing the aforementioned reactions can have the impact of improving thinking and broadening horizons. While textbook knowledge is slowly updated, course materials and lesson plans can be revised as needed to better introduce named reactions to assist in teaching. This allows the course to reflect the latest developments in the subject based on textbook knowledge, increase student interest and enthusiasm for learning, mobilize students' initiative, and creativity, enrich students' imagination, stimulate learning enthusiasm, and thus improve students' learning efficiency and teachers' teaching effectiveness. The teaching content is no longer limited to the textbook, and the teaching techniques become more scientific and emphasize the features of topic linkage, which also plays a significant role in supporting teachers' personal growth. Furthermore, polymers synthesized via specific reactions can be used in class assignments, quizzes, and even exams to assess polymer knowledge and structures. The knowledge and methods employed in the final solution remain belong to the textbook, allowing students to widen their horizons and cement their knowledge when completing assignments or tests. Named reactions in polymer chemistry frontier prospective teaching Two new named reactions have been employed for the first time in the synthesis of polymer backbones in recent years. Wan's group demonstrated the first effective application of the Barbier reaction in polymer synthesis in 2017, creating poly-(monobenzylmethanol), poly-(dibenzylmethanol), and poly-(triphenylmethanol).50 He also reported a new technique for polymerization in 2021, namely the usage of electrophilic nitro, and nitrite to generate C−N linkages for the construction of polyarylamine. A series of macromolecules, including o-polyaniline, p-polyaniline, m-polyaniline, para-polymethylaniline, and polynaphthylamine, were synthesized using Barbier-type nitro/nitroso addition polymerization,51 the majority of which are difficult to synthesize using other current polymerization methods. Brantley et al.52 first used the Doering LaFlame-Skatteböl rearrangement reaction for the construction of polymer backbones in 2020 and successfully prepared polymers with propadiene in the backbone, and the resulting materials exhibited unexpected photophysical properties (e.g., fluorescence), and the molecule, due to the presence of a more reactive propadiene segment, could facilitate subsequent reactions (e.g., [2 + 2] cycloaddition and gold-catalyzed multicomponent coupling),35 which makes this method promising.
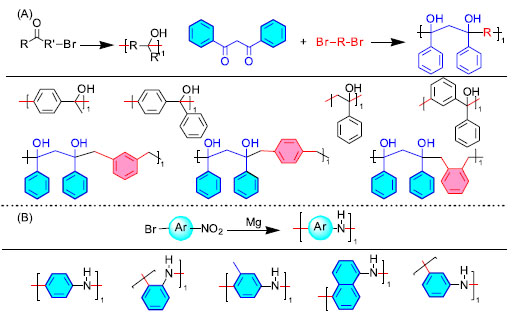 Figure 3. Application examples of Barbier reaction in polymer backbone synthesis50,51
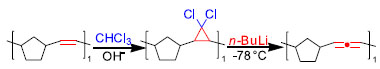 Figure 4. Application examples of Doering LaFlame-Skatteböl in polymer backbone synthesis52
The introduction of new named reactions for the construction of polymeric backbones can be beneficial in enhancing students' sense of innovation, cross-disciplinary skills, and heightened awareness of the body of knowledge according to cognitive psychology.53,54 In addition, the introduction of frontier knowledge is a good way to improve teaching effectiveness by stimulating students' interest in learning. Xue and Zhou55 have discussed the principles that should be grasped when introducing frontier knowledge, and the authors believe that the following two principles should be paid attention to in the teaching of polymer chemistry. Principle of diversity and novelty: polymer chemistry is the core curriculum of polymer materials majors, and the research fields encompassed by polymer materials are also more diverse, so it is necessary to further broaden the frontier knowledge and increase the diversity of frontier knowledge. For example, transition metal-catalyzed coupling reactions can be used as a clue to link frontiers related to conducting polymers, and D-A reactions can be used as a clue to link frontiers related to self-healing and thermally responsive materials. In addition, teaching materials for frontier knowledge should be kept up-to-date, otherwise, they cannot better reflect the characteristics of the "frontier". Teachers should always browse many information sources, including literature and scientific news, to constantly replenish and update the teaching materials of frontier knowledge in the discipline. The principle of inspiration: the high level of teaching is "teaching people how to fish", and "stimulating interest and motivation" is the ultimate purpose of introducing frontier knowledge in basic chemistry teaching. The teacher should not spoon-feed students' knowledge, but rather inspire them to gain more frontier knowledge on their own. For example, when the Diels-Alder reaction was introduced, pupils were piqued. Nevertheless, rather than expanding on the Diels-Alder reaction, we encouraged students to consider the characteristics of the "self-healing elastomer" generated from it, as well as what scientific research laws are mirrored in its preparation and process. While inspiring students to acquire more frontier knowledge, teachers should also provide some guidance, such as introducing students to the library's paper and electronic resources, recommending students to follow websites, blogs, and WeChat sites that disseminate science news, participating in students' online discussions, and encouraging students to write popular science articles, etc., so that on the one hand, they can promote teaching through research, and on the other hand, they can become windows for students to see the frontier.
COMPARISON AND FEEDBACK In the realm of polymer chemistry education, various efforts have been made to enhance the teaching methodologies. Some have attempted to incorporate the historical development of polymers into their instruction,56 while others have sought to introduce polymer chemistry concepts at an earlier stage within the educational system.57 Additionally, there are those who have endeavored to integrate the concepts of inorganic polymers into the teaching framework of polymer chemistry.58 Regardless of the perspective taken, these endeavors represent concerted efforts aimed at improving the teaching of polymer chemistry. This paper takes a novel approach by exploring the application of named reactions in polymer chemistry education. By leveraging the inherent fascination associated with named reactions, this teaching methodology has received positive feedback. Its utilization has effectively infused the field of polymer chemistry education with an engaging and captivating instructional approach.
CONCLUSION Interest is the best teacher and the motivating force behind the invention.59 Improving the efficiency of classroom instruction requires stimulating students' enthusiasm for learning.60 The expansion of the field of application of classical organic named reactions not only supports organic chemistry development but also considerably expands the library of monomers for polymer synthesis, which in turn stimulates the development of the field of polymer synthesis. The creation of an innovative polymer backbone is extremely beneficial to the development of novel materials. Scientists' continuous pursuit of unique and complicated macromolecular structures has also resulted in the creation of a variety of efficient polymerization tools based on named reactions.11-13 The purpose of this work is to try to incorporate named reactions into the teaching of polymer chemistry. On the one hand, it can assist students in learning polymer chemistry in a more closely related way to the organic chemistry they have already learned, and it is worthwhile to promote it in the teaching of organic chemistry and organic synthetic chemistry for both students and teachers. On the other hand, we call for more polymer workers to review and study the existing reactions, and we look forward to more named reactions in the field of polymer synthesis.
REFERENCES 1. Furrow, M. E.; Myers, A. G.; J. Am. Chem. Soc. 2004, 126, 5436. [Crossref] 2. Dai, X. J.; Li, C. J.; J. Am. Chem. Soc. 2016, 138, 5433. [Crossref] 3. Pan Changduo, Y. J.; Yunan Chemical Technology 2022, 49, 140. [Crossref] 4. Jie, J. L.; Name Reactions, 3rd ed.; Springer Cham: United States, 2008, p. 1-3. 5. Kurti, L.; Czako, B.; Strategic Applications of Named Reactions in Organic Synthesis, illustrated ed.; Elsevier: California, 2005, p. 3-5. 6. Wang, S.; Urban, M. W.; Nat. Rev. Mater. 2020, 5, 562. [Crossref] 7. Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G.; Chem. Soc. Rev. 2015, 44, 6684. [Crossref] 8. Jiang, X.; Feng, C.; Lu, G.; Huang, X.; Sci. China: Chem. 2015, 58, 1695. [Link] accessed in July 2023 9. Lu, D.; Zou, X.; Li, C.; High Perform. Polym. 2023, 35, 439. [Crossref] 10. Lu, D.; Zou, X.; Ye, L.; Polym. Adv. Technol. 2023, 34, 2097. [Crossref] 11. Koltzenburg, S.; Maskos, M.; Nuyken, O.; Polymer Chemistry, 1st ed.; Springer Berlin: Heidelberg, 2017, p. 3-5. [Crossref] 12. Carraher Junior, C. E.; Carraher's Polymer Chemistry, 10th ed.; CRC Press: Boca Raton, 2017, p. 3-8. 13. Hiemenz, P. C.; Lodge, P. T.; Polymer Chemistry, 3rd ed.; CRC Press: Boca Raton, 2020, p. 12-13. 14. Deng, X. Q. Y.; Basic Organic Chemistry (II), 4th ed.; Peking University Press: Beijng, 2017, p. 10-12. 15. Ohishi, T.; Sone, T.; Oda, K.; Yokoyama, A.; RSC Adv. 2019, 9, 40863. [Crossref] 16. Nojima, M.; Saito, R.; Ohta, Y.; Yokozawa, T.; J. Polym. Sci., Part A: Polym. Chem. 2015, 53, 543. [Crossref] 17. Wang, K.; Dong, N.; Liu, Z.; Shi, M.; Zhang, B.; Wang, J.; Chen, Y.; Polym. Chem. 2019, 10, 6003. [Crossref] 18. Inagaki, S.; Yamamoto, T.; Higashihara, T.; Macromol. Rapid Commun. 2020, 41, e2000148. [Crossref] 19. Iraqi, R.; Murad, A.; Aziz, S. B.; Abdullah, S.; Abdulwahid, R.; Polymers 2020, 12, 2791. [Crossref] 20. Lee, J.; Kim, H.; Park, H.; Kim, T.; Hwang, S. H.; Seo, D.; Chung, T. D.; Choi, T. L.; J. Am. Chem. Soc. 2021, 143, 11180. [Crossref] 21. Prabakaran, P.; Satapathy, S.; Prasad, E.; Sankararaman, S.; J. Mater. Chem. 2018, 6, 380. [Crossref] 22. Subnaik, S.; Sheridan, K.; Hobbs, C. E.; Macromol. Chem. Phys. 2021, 222, 200098. [Crossref] 23. Ai, Y.; Fan, Z.; Journal of Sichuan Normal University, Natural Science 2000, 23, 197. [Crossref] 24. Cristurean, D.; Schaumüller, S.; Strasser, P.; Haudum, S.; Himmelsbach, M.; Bechmann, M.; Brüggemann, O.; Teasdale, I.; Polym. Chem. 2021, 12, 3160. [Crossref] 25. Yue Guizhou, L. B.; J. Org. Chem. 2020, 40, 3132. [Crossref] 26. Liu, X.; Xiang, L.; Li, J.; Wu, Y.; Zhang, K.; Polym. Chem. 2020, 11, 125. [Crossref] 27. Li, B.; Wu, Y.; Wang, Y.; Zhang, M.; Chen, H.; Li, J.; Liu, R.; Ding, Y.; Hu, A.; ACS Appl. Mater. Interfaces 2019, 11, 8896. [Crossref] 28. Phang, S. W.; Daik, R.; Abdullah, M. H.; Polym. Test. 2004, 23, 275. [Crossref] 29. Cui, Y.; Xu, Z.; Li, H. Y.; Young, D. J.; Ren, Z. G.; Li, H. X.; ACS Appl. Polym. Mater. 2020, 2, 4512. [Crossref] 30. Zhang, M.; Zhou, Y.; He, M.; Zhang, T.; Ding, Q.; J. Appl. Polym. Sci. 2018, 135, 46335. [Crossref] 31. Guo, Z.; Wang, W.; Yang, Y.; Majeed, K.; Zhang, B.; Zhou, F.; Zhang, Q.; Polym. Chem. 2021, 12, 2009. [Crossref] 32. Desmaizieres, G.; Speer, M. E.; Thiede, I.; Gaiser, P.; Perner, V.; Kolek, M.; Bieker, P.; Winter, M.; Esser, B.; Macromol. Rapid Commun. 2021, 42, e2000725. [Crossref] 33. Parveen, S.; Kim, H.; Polym. Eng. Sci. 2018, 58, 1766. [Crossref] 34. Zhang, D.; Zheng, J.; Zhang, P.; Zhao, R.; Chen, Z.; Wang, M.; Deng, K.; Macromol. Chem. Phys. 2021, 222, 2100284. [Crossref] 35. Onwubiko, A.; Yue, W.; Jellett, C.; Xiao, M.; Chen, H. Y.; Ravva, M. K.; Hanifi, D. A.; Knall, A. C.; Purushothaman, B.; Nikolka, M.; Flores, J. C.; Salleo, A.; Bredas, J. L.; Sirringhaus, H.; Hayoz, P.; McCulloch, I.; Nat. Commun. 2018, 9, 416. [Crossref] 36. Ji, S.; Bruchmann, B.; Klok, H. A.; Macromolecules 2011, 44, 5218. [Crossref] 37. Jiang, Q.; Zhang, Y.; Du, Y.; Tang, M.; Jiang, L.; Huang, W.; Yang, H.; Xue, X.; Jiang, B.; Polym. Chem. 2020, 11, 1298. [Crossref] 38. He, Y.; Ma, W.; Yang, N.; Liu, F.; Chen, Y.; Liu, H.; Zhu, X.; Chem. Commun. 2021, 57, 7557. [Crossref] 39. Lin, J.; Bi, S.; Fan, Z.; Fu, Z.; Meng, Z.; Hou, Z.; Zhang, F.; Polym. Chem. 2021, 12, 1661. [Crossref] 40. Pilicode, N.; Naik, P.; Adhikari, A. V.; Polym. Eng. Sci. 2020, 60, 2550. [Crossref] 41. Bera, R.; Ansari, M.; Alam, A.; Das, N.; ACS Appl. Polym. Mater. 2019, 1, 959. [Crossref] 42. Lakouraj, M. M.; Mokhtary, M.; Polym. Int. 2009, 58, 1167. [Crossref] 43. Chen, J.; Jiang, L.; Li, C.; Fu, W.; Xia, Q.; Wang, Y.; Huang, Y.; Polym. Eng. Sci. 2020, 61, 662. [Crossref] 44. Yang, Z. J.; Yang, X. D.; Xinan Shifan Daxue Xuebao, Ziran Kexueban 2011, 12, 39. [Crossref] 45. Klara, K.; Hou, N.; Lawman, A.; Wang, L. Q.; J. Chem. Educ. 2013, 90, 401. [Crossref] 46. Zhang, J. Y.; Xia, C.; Wang, H. F.; Tang, C.; J. Energy Chem. 2022, 67, 432. [Crossref] 47. Weizman, H.; Nielsen, C.; Weizman, O. S.; J. Chem. Educ. 2011, 88, 1137. [Crossref] 48. Farmer, S. C.; Schuman, M. K.; J. Chem. Educ. 2016, 93, 695. [Crossref] 49. Moore, J. W.; Stanitski, C. L.; J. Chem. Educ. 2017, 94, 1603. [Crossref] 50. Sun, X. L.; Liu, D. M.; Tian, D.; Zhang, X. Y.; Wu, W.; Wan, W. M.; Nat. Commun. 2017, 8, 1210. [Crossref] 51. Su, M.; Li, T.; Shi, Q. X.; Xiao, H.; Bao, H.; Wan, W. M.; Macromolecules 2021, 54, 9919. [Crossref] 52. Galan, N. J.; Brantley, J. N.; ACS Macro Lett. 2020, 9, 1662. [Crossref] 53. Li, J.; Mei, C.; Xu, W.; Qian, Y.; Inf. Sci. 2015, 298, 447. [Crossref] 54. Kirschner, P. A.; Sweller, J.; Kirschner, F.; Zambrano, R. J.; International Journal of Computer-Supported Collaborative Learning 2018, 13, 213. [Crossref] 55. Xue, B.; Zhou, D.-X.; Huaxue Jiaoyu 2015, 36, 24. [Crossref] 56. Flores-Morales, P.; Requena, V. H. C.; Gatica, N.; Muñoz, C.; Pérez, M. A.; Rivas, B. L.; Sánchez, S. A.; Suwalsky, M.; Tapiero, Y.; Urbano, B. F.; J. Chem. Educ. 2017, 94, 1702. [Crossref] 57. Bopegedera, A. M. R. P.; J. Chem. Educ. 2017, 94, 1725. [Crossref] 58. de Lill, D. T.; Carraher Junior, C. E.; J. Chem. Educ. 2017, 94, 1674. [Crossref] 59. Yan, L.; Yang, L.; Guodong, Y.; Yulin, Z.; Jiahui, L.; Lirong, T.; Jing, X.; Experimental Technology and Management 2015, 09, 176. [Crossref] 60. Shuang, C.; Hengfu, W.; Modern Education Management 2014, 09, 100. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access