Revisão
| Chemical composition, biological activities and uses of anacardiaceae species: an updated review |
|
André Barreto Cunha*; Jorge Mauricio David*
Instituto de Química, Universidade Federal da Bahia, 40170-280 Salvador - BA, Brasil Recebido em 05/04/2023 *e-mail: jmdavid@ufba.br; andrebarretoc@outlook.com The present review, with 169 references, describes a critical updated compilation of studies regarding the Anacardiaceae family. Firstly, it is shown a detailed report of the chemical composition (essential oils, terpenoids, flavonoids, alkyl and alkenyl phenols, and other compounds) of species of all studied genera, followed by the biological properties (in vitro and in vivo activities) of extracts, enriched fractions, and pure new isolated metabolites. Furthermore, it is reported herein that some deposited processes developed with Anacardiaceae spp. (cosmetic and pharmacological compositions, besides some technological applications) as well as new findings about the biosynthesis of phenolic lipids, the primary chemical marker of the family. Consequently, these outcomes highlight the relevance of this family in developing natural products' chemistry from 2006 to now. INTRODUCTION Anacardiaceae is a family consisting of about 600 species distributed in 76 genera. The genera are subdivided into five tribes (Anacardieae, Dobineae, Rhoeae, Semecarpeae, and Spondiadeae). The plants of this family are known as sources of edible fruits and condiments such as mango, cashew, pistachios (Pistacia spp. ), sumac (Rhus coriaria) and pink peppercorns (Schinus terebinthifolia). Approximately 25% of genera present toxic phenolics, compounds responsible for several contact dermatitis. In general, the poisonous species of this family are restricted to the tribes Anacardieae, Rhoeae, and Semecarpeae.1,2 Phenolic and catecholic lipids are usual compounds present in these plants, which are usually responsible for their toxic properties, whether alone or in mixtures of different saturated or unsaturated aliphatic chains. These compounds are present in different plant parts and frequently occur in Rhus species. Thus, species of this family have been frequently studied from a chemical and biological point of view due to their potential as sources of new bioactive compounds. The most studied genera are Mangifera,Spondias,Lannea, Toxicodendron (Rhus),Schinus, Pistacia, Lithraea,Tapirira,Semecarpus,Melanorrhoea and Anacardium. However, most of Anacardiaceae species remain unknown regarding their chemical composition, alongside pharmacological and other biological activities. Although recent reviews about some classes or specific compounds in Anacardiaceae3,4 and genus Schinus5 were performed, there are just two reviews of all families, the last one dated from 2006.1,2 The current review with approximately 170 references is an update of the chemical composition, biological activities in extracts and pure compounds isolated from different Anacardiaceae plant species since 2006. Moreover, some processes, technological applications and new insights about the biosynthesis of phenolic lipids were also reviewed.
LITERATURE SEARCH STRATEGY A bibliographic survey of scientific articles published in indexed journals and deposited process patents was performed to develop this review. For this purpose, the databases SciFinder, Web of Science, Science Direct, PubMed, Scielo and Google Scholar were utilized. All articles published from 2006 to March 2023 were considered, including papers not considered in a previous review. In the quest "Advanced search" feature combined with the keywords "Anacardiaceae", "chemical composition", "bioactivity", "biosynthesis", and all the genera described in specialized literature were used.6 The titles of articles and patents found were scanned and organized in a file when considered meaningful. Afterward, duplicates were removed and, thus, the abstracts of the articles obtained were checked for relevant information as part of the inclusion strategy. Finally, all articles and patents were carefully read and, after reviewing, 149 papers and 21 patents were selected to compose the present work.
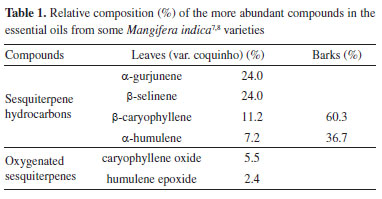
CHEMICAL COMPOSITION OF ANACARDIACEAE spp. Essential oils (EOs) and volatile other compounds (VOCs) Studies concerning to essential oils (EOs) and volatile organic compounds (VOCs) of Anacardiaceae family have been frequently developed, especially for edible species such as fruits and seeds. The EOs are usually obtained from plants' leaves, flowers and other aerial parts. The most recent works will be highlighted herein, including the compositions of these metabolites in different species and genera, the identification of new substances and other relevant information. The investigation of the EOs' chemical composition of Mangifera indica (var. "coquinho")7 indicated that the sesquiterpene hydrocarbons are the leading representative compounds (66.4%) against the oxygenated ones (8.7%), which have presented anticancer, antimicrobial and antioxidant activities. On the other hand, in barks of M. indica L. was reported8 that the sesquiterpene hydrocarbons reached 97.0% in an analog study (Table 1). The other components of these EOs are monoterpenes (≤ 2%).
Rhus cotinus L. (syn. Cotinus coggygria Scop. ) is a European tree commonly grown as an ornamental plant, presenting different cultivars due to the different purple foliage and flowers. The wood of this species presented economic importance, since it was formerly used to make the yellow dye called young fustic (fisetin), now replaced by synthetic dyes. The profile of EOs obtained from the same species varies according to the biome, the part of the plant (fresh aerial parts,9 leaves10 or flowering aerial parts11) and seasonally, even between regions that are close in latitude. Thus, the total composition of monoterpenes was not similar in both cases (Table 2).
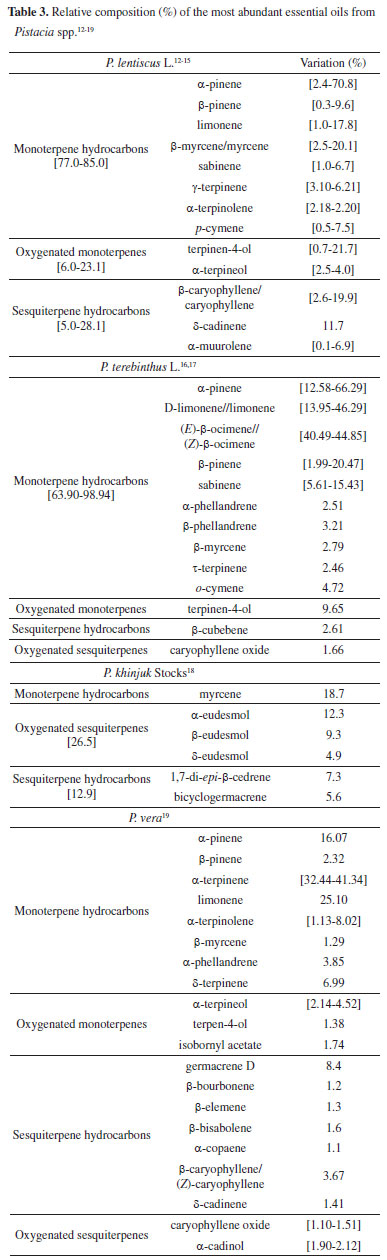
Pistacia spp. (such as P. lentiscus L. , P. vera, P. terebinthus L. , and P. khinjuk Stocks) are employed in Europe and the Mediterranean region as food, for cooking, and for other purposes (e.g. , the oleoresin). Consequently, this is the most probable reason for the expressive number of studies dealing with the EO composition of these species. The EOs profile of P. lentiscus is similar to species from different habitats12 (Southern Italy and Morocco, Tunisia, Greece or France), but distinct from other EO profiles obtained from specimens from Egypt, Sardinia Island/Italy and Spain, probably due to the different climate and seasonal changes, besides insect presence, physicochemical soil properties, extraction methods and others. Furthermore, mastic gum essential oils (MGEOs) of wild plants of P. lentiscus13 are quantitatively different compared to the cultivated plants, so that the tree age could be another affecting this chemical composition. Other subsequent studies14,15 present several data that confirm the exact behavior of the EOs profile. Similarly, the EOs content in P. terebinthus16,17 is also related to the plant organ and population origins. In these studies, the variability of the composition was carefully analyzed by the principal component analysis (PCA), and the conclusion is that abiotic (climatic, edaphic, chemical, among others) and biotic (genotypic diversity and nutritional variations) factors may be related to these variations. At last, since no previous published data deals with the P. khinjuk EO leaf profile, it was impossible to compare the current study with the EO composition of other P. khinjuk18 trees from other regions (particularly from Iran). Studies employing PCA (Principal Component Analysis) and HCA (Hierarchical Cluster Analysis) permitted to evaluate if EO constituents could reflect the chemotaxonomic relationships in Pistacia species. Based on the most abundant compounds present in the EOs (contents ≥ 3.5%), the groups were classified as chemotypes (i) Group A (α-pinene, β-pinene, limonene and terpinen-4-ol, P. lentiscus) and (ii) Group B (B1, α-terpinene, P. terebinthus; B2, limonene, P. vera).19 Table 3 summarizes the volatile compounds from Pistacia spp. , and the examination of the data clearly indicates the monoterpenes are the main compounds - especially α- and β-pinenes (in P. lentiscus), α-pinene, limonene, and β-ocimene (in P. terebinthus and P. vera) and myrcene and eudesmol (in P. khinjuk).
Spondias L. is a genus with about ten species, occuring mainly in Asia, three or four species native to the Neotropics, most of them produce edible fruits. A previous study with S. pinnata from east India showed that the major VOCs of whole green fruits were isopropyl myristinate (36.85%), isophorone (6.55%), limonene (4.46%) and linalool (3.57%).20 However, the EOs from fruits of specimens growing in Egypt was composed mainly of long-chain alkanes (51.1%) besides fatty acid esters (25.7%). The relative most predominant component was n-nonacosane (25.0%).21 Therefore, these results indicate that the profile of the significant constituents of EOs in the green fruits, ripe fruits and fruit peels can change with the plant part studied, even though the extraction methods or geographic locations could also influence such differences, which may partly determine the variation in bioactivity.22 Variations in the EO compositions could be related to the investigated species' cultivation, vegetative stage, source or seasonal growing. Furthermore, an increase in the oxygenated monoterpenoid amount, as well as a decrease in the sesquiterpenoid hydrocarbons content, was observed due to the dehydrating of the leaves, while the contents of some minor metabolites (geraniol, eugenol, borneol, terpinen-4-ol, besides others) were stable in the two oils, although were present in small quantities (< 1.0%).23 Table 4 summarizes the data and presents additional compounds of some Spondias spp.
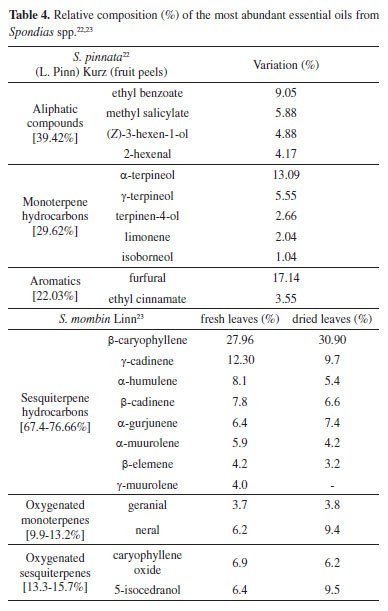
Table 5 summarizes the last updates in the VOCs' content of Schinus species.24-38 Different parts of Schinus terebinthifolia Raddi (sin.: Schinus terebinthifolia Raddi) and S. molle L. are widely studied, probably due to the employment of these species as folk medicines, and the fruits are used as spicier (pink pepper). The studies with the composition of S. terebinthifolia leaves EOs corroborated with seasonal variation previously observed. The oil obtained from specimens harvested in March showed a high concentration of myrcene (15.4%) and (E)-caryophyllene (14.7%); in July, these constituents represented only 0.8% and 2.7% (respectively) of the total oil. Germacrene-D content increased from 8.8% in March to 21.0% in July, whereas α-phellandrene, undetectable in oils collected in March, rose to 18.2% in July. The EOs obtained in July contained 15.5% of oxygenated sesquiterpenes, and these compounds are present in only 5.8% in the oils obtained from March studies.31
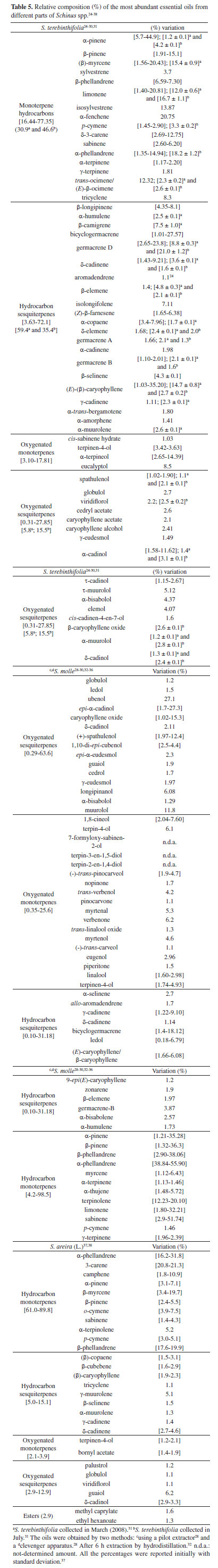
Other Schinus species are frequent sources of essential oils (S. longifolia,S. fasciculata,S. lentiscifolius and S. weinmannifolius).39-41 Likewise, in the former examples, the differences between the found EOs profiles are related to seasonal factors, extraction methodologies and geographical origin. Anacardium genus always presents commercially and economically important species, which have justified extensive studies with its main species, including their flavor-related volatile compounds. Studies with Brazilian A. occidentale L. oil, occurring in different regions, indicate differences in the chemical compositions of major compounds, whose differences are probably associated with genetic variability amongst the populations grown at each location. In the leaf species collected in Minas Gerais state (Brazil), (E)-caryophyllene (15.4%), germacrene-D (11.5%) and α-copaene (10.3%) are the main components. On the other hand, the major compounds from plants cultivated in Pará state (Brazil) were (E)-β-ocimene (28.8%) and α-copaene (13.6%). Compared with specimens collected in Nigeria, the composition is also different, and those were composed mainly of β-phellandrene (42.7%).31 Table 6 also includes the composition of the VOCs of other Anacardiaceae species that were determined for the first time.42-45
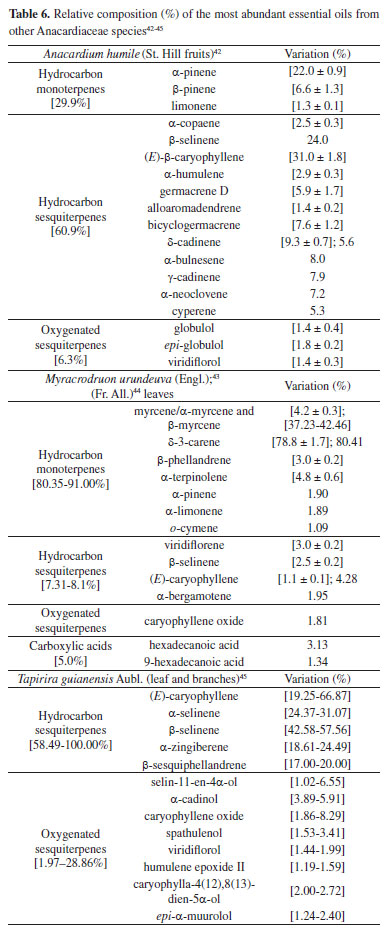
The literature reports presence of mixtures of essential oils and VOCs in other Anacardiaceae species, such as Pleyoginium timorense (Dc. ) Leenh,46 Pseudospondias microcarpa (A. Rich) Engl.47 and Sclerocarya birrea subsp. Caffra.48,49 In these examples, the most predominant metabolites in P. timorense fruits46 were D-limonene (64.51%), γ-terpinene (5.60%), α-copaene and (E)-caryophyllene (4.74%). In P.microcarpa fruits,47 α-terpinol and borneol (22.9% and 8.2%, for the epicarp), besides vaccenic acid and ascorbic acid 2,6-dihexadecanoate (20.1% and 29.8%, for the hull), caryophyllene oxide and α-humulene (8.4% and 6.8%, for the seed) and α-humulene and β-caryophyllene (9.4% and 6.4%, for the kernel) were the main compounds detected. Lastly, in Sclerocaryabirrea fruits,48 β-caryophyllene and α-humulene (91.3% and 8.3%) were the major compounds of fruit pulp. However, in head-space studies with the whole fruit, heptadecene (16.1%), benzyl 4-methylpentanoate (8.8%), benzyl butyrate (6.7%), (Z)-13-octadecenal (6.2%), cyclopentadecane (5.7%) and (Z)-3-decen-1-ol (8.4%) were the most abundant VOCs. Otherwise, it should be highlighted that the EOs composition of S. birrea (A. Rich) Hochst leaves from Benin were different according to the season.49 Thus, in hot period, the major constituents were 7-epi-α-selinene (38 ± 0.03%), α-muurolene (25 ± 0.03%), valencene (17 ± 0.06%), β-selinene (4.3 ± 0.01%), β-caryophyllene (3.2 ± 0.02%), allo-aromadendrene epoxide (1.5 ± 0.03%) and 14-hydroxy-α-humulene (1.5 ± 0.03%), but in the cold season the EOs was characterized by 7-epi-α-selinene (51.7 ± 0.12%), β-selinene (15.1 ± 0.2%), valencene (12.9 ± 0.05%), α-selinene (8.1 ± 0.03%) and β-caryophyllene (1.8 ± 0.02%). These results constitute the first report of these components in this species. Terpenoids and steroids Terpenoids are the most abundant class of natural products found in plants and have particular importance due to their role in plant physiology, biological properties and some industrial uses. They are present in different Anacardiaceae genera, and some isolates from this family are presented in Figure 1. The isolated compounds of these subclasses are well-known in plants in general as well as in Anacardiaceae spp. Compounds 1-8 were obtained from Mangifera mekongensis,50 so that the esters 1 (mekongsterol A), 2 (mekongsterol B) and 3 (β-sitosteryl-3-O-β-D-glucopyranosyl-6-O-palmitate) constitute novel derivatives, whereas stigmastane-3,6-dione (4), ambonic acid (5), ambolic acid (6), mangiferonic acid (7) and mangiferolic acid (8) are common in Mangifera. Besides, compounds 9-10 were obtained from Mangifera pajang Kosterm. ,51 metabolites 11-14 from S. terebinthifolia52 and daucosterol (15) from Schinopsis brasiliensis Engl.53 It is noteworthy that 14 was named as schinol and possesses a structure different from the previously registered compound named schinol (CAS #6813-07-6). The structure of compound 14 is previously known as the name of 3-epimasticadienolic acid (CAS #31539-04-5). The substances 11-13, found in the fruit oil of S. terebenthifolia, can be associated with the demonstrated antioxidant activity of the species, to the inhibition of NO synthase production and to antimicrobial properties, as well as 14 is related to antifungal activity52 against Paracoccidioidesbrasiliensis. From the roots of Dobinea delavayi (Baill. ) Baill. were isolated several sesquiterpenes, including new compounds (16-23),54 and ergostane-type compounds (24-27) were obtained from the stem bark of Antrocaryon klaineanum Pierre.55 The novel compound antrocarine E (24) was obtained with the known substances (7α)-7,20-dihydroxyergosta-4,24(28)-dien-3-one (25), (6α,7α)-6-methoxyergosta-4,24(28)-dien-7-ol (26) and (6α,7α)-ergosta-4,24(28)-diene-6,7-diol (27). Lastly, the new steroid-type compound named 3-oxolanosta-1,20(22)-dien-26-oic acid (28) was isolated from the galls of Pistacia integerrima Stewart.56
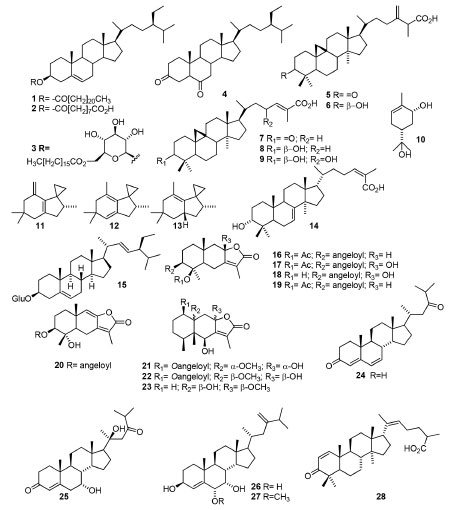 Figure 1. Structures of terpenes and terpenoids obtained from plants of different Anacardiaceae species
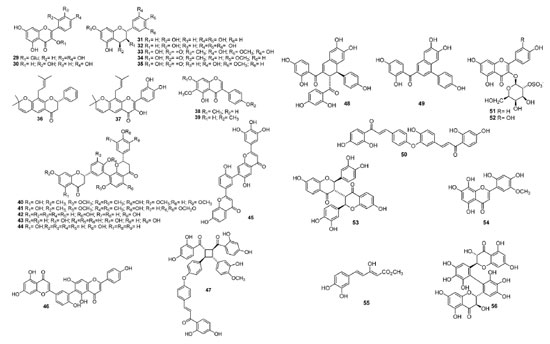
Flavonoids and biflavonoids Flavonoids are common in all plant kingdom, but biflavonoids are restricted in some families, including Anacardiaceae.1 Figure 2 presents an update of the presence of this class (29-39) in species of this family. They were isolated from from Lanneacoromandelica and L. acida. 57 From Semecarpus anacardium Linn. the biflavonoids nallaflavanone (40), anacarduflavanone (41), jeediflavanone (42), galluflavanone (43), tetrahydroamentoflavanone (44) and semecarpuflavone (45) were obtained.58 This flavonoid subclass is common in Anacardiaceae spp. Robustaflavone (46) was obtained for the first time from the leaves of S. terebinthifolia59 and some chalcone derivatives - schinopsone A (47) and schinopsone B (48), besides two known biflavonoid derivatives53 (49, 50) - were isolated from the roots of Schinopsis brasiliensis Engl. Moreover, kaempferol-3-O-β-(2"-sulphategalactopyranoside) (51) and quercetin-3-O-β-(2"-sulphategalactopyranoside) (52)60 were obtained for the first time from aqueous methanol leaf extract of Harpephyllum caffrum. In addition, a novel dimer (53) C-3/C-3'' of butin (3',4',7-trihydroxyflavanone) was isolated from C. coggygria Scop. wood61 alongside other known compounds (catechin, fisetin, quercetin, butein, sulfuretin, fustin, dihydroquercetagetin, and eriodictyol). From MeOH and EtOH antioxidant extracts of Pistacia terebinthus L. fruits62 it was isolated the new flavone 2-(2,4-dihydroxy-5-methoxyphenyl)-5,7,8-trihydroxy-4H-1-benzopyran-4-one (54) besides other known flavonoids (apigenin, luteolin, quercetin and luteolin-7-O-glucoside). On the other hand, the novel hispolone derivative 55 (methyl 5-(3,4-dihydroxyphenyl)-3-hydroxypenta-2,4-dienoate)63 was obtained from the mushroom Inonotus hispidus growing on Pistacia atlantica as well as hispolone, hispidin and other phenolic compounds. The compound 56 (named acuminatanol)64 was the first 2'2'''-bis-dihydrobiflavonol isolated from the aqueous extract of Trichoscypha acuminata, being the first example of a bis-dihydroflavonol linked exclusively via the B-rings at C-2' and C-2''' positions. At last, the phytochemical investigation of the leaves of Sorindeia juglandifolia A. Rich. led to the obtention of a new C-glucosylflavone (2'',6''-di-O-acetyl-7-O-methylvitexin),65 besides other seven known compounds.
Alkyl and alkenylphenols Alkyl and alkenylphenols, also known as phenolic lipids, are chemotaxonomic markers of various species of Anacardiaceae. In general, they present a salicylic acid moiety, but some are decarboxylated structures. Figure 3 presents the structures of several alkyl and alkenylphenols isolated from Anacardiaceae spp. Ozorcardic acids A (57) and B (58), alongside anacardic acid (59), were obtained for the first time from Ozoroa pulcherrima Schweinf.66 Furthermore, 3-((7Z,10Z)-pentadeca-7,10-dien-1-yl)benzene-1,2-diol (60) and 3-((8Z)-pentadec-8-en-1-yl)benzene-1,2-diol (61) are kwown compounds now obtained from S. anacardium58 and the new alkyl resorcionols (,Z)-5-(trideca-4,7-dienyl)-benzeno-1,2-diol (62), (Z)-5-(trideca-4-enyl)-benzeno-1,2-diol (63), (Z,Z)-5-(pentadeca-6,9-dienyl)-benzeno-1,2-diol (64), (Z,Z)-5-(trideca-5,8-dienyl)-benzeno-1,2-diol (65) and (Z)-5-(heptadec-6-enyl)-benzeno-1,2-diol (66) from Lithraea molleoides67 Vell. Eng. Besides, 3-(2-(heptan-2-yl)-3-methylnonyl)phthalic acid (67) and 2-hydro-6-[(8'E, 11'E, 14'E)-22'-hydroxydocasa-8',11',14'-trienyl] benzoic acid (68) were obtained from sheets of Spondias mombin.68 The presence of (E)-double bonds and branched alkyl chains in 67 and 68 are unusual, whose detailed analysis of the NMR and MS data published indicates the need of new experiments to corroborate with the published unusual structures for these compounds. Moreover, three new dihydrobenzofuranoids [2-[(10'Z)-dodec-10'-enyl]-dihydro-1-benzofuran-5-ol (69), 2-[(10'Z)-tridec-10'-enyl]-dihydro-1-benzofuran-5-ol (70) and 2-[(10'Z)-pentadec-10'-enyl]-dihydro-1-benzofuran-5-ol] (71) were isolated from Tapiriraguianensis seeds.69 On the other hand, unusual dimeric alkylresorcinol named integracin E (72) was obtained from the stem barks of Swintonia floribunda, besides propyl ferulate.70 At last, gentisic acid derivative 73 (mycronic acid) has been isolated for the first time from Micronychia tsiramiramy roots71 with several known compounds previously isolated.
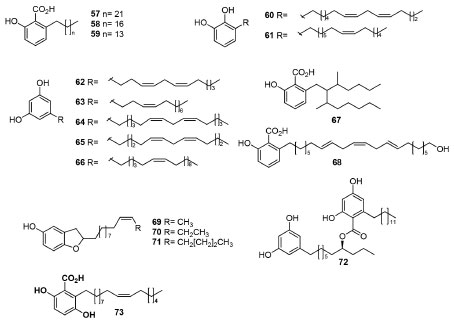 Figure 3. Alkyl, alkenylphenols and acids isolated from several Anacardiaceae species
Miscellaneous compounds isolated from Anacardiaceae Many other types of metabolites that can occur in Anacardiaceae spp. and were reported in the period of this review update (Figure 4), such as the simple phenolic derivatives butein (74) and anacardoside (75) from Semeacarpus anacardium Linn.58 Besides, for the first time 1,2,3,4,6-penta-O-galloyl-glucopyranoside (PGG, 76) was isolated from Schinusterebeinthifolia59 and three new metabolites [1,2-benzenedicarboxylic acid-mono(2-ethylhexyl)ester (77), (9E,12E)-tetradeca-9,12-dien-1-yl acetate (78) and 3-chloro-N-(2-phenylethyl)propanamide (79)], the last two atypical compounds, from Mangiferaindica.72 (+)-Pinoresinol (80), syringaresinol (81) and (+)-epi-pinoresinol (82) were obtained from the stem barks of Swintoniafloribunda70 and the antioxidant compounds as the novel biaurone disulfuretin (83), sulfuretin (84) and sulfurein (85) were isolated of two separate collections of Cotinuscoggygria (R. cotinus),73 all of them for the first time in these genera. Moreover, the new lignan (+)-(8S,8'S)-5'-metoxi-4,4'-di-O-methylsecoisolariciresinol (86)74 was obtained from stems of Buchanania lucida. Other several studies described the isolation of many novel special metabolites, as the compounds 2,6,3',4'-tetrahydroxy-4-methoxybenzophenone (87), 2,6,4'-trihydroxy-4,3'-dimethoxybenzophenone (88) and dobiniside A (89) from the roots of Dobineadelavayi,75,76 3-methoxyellagic acid 4-O-galactopyranoside60 (90) from the leaves of H. caffrum and the fatty acid ester 91 from Cyrtocarpa procera77 Kunth (besides other known analogues). The new 1,4-benzoquinone derivative (92), which can be consider an alkenyl phenol derivative, was isolated from the root of M. tsiramiramy,71 and the novel benzofuran lactone 93 (rhuscholide A)78 was isolated from the stems of Rhus chinensis with other known compounds. At last, the new bischromanone 94 has been obtained from the stems of Semecarpus caudata79 alongside five known flavonoids (quercetin, naringenin, taxifolin, (+)-eriodictyol and 3,4',7-trihydroxyflavone) and two novel long-chain alkyl compounds 9,11-dihydroxyoctadecan-7-one (95) and (-)-3-hydroxydecyl eicosanoate (96) from the galls of Pistaciaintegerrima Stewart.56 The authors signed compound 95 as rel-(+)-(9R,11R) enantiomer; however, they did not present spectrometric data supporting the proposed stereochemistry. Bis(2-ethylhexyl) phthalate is a plasticizer and compound 77 could not be a natural product, as pointed out by the authors. However, once there is no evidence of optical light deviation of 77, a partial hydrolysate was synthesized from the commercial phthalate. For compounds 78 and 92, there are also no spectrometric evidence of the stereochemistry and carbon position of the double bonds of the linear carbon chains.
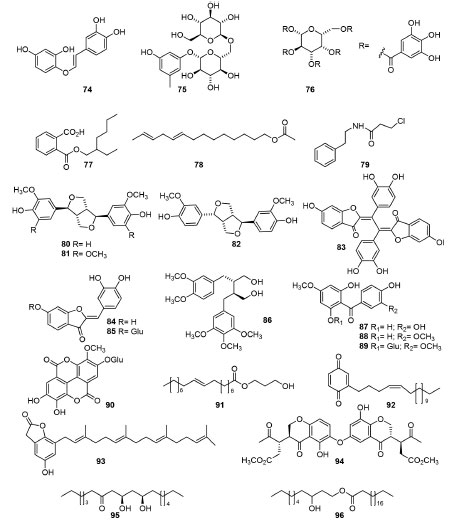 Figure 4. Miscellaneous compounds obtained from different Anacardiaceae spp
In conclusion, we could highlight the occurrence of β-sitosteryl-3β-glucopyranoside-6'-O-fatty acid esters, β-sitosterol, phytol, a mixture of phytyl fatty acid esters and β-sitosteryl fatty acid esters, chlorophyll, squalene, the compound 59 and other long-chain constituents in the CH2Cl2 extract of Dracontomelon dao (Merr. & Rolfe)80 leaves, as well as the isolation of 15 together with gallic acid and ethyl gallate from the EtOH extract of Mauria heterophylla.81
BIOLOGICAL ACTIVITIES Anacardiaceae family presents several species that produce compounds with different biological properties. Therefore, in the last decades, numerous studies have employed extracts and some isolated metabolites presenting in vitro and in vivo activities, mainly as radical quenching, antinociceptive and anti-inflammatory, as well as against microorganisms/strains, cell lines and viruses. Biological activities of extracts of Anacardiaceae spp. In vitro studies Lannea spp.82,83 biological studies such as aqueous extracts of L. barteri Engl. bark82 have presented antibacterial activity against Pseudomonas aeruginosa (MIC = 6.25-25.00 mg mL-1, LBE 6.25; 12.5; 25.0; 50.0 and 100.0 mg mL-1) and Acinetobacter baumannii (MIC = 25.00-43.75 mg mL-1, LBE 6.25; 12.5; 25.0; 50.0 and 100.0 mg mL-1), including MIC/MBC = 1.0 in all cases. These biological properties are probably due to phenolic/polyphenolic compounds in extracts, whose results may justify the plant's traditional use against urinary infections. Moreover, the ethanolic extract of L. velutina A. Rich83 has presented antioxidant (% DPPH inhibition: 52.81 ± 2.16; % Fe3+ reducing power/FRAP: 1.74 ± 0.45 mmol EAA 10 g extract-1) and antimicrobial activities (against Gram-positive and Gram-negative bacteria strains, with inhibition diameters greater than 8 mm), which is related to the flavonoid (1.770 ± 0.005 mg eq. Quercetin 10 g extract-1) and polyphenol (969.67 ± 8.23 mg GAE g extract-1) contents. Concerning the studies dealing with M. indica,84-87 it is known that compounds from this plant present many biological activities, typically related to mangiferin (97) and other polyphenolic compounds. The antibacterial activity84 of (seed) mango kernel extracts were attributed to 2,4-bis(1,1-dimethylethyl)phenol (98), and the inhibitory effect85 over PLA2 (phospholipase A2), hyaluronidase and LAAO (L-amino acid oxidase) is associated with PGG (99), which selectively block the PLA2 and LAAO active sites (Figure 5). Observed anticancer proprieties86 are possibly due to mangiferin, and other activities86,87 (e.g. , antidiabetic, antioxidant, and antimicrobial) might be associated with different compounds, such as aglycones, saponins and terpenes.
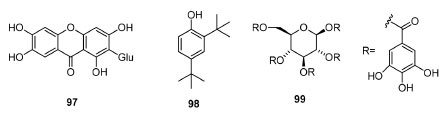 Figure 5. Some bioactive compounds from Mangifera indica
Crude extract and leaf EtOAc fraction of Pistacia spp. and P. atlantica Desf. displayed, simultaneously, a stronger antioxidant activity88 (DPPH assay: IC50 = 0.0273 ± 0.0001 and 0.0419 ± 0.0010 mg mL-1) in comparison on BHA and ascorbic acid (IC50 = 0.08 ± 0.002 and 0.06 ± 0.002 mg mL-1) in DPPH assay due to the presence of flavonoids and tannins. Pistaciaintegerrima Rechinger f. stems EtOAc and CHCl3 fractions extracts89 exhibited low to moderate antitumor activity, with dose-dependent cell viability (97.4-100% inhibition of MCF-7 cells lines by at 200 μg mL-1) as well as antifungal activities. Moreover, the anti-melanogenic activity90 of P. atlantica subsp. kurdica extracts showed significant inhibition of tyrosinase activity and an ensuing reduction of melanin synthesis, what is potentially valuable for treatments for skin hyperpigmentation disorders and new advances in the cosmetic industry. In conclusion, EtOH extracts of in vitro samples (under NaCl stress) and in vivo (grown naturally) of P. khinjuc specimens91 were compared regarding their antioxidant and antimicrobial properties and, according to the results, samples from in vivo specimens generally presented higher activities than in vitro counterparts. Rhusparviflora aqueous leaf extract was used as a medium (with 0.1 mol L-1 solution of zinc acetate dehydrate) in ZnO nanoparticles synthesis,92 which exhibited potential antimicrobial activity against S. aureus,P. aeruginosa,A. niger and C. albicans. Likewise, the MeOH:CH2Cl2 (1:1), MeOH and aqueous extracts of R. vulgaris Meikle stem bark were bactericidal/bacteriostatic against different microorganisms,93 in such a way that MeOH extract showed significant activity toward MRSA/methicillin-resistant Staphylococcus aureus (MIC 0.391 mg mL-1 and MBC 1.563 mg mL-1). The authors pointed these results supports traditional use of R. vulgaris as a toothbrush. On the other hand, extracts' cytotoxicity and mild skin damage warrant further research, so R. vulgaris can be recommended to develop effective and safe mouthwashes. Lastly, there are several other Rhus spp. who also have shown many mild biological properties94 (antiviral, antimutagenic, antioxidant, hypoglycemic, antitumour, antimalarial etc. ) which depend on their constituents, among which phenolic compounds, flavonoids/biflavonoids and glycosides are the primary bioactive metabolites. Schinus genus is widely present in folk medicine and, in a study with S. molle ripe fruits,95 the hexane and petroleum ether extracts were tested and showed antifungal activity against Botrytiscinerea, whose activity was attributed to a composition of oleic and linoleic acids and monoterpenes. The petrol extract was weakly active (at 1000 ppm), although there was a higher suppression for the fungi at this concentration according to the extract. Likewise, different leaf extracts and fractions of S. lentiscifolius were tested for the first time against five Gram-positive, three Gram-negative bacteria and four yeasts,96 which displayed a broad spectrum of weak antibacterial activity with MIC ranging 125 to 250 μg mL-1, but a meaningful antifungal activity (MIC = 15.5-25 μg mL-1). The EtOAc fraction was the most active, and various compounds were isolated from it, among which the most active metabolite was the moronic acid (100) (MIC = 1.52-3.12 μg mL-1). Sequentially, 100 was submitted to derivatization (Figure 6) to evaluate the role of carbonyl(C-3) and carboxyl(C-28) groups regarding the activity. The methyl ester derivative of moronic acid (101), obtained by treatment with diazomethane, was more active against Cryptococcus neoformans (MIC = 50 μg mL-1). Schinus terebinthifolia is the species more studied, and the last decade studies have shown its antimycobacterial activity against Mycobacterium bovis BCG, alongside a significant inhibitory effect on the nitric oxide production (IC50 19.23 ± 1.64 μg mL-1) and mycobacterial growth (IC50 14.53 ± 1.25 μg mL-1),97 what is probably due to the flavonoids therein.
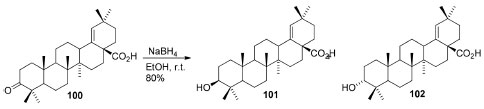 Figure 6. Scheme of reduction of moronic acid (100) to morolic (101) and acridocarpusic (102) acids
Similarly, some species of Spondias have exhibited many applications and useful therapeutic properties. Stem bark aqueous and methanolic extracts of S. mombin98 were evaluated concerning the dose-dependent antioxidant activity, whose outcomes indicated that MeOH extract presented the highest level of active constituents (total phenolic and flavonoids), being more active than the aqueous according to DPPH and FRAP scavenging assays and FTC method. Besides, S. tuberosa hexane leaf extracts were studied and presented antioxidant and antifungal activities.99 Flavonoids, hydrolysable tannins, saponins and terpenes were identified by TLC and HPLC analysis in the extracts and, likewise, fatty acid methyl esters (saturated and unsaturated) by 1H NMR data as the main components. The extract showed mild activity in DPPH assay (IC50 = 234.00 mg mL-1) and moderate by ABTS method (IC50 = 123.33 μg mL-1). Moreover, it was also weakly active against C. albicans and glabrata (MIC50 2.0 and 0.078 mg mL-1, respectively). Finally, an active fraction from the hydromethanolic extracts of S. pinnata stem bark exhibited a high antioxidant effect and radical scavenging potential against ROS and RNS, including the reducing power and inhibiting lipid peroxidation (Fe2+ in vitro chelation and ferritin ion release assays).100 In addition, the phytochemical composition of Searsia chirindensis leaf101 organic extracts indicated presence of antibacterial compounds with activity against Gram-negative (Campylobacter jejuni,E. coli and Shigella flexneri) and Gram-positive (S. aureus) strains. From the the most active extract (EtOAc) were obtained methyl gallate, myricetin-3-O-arabinopyranoside, myricetrin-3-O-rhamnoside, kaempferol-3-O-rhamnoside and quercetin-3-O-arabinofuranoside. All the compounds showed antibacterial activity against all bacterial strains tested (MIC = 30-250 μg mL-1), whose activities corroborate to the ethnomedicinal use of S. chirindensis against diarrhoea. Furthermore, different phytocompounds from the aqueous-MeOH extract (70%) leaf extract of Searsia lancea were evaluated for antibacterial properties (MIC) against four bacterial strains (Enterococcus faecalis, Klebsiella pneumoniae, Neisseria gonorrhoeae and S. aureus).102 Thus, an EtOAc chromatographic sub-fraction demonstrated good antibacterial properties (MIC range: 31-61 μg mL-1 against E. faecalis and S. aureus) and, based on uncommon GC-MS analysis for medium polar extracts, 81.5% of it consisted of broad-spectrum antibacterial compounds tetracosanol (43.98%) and nonadecanol (37.5%). Therefore, these current findings may support the traditional use of S. lancea leaves to manage gastro-intestinal disorders as well as gonorrhea. In conclusion, the study of the total extract (a XO inhibitor in vitro) of Terminthia paniculata (Sanyeqi)103 and its active fractions yielded six chalcone-flavonone heterodimers (Figure 7). Termipaniculatones A (103) and E (108) showed XO inhibitory activity (IC50 = 55.6 and 89.5 μmol L-1, respectively), which took effects via a mix-type mode. Regarding to their action mechanisms, a molecular modeling study revealed that termipaniculatone A (103) was well located into the active site of XO by interacting with Glu802, Arg880, Thr1010 and Val1011 residues. At last, this is the first time wherein the anti-acute gouty arthritis properties of T. paniculata and the characteristic biflavonoids as active constituents were related, which provides valuable information for searching new XO inhibitors from natural sources.
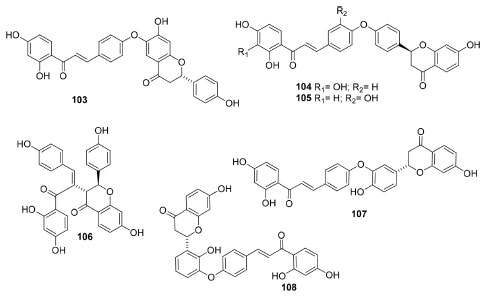 Figure 7. Chalcone-flavones from Terminthia paniculata
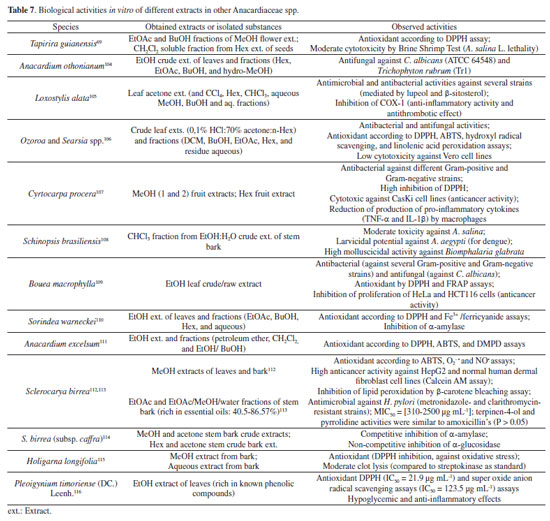
Other biological activities of extracts and enriched fractions from different Anacardiaceae spp.69,104-116 are summarized in Table 7.
In vivo studies The in vivo antioxidant extracts of Lannea stuhlmannii and L. humilis were analyzed by HPLC-PDA-ESI-MS/MS and permitted to annotate 22 specialized metabolites, including sulphated flavonoids (Figure 8).117 The antioxidant behavior of the extracts was observed through the reduction of high levels of AST (serum aspartate aminotransferase) and total bilirubin by the attenuation of deleterious histopathologic changes in the liver (induced by D-GalN) or the protection of hepatocytes from apoptosis, besides an increased expression of Bcl-2 (anti-apoptotic protein). Moreover, molecular docking evaluation showed that some identified compounds from both plants could bind to the Bcl-2:Bim (BH3) interface by hydrophobic interactions (or hydrogen and ionic bonds) with an appreciable binding free energy, whose properties are due to the presence of flavonoids and proanthocyanidins. However, the correct stereochemistry of the catechins were not determined. On the other hand, the diuretic and saluretic effects of an aqueous decoction (LMaq) and EtOAc extract of L. microcarpa barks in comparison to amiloride's and furosemide's were reported, in such a way that their mechanism of action seemed more analogous to the furosemides.118 In this study, it was verified that the diuretic activity (urinary excretion) of LMaq was dose-dependent and that the administration of extracts provided the selective elimination of Na+ concerning the stabilizing excretion of K+, confirming that L. microcarpa extracts may be a promising alternative for the therapeutic management of renal and cardiovascular pathologies.
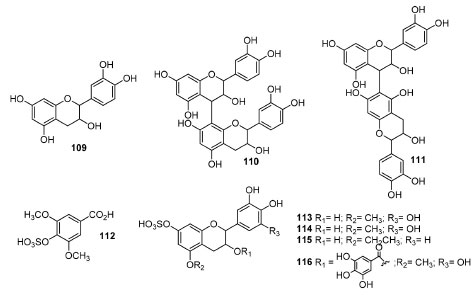 Figure 8. Compounds from active extracts of Lannea stuhlmannii and L. humilis
The leaf aqueous extract in vivo activities of Mangiferaindica presented in vivo antidiabetic and hypolipidemic activities, which significantly decreased the total serum cholesterol, triglycerides (89.75 ± 0.46%) and very low-density lipoprotein (17.95 ± 0.09%) in rats (200 mg kg-1 body weight, p.o. ) and, simultaneously, increased high-density lipoproteins (30.21 ± 2.59%). The results were almost comparable to those of atorvastatin.119 Furthermore, studies of the analgesic properties EtOH, and petroleum ether extracts of M. indica dried leaves120 indicated antinociceptive activity (oral dose of 200 mg kg-1 of body weight, with a writhing inhibition of 44.5-51.7% and 41.6-50.0%, respectively), while CCl4 presented a mild effect (writhing inhibition of 25-30%). Nevertheless, no investigation was performed to lead to identify the bioactive compounds. The hypoglycemic effect of MeOH:H2O extract of Pistacia spp. (e.g. ,P. khinjuk) were evaluated in six groups of Swiss albino mice previously treated with alloxan monohydrate (except the normal group).121 In normoglycemic mice, the plant extract showed statistically significant hypoglycemic activity at 200 and 500 mg kg-1 and the blood glucose level decreased. On the other hand, the aqueous ethanolic extracts of leaves of Sclerocaryabirrea were tested on basal plasma glucose (BPG) and oral tolerance glucose in mice, which significantly reduced peak of hyperglycemia at 100 mg kg-1 body (p < 0.001), though it did not have a relevant hypoglycemic effect on BPG. Moreover, this study reported that the co-administration of S. birrea aqueous EtOH extracts with analogous extracts of G.sylvestre (Asclepiadaceae) enabled a greater cutback on hyperglycemia (47%) compared to the S. birrea extract alone (36%).122 These species are sources of flavonoids, saponosides, tannins and other bioactive metabolites, whose combined use of these plants would be an asset in treating diabetes. Aqueous extract and enriched flavonoid fraction (FF) of the EtOAc of Rhus trilobata were evaluated as a potential alternative against colorectal adenocarcinoma cells and other types of cancer.123 The toxicological effect of the extracts was determined in female BALB/c mice after 24 h and 14 days of intraperitoneal administration of 200 mg kg-1 of both extracts. Besides, UPLC-PAD-MSE permitted to detect the most abundant compounds in the active extracts. Known compounds such as methyl gallate, epigallocatechin 3-cinnamate, quercetin 3-(2"-alloylglucosyl)-(1→2)-alpha-L-arabinofuranoside, β-PGG (100), 4-O-digalloyl-1,2,3,6-tetra-O-β-D-galloylglucose, myricetin 3-(4"-galloylrhamnoside) and fisetin were annotated, which possibly are responsible for the activity. The evaluation of toxicity did not reveal meaningful anatomical changes nor histological damages. Similarly, the total flavonoid content of Rhuscotinus (e.g.Cotinus coggygria)124 showed a potent in vivo antitumor effect in xenograft animal models of ectopic glioblastoma against several lineages of highly malignant cells (IC50 = 93.57-128.49 μg mL-1). This activity (tumor's volume reduction at 25 and 50 mg kg-1 CCF) was analogous to that temozolomide (positive control). The compounds present in the extract inhibited the growth of tumors in mice in a day-dependent pattern (7-28 days, p < 0.05). Spondiaspinnata stands out regarding in vivo bioactivity. The investigation of the antioxidant effect of aqueous bark extract125 (through evaluation of the activity of several enzymes in STZ-diabetic rats) showed that AEsp decreased, (i) the LPO (by 17%) and (ii) the alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) activities by 20, 17 and 36%, respectively. However, the (i) liver reduced liver glutathione (GSH) content and (ii) the activities of glutathione reductase, glutathione peroxidase and glutathione S-transferase were increased by 43, 44, 69 and 52%, respectively (p < 0.05 at a dose of 1.00 g kg-1). Furthermore, the EtOAc extract of S. pinnata's stem heartwood exhibited a hepatoprotective effect126 in rats under CCl4-injury induction. The results showed that this extract brought back the altered serum levels of some biochemical markers (SGPT/serum glutamyl pyruvate transaminase, SGOT/serum glutamyl oxalacetic acid transaminase, ALKP/alkaline phosphatase and bilirubin) to near normal range according to a dose-dependent mechanism. Finally, we could highlight in the study of the antipyretic potentials127 of the acetone and EtOH extracts of S. pinnata stem bark were evaluated. The ethanol extract (at 200-400 mg kg-1 p.o) presented a substantial reduction in yeast-induced elevated temperature in mice (along 1 h up to 5 h) in a dose-dependent manner, being compared to paracetamol. The possible mechanisms of action of S. pinnata stem bark extracts and the bioactive compounds still need to be further elucidated. Moreover, in studies with Buchnania lanzan,128 the MeOH leaf extract exhibited a significant neuroprotective activity (against AlCl3 induced Alzheimer's in Albino Wistar rats) in two different doses (200 and 400 mg kg-1 day-1, orally for four weeks). The evaluation of learning and memory outcomes indicated that the leaf part of B. lanzan was more active in attenuating memory deficits than other parts. Furthermore, its mechanism of memory retention seems to be similar as compared to standard drugs, which might allow this plant has potential in the treatment of cognitive dysfunctions connected with neurodegenerative disorders. Lastly, the crude MeOH extract of barks of Holigarna longifolia Roxb. and its chromatographic fractions demonstrated neuroprotective activities by increase of phenobarbitone-induced sleeping time of mice, as well as a substantial inflammation inhibitory efficacy compared to positive control. In addition, only MeOH extract provoked a significant antinociceptive activity by inhibiting abdominal writhes produced by AcOH compared to standard analgesic diclofenac sodium, whose outcomes indicate that H. longifolia might be a promising neuroprotective plant.115 Biological activities of isolated compounds from Anacardiaceae spp. In vitro studies Firstly, it can stood out that catechin-3-O-rhamnoside, a flavonoid isolated from Lanneakerstingii Engl. (EtOAc stem bark extract)129 for the first time, exhibited antimicrobial (diffusion and broth dilution methods) and antioxidant (by DPPH scavenging assay) activities. This compound presented a selective activity against several bacteria and fungi (Candida spp. ) with MIC ranging from 6.25 μg mL-1 (for S. aureus and MRSA,B. subtilis,E. coli,K. pneumonia and S. dysentariae) to 12.5 μg mL-1 (for S. typhi- S. enterica,C. albicans and C. tropicalis), while the MBC/MFC (minimum bactericidal/fungicidal concentrations) ranged from 12.5 to 50.0 μg mL-1. Moreover, these activities are higher than chloramphenicol and positive nystatin controls, probably due to the flavonoid skill to complex with bacterial cell walls and extracellular soluble proteins. Two new prenylated flavonoids 117 and 118 (Figure 9), alongside four known compounds (myricitrin, betmidin, lupeol and sitosterol) isolated from L. alata Engl. roots, might be associated to a good antibacterial and dose-dependent DPPH scavenging activity.130 Both glycosides presented better antioxidant activity than 117 and 118 and betmidin showed the best antimicrobial activity among all tested metabolites. The presence of 3-O-arabinose glucoside might be associated to the activity of betmidin against Gram-positive bacteria. Similarly, the arabinofuranoside's antioxidant effect (followed by the rhamnopyranoside), which was compared to ascorbic acid in high concentrations, corroborates with the ethnomedicinal uses of L. alata in the management of Gram-positive bacteria sicknesses. Structural features of 117 and 118 are narrowly related to their properties, such that the lower antioxidant (compared to the glycosides) behavior may be due to the presence of cyclized prenyl moieties thereon. Nevertheless, flavonol 117 is more active than 118 against Gram-negative strains (Pseudomonas spp. ), what can be related with its planar C2-C3 double bond and suitability to this activity.
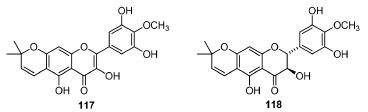 Figure 9. Structures of lanneaflavonol (117) and dihydrolanneaflavonol (118) isolated from L. alata roots
Lupeol and a mixture of phenolic lipids (mainly urushiols, with minor amounts of an alkenylphenols) from Schinopsislorentzii (Griseb. ) Engl. and S. haenkeana Engl. showed antifungal (against Fusarium graminearum and F. verticillioides) and antimicotoxigenic effects. In this study, the phenolic lipids were more active than lupeol against Fusarium spp. presenting MIC50 31 to 28 μg g-1 for F. graminearum and 165 to 150 μg g-1 for F. verticillioides.131 Besides, the antimicotoxigenic activity was higher than that of ferulic acid, since the fumosinin and deoxynivalenol production was thoroughly inhibited by all bioactive metabolites even at lower concentrations. This activity is relevant to controlling these toxigenic fungi, owing to the stimulation of mycotoxin biosynthesis by several commercial antifungals. The in vitro activities of several EOs of branches, fruits and leaves of Rhus typhina L. wood132 (from Northeast Italy) exhibited high antimicrobial activity in vitro against C. albicans (inhibition zone 22.6-35.0 mm, MIC 0.02 mg mL-1), although only the EOs from leaves and fruits were active against E. coli ATCC (inhibition zone 17.6-22.5 mm, MIC 0.064 mg mL-1). Furthermore, the antioxidant effect (DPPH assay) of leaf and fruit EOs was superior to that EOs of the branches, as indicated by their respective IC50 values (2.29 ± 0.10 μg mL-1, leaves; 2.54 ± 0.10 μg mL-1, fruits; 5.80 ± 0.18 μg mL-1, branches). Biological studies with Mangifera indica133 have shown a wide range of applications of active extracts and isolated compounds such as 97. Mangiferin (1,3,6,7-tetrahydroxyxanthone-C2-β-D-glucoside) is a pharmacologically active metabolite present in high yields in M. indica (bark, roots, fruits, and leaves) and exhibits diverse biological properties, among which are the antibacterial and cytotoxic/anticancer activities. A study reports that the solution of 97 was found to exert promising activity against both Gram-positive and Gram-negative bacteria, which is particularly relevant because known antibiotics resistance. Besides, the cytoprotective potential of 97 for hematopoietic cells from leukemogenesis was verified based on the decreased olive tail moment (OTM) and micronucleus (MN) frequency, so that 97 probably reduces DNA damage in the etoposide-treated mononuclear cells.133 Moreover, a study for the characterization of epicuticular leaf DCM extracts and several derivatives of Lithrea caustica (Molina) Hook and Arn. showed that litreol ((3-[pentadecyl-10-enyl-catechol]) and some derivatives behave as inhibitors of 15 soybean and 5 human lipoxygenases (15-sLOX and 5-hLOX).134 The highest activities were exhibited by litreol (IC50 = 54.77 μmol L-1, against s-LOX; 2.09 μmol L-1, against h-LOX) and 3-pentadecylcathecol (IC50 = 55.28 μmol L-1, against s-LOX; 2.74 μmol L-1, against h-LOX), in such a way that the respective kinetic studies indicated a mixed and selective inhibition mechanism to 5-hLOX. Besides, the pistagremic acid (119) isolated from the dried galls of Pistaciaintegerrima Stewart exhibited an inhibitory effect against α-glucosidase in vitro against yeast (IC50 = 89.12 ± 0.12 μmol L-1),135 confirming former molecular docking simulations. Thus, a molecular binding mode was explored, and the results indicated hydrogen bonding interactions between this triterpene and significant amino acid residues surrounding the catalytic site of the α-glucosidase, which could be mainly responsible for their role in potent inhibitory activity. Therefore, 119 (Figure 10) showed a promising potential to be further investigated as a new lead compound for better management of diabetes.
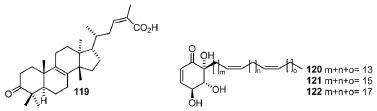 Figure 10. Pistagremic acid (119) isolated from Pistacia integeriima and of pleiogenones A-C (120-122) obtained from Pleiogynium timoriense
The investigation of a DCM extract of the bark of Pleiogynium timoriense against the A2780 ovarian cancer cell line (A2780 OCCL) indicated an IC50 value of 1.3 μg mL-1. Bioassay-directed fractionation of this extract yielded the three new bioactive trihydroxyalkylcyclohexenones (Figure 10) named pleiogenones A (120), B (121) and C (122), which showed a higher antiproliferative activity against the A2780 OCCL presenting IC50 of 0.8, 0.7, and 0.8 μg mL-1, respectively.136 Compound 72, named integracin E, obtained from the stem barks of Swintonia floribunda,70 also presented a potent tyrosinase inhibitory activity with an IC50 value of 48.2 μmol L-1. Likewise, the bioactivity-guided fractionation of EtOAc leaf extract of Poupartia borbonica J.F.Gmel. furnished three novel alkyl cyclohexenone derivatives (123-125)137 with absolute configurations assigned (Figure 11). These compounds were active against 3D7 and W2 Plasmodium falciparum strains (IC50 = 0.55-1.81 μmol L-1) and exhibited in vitro cytotoxicity against WI38 human fibroblasts and human cervical cancer (HeLa) cell lines (WST-1 assay), but no hemolytic activity was observed for the extract and pure metabolites. Besides, the MeOH extract was also evaluated, and it displayed moderate antiplasmodial properties in vitro, which might be attributed to its flavonoid content, including the unknown compound 3'-O-hydroxysulfonylquercetin (126). Moreover, studies with Tapirira guianensis leaves allowed the obtention of these compounds, which seems to be precursors of alkyl and alkenyl phenols (127-130). The cyclohexene derivatives 127 and 128 were in mixture and they also showed against P. falciparum strains (IC50 = 4.7 ± 0.3 and 5.4 ± 1.7 μmol L-1) against F32 and FcB1 strains. This mixture was also active against Leshimania amazonensis (IC50 = 1.0 ± 0.1 μmol L-1), S. aureus (IC50 = 75.4 μmol L-1) and S. epidermidis (IC50 = 17.6 μmol L-1).138
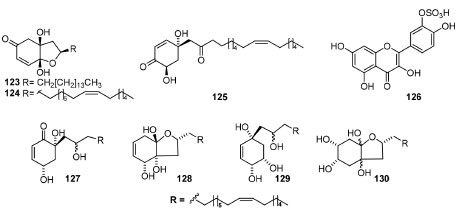 Figure 11. New bioactive alkyl and alkenyl cyclohexenone derivatives and sulphorated quercentin from P. borbonica and alkenyl derivatives from T. guianensis
In sequential studies with P. borbonica, the cytotoxicity and pharmacological activities139 of poupartone B (123) were deeply evaluated. A real-time live-cell imaging of different human cancer cell lines and normal fibroblasts treated with 123 was carried out. Thus, this compound showed a potent inhibition of cell proliferation associated with the induction of cell death. Besides, 123 (at 1-2 μg mL-1) induced a rapid retraction of cellular protrusions associated with cell rounding, massive cytoplasmic vacuolization, loss of plasma membrane integrity and plasma membrane bubbling, ultimately leading to paraptosis-like cell death. These results highlight the cytotoxicity of 123 against several in vitro cancer cell lineages. The stem bark CHCl3 extract of Protorhus longifolia (Benrh. ) Engl. Furnished the known 3-oxo-5α-lanosta-8,24-dien-21-oic acid (131) and 3β-hydroxylanosta-9,24-dien-24-oic acid (132), which were screened for several activities (Figure 12).140 These compounds showed satisfactory anti-platelet aggregation activities dose dependent, so that 131 showed the highest activity (IC50 = 0.99 mg mL-1) on the thrombin-induced platelet aggregation.
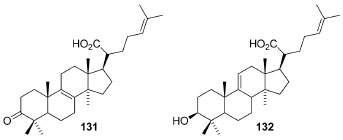 Figure 12. 3-oxo-5α-lanosta-8,24-dien-21-oic and 3β-hydroxylanosta-9,24-dien-24-oic acids with anti-platelet aggregation activities from P. longifolia
Two different studies with Semecarpus anacardium afforded the obtention and 3-(8'(Z),11'(Z)-pentadecadienyl)-catechol (SA-3C) isolated from the plant kernel141 and tetrahydroamentoflavone142 from the seeds. The alkylphenol showed cytotoxic activity against tumor cell lines with IC50 values lower than doxorubicin and even multidrug resistant tumor cell lines were equally sensitive to SA-3C. Besides, it induced apoptosis in human leukemia cell lines in a dose-dependent pattern, showed synergistic cytotoxicity with doxorubicin and induced the cell cycle arrest at S- and G2/M-phases, what was correlated with inhibition of checkpoint kinases. On the other hand, tetrahydroamentoflavone (THA) exhibited a strong inhibitory effect against xanthine oxidase (XO), what was investigated through a Lineweaver-Burk (LB) plot for the XO inhibition of THA and allopurinol constructed from the kinetic data. In this case, IC50 values of THA and allopurinol for XO inhibition were 92 and 100 nmol L-1 and their corresponding values for Ki were 0.982 and 0.612 μmol L-1. In conclusion, the fruit extract of Sorindeiajuglandifolia furnished two bioactive simple compounds identified as 2,3,6-trihydroxybenzoic acid and methyl 2,3,6-trihydroxybenzoate.143 These compounds showed inhibitory effects against P. falciparum W2 (IC50 16.5 μmol L-1 and 13.0 μmol L-1) and falcipain-2 (IC50 35.4 and 6.1 μmol L-1), respectively. In vivo studies with pure compounds To date, there are few examples of in vivo studies of isolated metabolites of Anacardiaceae. For instance, compounds isolated from EtOH extracts of leaves of Schinus polygamous C. (3-O-acetyllupeol, β-sitosterol, lupeol, gallic acid, methyl gallate, kaempferol, quercetin-3-α-O-rhamnoside and its aglicone quercetin) were submitted to hepatoprotective, antioxidant and curative in vivo studies. Lupeol and gallic acid were evaluated by oral administration in adult male albino rats (50-100 mg kg-1) and both compounds showed a significant protection against CCl4-induced liver damage. Besides, a remarkable antioxidant effect (> 90% for both compounds, measured by the activity of enzyme reduced glutathione) was observed.144 In addition, the in vivo study of antihyperglycemic activity of the methyl ester of 3β-hydroxylanosta-9,24-dien-21-oic acid (132) from P. longifolia stem bark extract showed antihyperglycemic behavior in an STZ-induced diabetes rat model. This compound showed hypoglycemic effect by reducing blood glucose levels by 37% and improved glucose tolerance in the diabetic rats. Furthermore, a relatively higher hepatic glycogen content, alongside hexokinase and glucokinase activities (with a decrease in glucose-6-phosphatase activity), was observed in the triterpene-treated diabetic group when compared with the diabetic control group. The treatment increased antioxidant status of the diabetic animals, as well as the activity of superoxide dismutase and catalase along with a decrease in malondialdehyde content.145 At last, different compounds aforementioned have also exhibited in vivo bioactivities; termipaniculatone A103 (103) possesses anti-hyperuricemic and anti-inflammatory activities in mice, 2,3,6-trihydroxy benzoic acid143 is active against P. berghei strain B, with mean parasitaemia suppressive and curative doses of 44.9 mg kg-1 and 42.2 mg kg-1, respectively. The 3β-hydroxylanosta-9,24-dien-24-oic acid140 (132) showed a strong inhibition of the acute inflammation of rat paw but in a higher concentration (500 mg kg-1), while pourpatone B (123) showed in vivo antimalarial (P. berghei) growth inhibition at a dose 15 mg kg-1 day-1 i.p.137
PROCESSES AND PRODUCT PATENTS BASED ON ANACARDIACEAE spp. Employment in cosmetics During the last two decades three cosmetic formulations were developed based on compounds isolated or present from Anacardiaceae spp. extracts. Ellagic acid or its derivatives, essential oils or foaming agents were employed in formulations with surfactants, thickening agents and other constituents. All evaluate compositions showed a good anti-dandruff effect mainly caused by Malassezia (Pityrosporum spp. yeasts).146 Likewise, a hair styling composition in the form of foam relating to a process for shaping keratin fibers was also developed, comprising the application of at least one "mousse" composition, including one or several fresh fruit juices, including species of Anacardiaceae (mango) and/or surfactants. This type of cosmetic formulation was mainly made in an aqueous medium or organic hydrophilic solvent (linear or ramified alcohols), in such a way as to allow to take advantage of some active constituents that are naturally present in fresh fruits (vitamins, α- and β-hydroxyacids, antioxidants, and anti-inflammatories), which have beneficial properties for the hair and scalp.147 At last, a topical cosmetic formulation was elaborated, in which the dedifferentiated plant cells are elicited in vitro following a cycle of successive darkness and lighting periods under a CO2 atmosphere.148 The compositions with Anacardiaceae spp. and other plants permitted to observe an anti-aging effect, a protective effect for the skin and an antioxidant effect, as well as antifungal and antiradical properties. Patents of medical and other biological uses Several formulations comprising a hydroxylated fatty acid (such as ricinoleic acid) or a triglyceride containing hydroxylated fatty acid (e.g. , castor oil) were combined with the liquid from the cashew nut peel (A. occidentale and others) and/or alkyl phenols or anacardic acids and derivatives, which have presented broad antimicrobial against Gram-positive and Gram-negative bacteria, fungi and protozoans. In this case, for the first time was presented that ricinoleic acid might behave as an oral antibiotic and an antiprotozoal agent, which was followed by extremely low toxicity in comparison with other antibiotics. Therefore, these formulations are developed to the prevention and treatment of pathogenic processes in people and animals (by oral, topical or parenteral administration), as well as to control fermentation (made with yeast - S. cerevisiae) and as an antifungal for food and seeds.149 An antiviral composition with antiretroviral properties for treating HIV patients, which was made with acetic acid and coconut extracts, a solution of mineral salts (e.g. , seawater) and other plant extracts of Spondiasmombin barks, Liliaceae (Smilax medica roots) and Euphorbiaceae (roasted castor beans, Ricinus spp. ), was developed.150 The results of these formulations indicated an inhibition of the HIV-1 reverse transcriptase activity (which may exceed 90% compared to the activity measured in the controls) and a decrease in the cytopathogenic effect of HIV-1 in infected cells after the treatment with the antiviral compositions. Besides, the absence of toxicity was observed in mice essays. On the other hand, a preparation for in vivo and in vitro applications based on an aqueous Mangiferaindica fruit extract called sirtuin 1 (SIRT1) is claimed to be an activating agent,151 which may be used to reduce the risk of obesity, type-II diabetes, high blood lipid levels, arteriosclerosis, and heart illnesses, as well as a cellular and DNA protector. In addition, an aqueous ethanolic dilution of juices or extracts derived from some plants were transformed into a paste/jelly/jam/cake/cream puff/chocolate to be used as functional foods and had anti-stress (e.g. , induced by gastric ulcers) and adaptogenic activities. The extracts of M. indica (65.0-75.0 wt. %), Withaniasomnifera (3.5-5.0 wt. %, Solanaceae), Aspargusrecemosus (3.5-5.0 wt. %, Asparagaceae), Amaranthushypochondriacus (10.0-20.0 wt. %, Amaranthaceae) and Evolvulusalsinoides (0.2-0.6 wt. %, Convolvulaceae)152 did not provoke mortality in any of the rats' treated groups, as well as behavior's abnormalities in the animals exposed with herbal preparations. The results of these formulations showed antiulcer proprieties (since they reduce the ulcer index and decrease its severity) and exhibited antioxidant effect by the decrement of lipid peroxidation, the rising of catalase levels and the enhancement of superoxide dismutase activity. Another invention related to an herbal formulation prepared with several plants (including M. indica)153 developed for the diabetes prevention and treatment, as well as associated damages, was proposed in the period. The formulation developed therein might not only control type-II diabetes, but also offer reversal possibilities in prediabetes and, thereby, possible prevention for diabetes mellitus and its complications. The plant extract composition provides good glycemic management and reduces the glycosylation of hemoglobin, controls the total cholesterol levels, improves cardiovascular health by decreasing hypertension and enhances wound healing of diabetic ulcers. Besides, another minor effect is the reduction systolic and diastolic blood pressure, prevention of oxidative stress and minimization of hypertensive drug dependency. Compositions and extracts based on Schinus terebinthifolia and one or more compounds present in this plant. In certain embodiments, the formula is administered in combination with another antibiotic agent. 154 These formulations prevent bacterial infections (e.g. , caused by Staphylococci sp. ), besides other skin damages, acne and other corresponding applications. Their main compounds herein were annotated by LC-FTMS (flavonoids, triterpenoids and steroidal sapogenins, among others), which are known to have many known biological properties. Anacardic acids were used as an effective formulation for treating anemia and low blood pressure. In these formulations is including at least one anacardic acid of C-15 alkyl/alkenyl chain (59 and the ∆8,9 alkene derivative) isolated from roots and barks aqueous extract of Ozoroa paniculosa.155 The composition was active for oral, rectal, nasal, vaginal or parenteral (subcutaneous, intramuscular, intravenous or intradermal) administration, both to humans and animals. Besides, six active fractions of Anacardiaceae and Asteraceae spp. were employed to elaborate herbal compositions to treat infertility in men presenting no-side effects.156 The inventors stated that these formulations restored vigor, increased sexual libido, exhibited a healing effect on X and Y chromosome-related diseases and granted other corresponding benefits in synergistic plant compositions. Some like-urushiol derivatives were synthesized (e.g. , 3-n-pentadecyl catechol, and/or 3-n-heptadecyl catechol), both similar that isolated from poison ivy.157 They are potential compositions for the prevention and/or prophylactic treatment of contact dermatitis caused by poison ivy and poison oak and other ACD causing plants of the family Anacardiaceae and Ginkoaceae. The embodiments were effective for tolerizing and desensitizing mammals, including humans. They comprise esters from urushiols and amino acid, or combination of amino acids (i.e. di, tri, or poly peptide residue), a carbamate forming compound, a sulphate or phosphate ester or even an ester of a dicarboxylic acid, resulting in a salt forming compounds with water soluble characteristics to facilitate the topic use. Different insecticidal compositions were prepared in liquid, dehydrated and lyophilized forms, wherein several plants of various families (e.g. ,Pistacia vera, Anacardiaceae) were included, whose constituents (polypeptides alone vs. binary systems) expressed as polypeptides/polynucleotides showed pesticidal properties.158 These combinations showed to be more active than the components individually, whose results are promising for agriculture, ecology, biotechnology and other scientific applications. The efficacy of the gum of Odina wodier Roxb. (i.e. ,Rhus odina), an Asian plant that presents many applications in folk medicine, was evaluated for the first time as a tablet binder.159 Hitherto, the potential binding capability and an emulsifier have already been studied to stabilize emulsions. Chemical analyzes appointed to the presence of carbohydrates but the absence of tannins and peroxidase enzyme in the "gum odina" compositions, what removed the possibility of oxidative degradation of gum as excipient. The gum shows stable in liquid conditions and no toxicity was observed. These results showed that "gum odina" could be used as pharmaceutical excipients (e.g. , tablet binder or emulsifier), being effective in minimal amounts compared to the standard tablet binders. Furthermore, other studies with Anacardiaceae spp. were developed with new compositions or products that might be applied as (i) antimicrobial coating films for filters and air conditioning equipment (e.g. , branch/leaf/rhizome/bark aqueous extracts of different plants, including Pistacia spp. ),160 (ii) in procedures for a concentration of xanthones at high pressure on a semi-industrial scale using extracts of several plants (e.g. ,M. indica)161 or (iii) to increase the content of desired ingredients in crops (such as fruits and vegetables) by applying succinate dehydrogenase inhibitors (SDIs).162 Thus, the embodiments of invention (i) were intensely active against harmful microorganisms in the living environment, promoting its cleanliness and preventing microorganisms' degradation. On the other hand, the use of a hydrophobic stationary phase mixed to a supercritical eluent (pure CO2 vs. a mix of CO2 with a polar cosolvent in isocratic or gradient mode) in (ii) allowed the obtention of phenolic acids, benzophenones, flavonoids and xanthones in high amounts (mainly 97, 5× higher than in original leaf extract), whose process was efficient and avoided losses in the bioactivity of the fractionated substances. At last, the study showed the behavior of several SDIs against various plant species (including mango, sumac, and pistachio), whose results indicated that different types of natural metabolites might have their contents increased thereon, since the SDI is applied to the crop prior to the harvest and at a rate ranging from 1 to 250 g ha-1. Several formulations, including at least one plant of Asteraceae, Lamiaceae, Anacardiaceae (P. lentiscus) and Cistaceae families in treating varroatosis (by Varroadestructor, varroa mite) in bees were elaborated.163 This invention achieves a composition that is harmless for bees and humans and effective in a short time on mites in both phoretic and reproductive phases in opened/operculated cells. Besides, antioxidant formulations from Anacardiaceae spp. (in particular, Sclerocarya birrea) were obtained by maceration/extraction of roots, bark, leaves, fruits or their parts (endocarp, mesocarp, epicarp) and using different solvents, whose results suggested that the prepared extracts had outstanding oxidative stability and showed a good antiradical behavior.164 Other plant compositions, including Anacardiaceae species prepared with unrefined oils (from natural seedlings) free of phorbolic esters and trans fatty acids, were developed for cosmetic, dermatologic, dietetic, insecticide, pharmaceutical, veterinary and eating uses.165 These compositions exhibited satisfactory outcomes as an antimicrobial/antifungal, germ inhibitor and for the management of cellular functions, including the potential to be employed in external and internal medications. In conclusion, in the same way a formulation comprising sumac (Rhus spp. ) and oregano can be highlighted166 once it can be used as a preservative agent to prevent or slow down the deterioration of food products (e.g. , for wet and dry baked products). These formulations allowed the storage time of the baked products to be significantly extended, substantially delaying both the appearance of mold (mainly on wet baked goods) and the rancidity, what probably is related to the presence of polyphenols.
PROGRESS IN BIOSYNTHESIS OF PHENOLIC LIPIDS Phenolic lipids and derivatives are chemotaxonomic markers of this family, but their biosynthesis is still not completely enlightened.1 More recent publications have given new contributions, such as the structure and function of polyketide biosynthetic enzymes (PKSs) and the strategies for producing several polyketides.167 The results indicated that the type III PKSs have involved in the processes of polyphenols and phenolic lipids biosynthesis in plants, bacteria and fungi. Hence, type III PKSs synthesize a broad group of metabolites, since they differ in their preference of starter and extender substrates, the number of condensation steps and the mechanism of intramolecular cyclization of poly-β-keto intermediates. In vitro biochemical analysis using Azotobacter vinelandii bacterium strains was formerly conducted since alkylresorcinols and alkylpyrones are the major lipids of A. vinelandii cyst membranes. Gene disruption analyzes showed that the ars gene cluster is essential for biosynthesis, which consists of two types I fatty acid synthase (FAS) genes (arsA,arsD) and two types III PKSs (arsB,arsC). Thus, it was observed that the reactions of arsA,arsB, and arsD lead to alkylresorcinols. In contrast, the reactions of arsA,arsC, and arsD lead to alkylpyrones, once arsB catalyzes the decarboxylative C2-C7 aldol condensation to produce alkylresorcinols and arsC catalyzes the C5 oxygen-C1 lactonization to synthesize alkylpyrones (Figure 13). These features are due to the specific amino acid residues at the type III PKSs active site cavity (Trp281 to alkylresorcinols and Gly284 to alkylpyrones).167
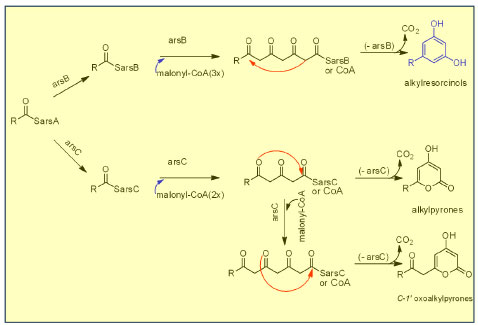 Figure 13. The biosynthetic pathway of phenolic lipids according to the experiments with Azotobacter vinelandii (adapted from Miyanaga)167
Similarly, a study of characterization of an orphan Type III polyketide synthase (PKS/CepA) in uncultivated Entotheonella sponge (Theonella spp. ) provided new information in phenolic lipids' biosynthesis, as well as the metagenomic features related. Three PKS18 aminoacid residues (Thr144, Cys205, and Ala209) were crucially involved in its substrate preference (i.e. , alkylresorcinols vs. alkylpyrones, according to the long-chain alkyl units binding). Nevertheless, for the enzyme BpsA the PKS-like substrate-binding tunnel is composed of Thr, Cys and Phe residues at the corresponding positions. Based on their bioinformatic analyzes, seems CepA was most likely a resorcinol synthase that accepted just long-chain fatty acid starters (133-138) directly from several coenzyme-A precursors (Figure 14(a)). In optimized enzymatic assays, two alkylresorcinols, and three alkylpyrones (139-143) were obtained (Figure 14(b)). At last, the in vitro experiments demonstrate that CepA factor acts as a phenolic lipid synthase, processing long-chain fatty acid acyl-CoA and malonyl-CoA thioesters, wherein the product range includes tetraketide resorcinols as well as tri- and tetraketide α-pyrones, which were detected for the first time in theonellid sponges of Entotheonella species.168 However, the presence of alkylpyrones might indicate incoherence with the bioinformatic prediction.
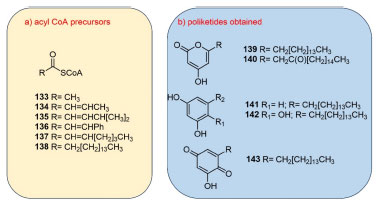 Figure 14. (a)Structures of potential PKS starters used as test substrates. (b)Proposed structures to the tri- or tetraketides obtained by optimized enzymatic assays using theonellid sponges (CepA pathway)
In conclusion, the catalytic activity of O-methyltransferase SrsB in the decarboxylative methylation of alkylresorcylic acid (ARA) along phenolic lipid biosynthesis by Streptomyces griseus (or S. lividans)169 was investigated, whose operon (Srs) encodes a type III PKS and a flavoprotein hydroxylase. Former studies have reported that SrsA enabled the production of an ARA as a direct product rather than a corresponding alkylresorcinol (ARC), while SrsB produced alkylresorcinol methyl esther (ARME) in the presence of S-adenosyl-l-methionine (SAM). However, SrsB has been shown incapable of catalyzing the O-methylation of ARC, suggesting that ARA was the substrate of SrsB, whose conversion to ARME might take place by (i) the O-methylation of the OH-group (C-6) or (ii) the decarboxylation of the neighboring carboxyl group (C-1). These studies proposed that O-methylation was coupled with decarboxylation, so that SrsB catalyzed the feasible SAM-dependent decarboxylative methylation of ARA, which is the first report of a methyltransferase with this catalytic behavior in an in vitro assay (Figure 15).
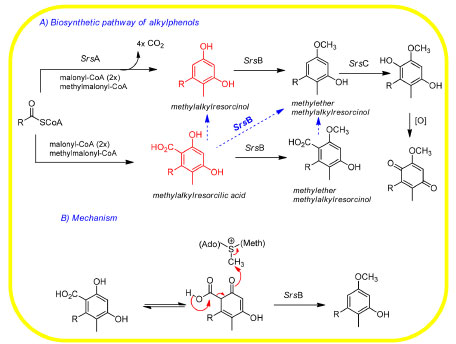 Figure 15. (A) Scheme of the Srs biosynthetic pathway and (B) proposed mechanism of biosynthesis of alkylresorcinol methyl ester by SrsA and SrsB with the mediation of S-adenosyl-L-methionine (SAM) (adapted from Nakano)169
CONCLUSIONS This review presents a detailed report regarding the chemical composition and biological activities of pure compounds and extracts of the Anacardiaceae family. Technological prospection was also detailed with a variety of patents. Furthermore, new significant information about phenolic lipids biosynthesis was finally pointed out. However, for plants from this family, the biosynthesis of the chemical markers remains unclear, once the biosynthesis update was carried out with microorganisms. This botanical family is significant to pharmacology, the chemistry of natural products and corresponding scientific fields. Therefore, based on the data and the new findings shown hither, further research with the Anacardiaceae family was needed, both to isolate new bioactive compounds and elucidate the compounds responsible for the biological activities, as well as towards alternative contributions to biosynthetic studies of chemotypes/chemotaxonomic markers in this family.
ACKNOWLEDGMENTS The authors thank CNPq-Conselho Nacional de Desenvolvimento Científico e Tecnológico (# 302848/2022-3), INCT Energia e Ambiente, and CAPES-Conselho de Aperfeiçoamento de Pessoal de Nível Superior (finance code 001) for financial suport and scholarship.
REFERENCES 1. Correia, S. J.; David, J. P.; David, J. M.; Quim. Nova 2006, 29, 1287. [Crossref] 2. Baer, H. In The Poisonous Anacardiaceae; Kinghorn, A. D. , eds.; Columbia Univ. Press: New York, 1979, p. 161-170. 3. Schulze-Kaysers, N.; Feuereisen, M. M.; Schieber, A.; RSCAdv. 2015, 5, 73301. [Crossref] 4. Singh, S. K.; Sharma, V. K.; Kumar, Y.; Kumar, S. S.; Sinha, S. K.; Herba Pol. 2009, 55, 126. [Link] accessed in August 2023 5. El-Nashar, H. A. S.; Mostafa, N. M.; Abd El-Ghffar, E. A.; Eldahshan, O. A.; Singab, A. N. B.; Nat. Prod. Res. 2022, 36, 4839. [Crossref] 6. Mobot Research, https://www.mobot.org/mobot/research/apweb/genera/anacardiaceaegen.html, accessed in August 2023. 7. Simionatto, E.; Peres, M. T. L. P.; Hess, S. C.; da Silva, C. B.; Chagas, M. O.; Poppi, N. R.; Prates, C. B.; Matos, M. F. C.; Santos, E. C. S.; de Carvalho, J. E.; J. Essent. Oil Res. 2010, 22, 596. [Crossref] 8. Nyegue, M. A.; Kemegne, G. A.; Kamdem, S. L. S.; François-Xavier, E.; Menut, C.; Nat. Prod. Commun. 2018, 13, 903. [Crossref] 9. Joshi, S. C.; Mathela, C. S.; J. Nat. Prod. Plant Resour. 2014, 4, 39. [Link] accessed in August 2023 10. Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Çenet, M.; Öztürk, B.; Balcilar, M.; Nat. Prod. Res. 2014, 28, 2150. [Crossref] 11. Fraternale, D.; Ricci, D.; Nat. Prod. Commun. 2018, 13, 1175. [Crossref] 12. Negro, C.; De Bellis, L.; Miceli, A.; J. Essent. Oil Res. 2015, 27, 23. [Crossref] 13. Tabanca, N.; Nalbantsoy, A.; Kendra, P. E.; Demirci, F.; Demirci, B.; Molecules 2020, 25, 2136. [Crossref] 14. Abed, A.; Rachid, D.; Catherine, M.; Ismahene, S.; Aicha, L.; Nadia, B.; Hichem, D.; Charef, R.; Mehdi, E.; Farida, T.; Mounira, O. K.; Lahouari, D.; Int. J. Biosci. 2017, 10, 146. [Crossref] 15. Komaitis, M.; Gardeli, C.; Papageorgiou, V.; Mallouchos, A.; Theodosis, K.; Food Chem. 2008, 107, 1120. [Crossref] 16. Hadjari, A.; Pulaj, B.; Mustafa, B.; Nelson, K.; Quave, C. L.; BMC Complementary Altern. Med. 2016, 16, 147. [Crossref] 17. Ulukanli, Z.; Karabörklü, S.; Öztürk, B.; Çenet, M.; Balcilar, M.; J. Food Process. Preserv. 2014, 38, 815. [Crossref] 18. Abolghasemi, A.; Shojaaddini, M.; Tajabadipour, A.; Sefidkon, F.; J. Essent.Oil Bear. Plants 2018, 21, 796. [Crossref] 19. Ismail, A.; Lamia, H.; Mohsen, H.; Samia, G.; Bassem, J.; Sci. Int. 2013, 1, 148. [Crossref] 20. Satpathy, G.; Tyagi, Y. K.; Gupta, R. K.; Food Res. Int. 2011, 44, 2076. [Crossref] 21. Sameh, S.; Al-Sayed, E.; Labib, R. M.; Singab, A. N. B.; Ind. Crops Prod. 2019, 137, 468. [Crossref] 22. Ren, L.; Jing, Y. J.; Zhen, S. X.; Fei, W. Y.; Corlett, R. T.; Kai, X. Y.; Bin, H. H.; Molecules 2020, 25, 343. [Crossref] 23. Oladimeji, A. O.; Aliyu, M. B.; Ogundajo, A. L.; Babatunde, O.; Adeniran, O. I.; Balogun, O. S.; Pharm. Biol. 2016, 54, 2674. [Crossref] 24. Lago, J. H. G.; Santana, J. S.; Sartorelli, P.; Guadagnin, R. C.; Matsuo, A. L.; Figueiredo, C. R.; Soares, M. G.; da Silva, A. M.; Pharm. Biol. 2012, 50, 1248. [Crossref] 25. Gazim, Z. C.; Bortolucci, W. C.; de Oliveira, H. L. M.; Silva, E. S.; Vilas Boas, M. R.; de Carvalho, T. M.; Campo, C. F. A. A.; Gonçalves, J. E.; Piau Júnior, R.; Aust. J. Crop Sci. 2018, 12, 1645. [Crossref] 26. Fernandes, M. Z. L. C. M.; Zanini, S. F.; Affonso, C. R. G.; Fernandes, R. M.; de Oliveira, J. M. G.; Martins, M. C. C.; de Lima, S. G.; Souza Júnior, G. R.; J. Braz. Chem. Soc. 2012, 23, 180. [Crossref] 27. Sartorelli, P.; Santana, J. S.; Guadagnin, R. C.; Lago, J. H. G.; Pinto, E. G.; Tempone, A. G.; Stefani, H. A.; Soares, M. G.; da Silva, A. M.; Quim. Nova 2012, 35, 743. [Crossref] 28. dos Santos, A. C.; Rossato, M.; Agostini, F.; Serafini, L. A.; dos Santos, P. L.; Molon, R.; Dellacassa, E.; Moyna, P.; J. Essent. Oil Bear. Plan. 2009, 12, 16. [Crossref] 29. Pawlowski, A.; Kaltchuk-Santos, E.; Zini, C. A.; Caramão, E. B.; Soares, G. L. G.; S. Afr. J. Bot. 2012, 80, 96. [Crossref] 30. Chaves, D. S. A.; Cavalcanti, A. S.; Alves, M. S.; da Silva, L. C. P.; Patrocínio, D. S.; Sanches, M. N.; de Souza, M. A. A.; Rev. Bras. Farmacogn. 2015, 25, 356. [Crossref] 31. Barbosa, L. C. A.; Montanari, R. M.; Demuner, A. J.; Silva, C. J.; Andrade, N. J.; Ismail, F. M. D.; Barbosa, M. C. A.; Molecules 2012, 17, 9728. [Crossref] 32. Simionatto, E.; Chagas, M. O.; Peres, M. T. L. P.; Hess. S. C.; da Silva, C. B.; Ré-Poppi, N.; Gebara, S. S.; Corsino, J.; Morel, A. F.; Stuker, C. Z.; Matos, M. F. C.; de Carvalho, J. E.; J. Essent.Oil Bear. Plants 2011, 14, 590. [Crossref] 33. Afifi, F. U.; Aboalhaija, N. H.; Awwad, O.; Khalil, E.; Abbassi, R.; Abaza, I. F.; Chem. Biodiversity 2019, 16, e1900388. [Crossref] 34. Budel, J. M.; Machado, C. D.; Raman, V.; Rehman, J. U.; Maia, B. H. L. N. S.; Meneghetti, E. K.; Almeida, V. P.; Silva, R. Z.; Farago, P. V.; Khan, I. A.; Rev. Bras. Farmacogn. 2019, 29, 1. [Crossref] 35. Rodilla, J. M.; Rocha, P. M. M.; Diéz, D.; Elder, H.; Guala, M. S.; Silva, L. A.; Pombo, E. B.; Molecules 2012, 17, 12023. [Crossref] 36. Machado, M. M.; de Campos, M. M. A.; Duarte, J. A.; Zambrano, L. A. B.; Quintana, L. D.; Rocha, M. B.; Schmitt, E. G.; Boligon, A. A.; de Oliveira, L. F. S.; J. Evidence-Based Complementary Altern. Med. 2018, 1, 1. [Crossref] 37. Descamps, L. R.; Chopa, C. S.; Ferrero, A. A.; Nat. Prod. Commun. 2011, 6, 887. [Crossref] 38. Murray, A. P.; Gurovic, M. S. V.; Rodríguez, S. A.; Murray, M. G.; Ferrero, A. A.; Nat. Prod. Commun. 2009, 4, 873. [Crossref] 39. Murray, A. P.; Rodríguez, S. A.; Murray, M. G.; Nat. Prod. Commun. 2008, 3, 1551. [Crossref] 40. da Silva, E. R.; Lazarotto, D. C.; Pawlowski, A.; Soares, G. L. G.; Rev. Cubana Plant. Med. 2019, 24, 783. [Link] accessed in August 2023 41. Hernandes, C.; Taleb-Contini, S. H.; Bartolomeu, A. C. D.; Bertoni, B. W.; França, S. C.; Pereira, A. M. S.; Nat. Prod. Commun. 2014, 9, 1383. [Crossref] 42. Cardoso, C. A. L.; Jeller, A. H.; Ré-Poppi, N.; Coelho, R. M.; Yasunaka, D. S.; Schleder, J. D. E.; J. Essent. Oil Res. 2010, 22, 469. [Crossref] 43. Carvalho, C. E. S.; Sobrinho-Júnior, E. P. C.; Brito, L. M.; Nicolau, L. A. D.; Carvalho, T. P.; Moura, A. K. S.; Rodrigues, K. A. F.; Carneiro, S. M. P.; Arcanjo, D. D. R.; Citó, A. M. G. L.; Carvalho, F. A. A.; Exp. Parasitol. 2017, 175, 59. [Crossref] 44. Tintino, S. R.; Figueredo, F. G.; Lucena, B. F. F.; Matias, E. F. F.; Leite, N. F.; Andrade, J. C.; Nogueira, L. F. B.; Morais, E. C.; Costa, J. G. M.; Coutinho, H. D. M.; Rodrigues, F. F. G.; Pharm. Biol. 2014, 52, 560. [Crossref] 45. Zoghbi, M. G. B.; Pereira, R. A.; de Lima, G. S. L.; Bastos, M. N. C.; Quim. Nova 2014, 37, 1188. [Crossref] 46. Said, A.; Omer, E. A.; El Gendy, M. A. M.; Fawzy, G.; Abd El-Kader, A. E.; Fouad, R.; J. Mater. Environ. Sci. 2018, 9, 2274. [Link] accessed in August 2023 47. Babouongolo, S. G.; Loumpangou, C. N.; Dao, E.; Simon, V.; Elouma Ndinga, A. M.; Ouamba, J. M.; J. Essent.Oil Bear. Plants 2021, 24, 421. [Crossref] 48. Viljoen, A. M.; Kamatou, G. P. P.; Baser, K. H. C.; S. Afr. J. Bot. 2008, 74, 325. [Crossref] 49. Kpoviéssi, D. S. S.; Gbaguidi, F. A.; Kossouoh, C.; Agbani, P.; Yayi-Ladekan, E.; Sinsin, B.; Moudachirou, M.; Accrombessi, G. C.; Quetin-Leclercq, J.; J. Med. Plants Res. 2011, 5, 4640. [Link] accessed in August 2023 50. Nguyen, H. X.; Le, T. C.; Van Do, T, N.; Le, T. H.; Nguyen, N. T.; Nguyen, M. T. T.; Chem. Cent. J. 2016, 10, 1. [Crossref] 51. Sukari, M. A.; Ahmad, S.; Ismail, N.; Ismail, I. S.; Abdul, A. B.; Abu Bakar, M. F.; Kifli, N.; Ee, G. C. L.; BMC Complementary Altern. Med. 2015, 21, 105. [Crossref] 52. Silva-Júnior, E. F.; Aquino, P. G. V.; Santos-Júnior, P. F. S.; Nascimento, I. J. S.; Gomes, E. A.; Silva, A. L. L.; Verissimo, R. C. S. S.; Aquino, T. M.; Araújo-Júnior, J. X.; J. Chem. Pharm. Res. 2015, 7, 389. [Link] accessed in August 2023 53. David, J. M.; Moreira, B. O.; Vilar, V. L. S.; de Almeida, R. N. S.; Morbeck, L. L. B.; Andrade, B. S.; Barros, R. G. M.; Neves, B. M.; de Carvalho, A. L.; Cruz, M. P.; Yatsuda, R.; J. Ethnopharmacol. 2022, 289, 115089. [Crossref] 54. Cheng, Z. Q.; Yang, D.; Ma, Q. Y.; Dai, H. F.; Huang, S. Z.; Yi, X. H.; Zhou, J.; Zhao, Y. X.; Planta Med. 2012, 78, 1878. [Crossref] 55. Fouokeng, Y.; Akak, C. M.; Tala, M. F.; Azebaze, A. G. B.; Dittrich, B.; Vardamides, J. C.; Laatsch, H.; Fitoterapia 2017, 117, 61. [Crossref] 56. Ahmad, S.; Ali, M.; Ansari, S. H.; Ahmed, F.; J. Saudi Chem. Soc. 2010, 14, 409. [Crossref] 57. Achika, J. I.; Trop. J. Nat. Prod. Res. 2018, 2, 442. [Crossref] 58. Semalty, M.; Semalty, A.; Badola, A.; Joshi, G. P.; Rawat, M. S. M.; Pharmacogn. Rev. 2010, 4, 88. [Crossref] 59. Formagio, A. S. N.; da Silva, M. M.; Iriguchi, E. K. K.; Kassuya, C. A. L.; Vieira, M. C.; Foglio, M. A.; de Carvalho, J. E.; Ruiz, A. L. T. G.; Souza, K. P.; Rev. Bras. Farmacogn. 2017, 27, 445. [Crossref] 60. Nawwar, M.; Hussein, S.; Ayoub, N.; Hashim, A.; El-Sharawy, R.; Lindequist, U.; Harms, M.; Wende, K.; Fitoterapia 2011, 82, 1265. [Crossref] 61. Antal, D. S.; Schwaiger, S.; Ellmerer-Mueller, E. P.; Stuppner, H.; Planta Med. 2010, 76, 1765. [Crossref] 62. Topcu, G.; Ay, M.; Bilici, A.; Sarikuerkcue, C.; Oezturk, M.; Ulubelen, A.; Food Chem. 2007, 103, 816. [Crossref] 63. Yousfi, M.; Djeridane, A.; Bombarda, I.; Chahrazed-Hamia; Duhem, B.; Gaydou, E. M.; Phytother. Res. 2009, 23, 1237. [Crossref] 64. Hu, J. F.; Garo, E.; Hough, G. W.; Goering, M. G.; Johnson, M. N.; Eldridge, G. R.; Tetrahedron Lett. 2007, 48, 5747. [Crossref] 65. Ndongo, J. T.; Mbing, J. N.; Bikobo, D. N.; Atchade, A. T.; Shaaban, M.; Pegnyemb, D. E.; Laatsch, H.; Z. Naturforsch. , C:J. Biosci. 2013, 68, 169. [Crossref] 66. Christelle, T. D.; Hussain, H.; Dongo, E.; Hermine, J. M. B.; Ahmed, I.; Krohn, K.; Nat. Prod. Commun. 2011, 6, 1133. [Crossref] 67. Catalano, A.; Lantaño, B.; Fabián, L.; López, P.; Am. J. Plant Sci. 2020, 11, 861. [Crossref] 68. Tokoudagba, J. M.; Gandonou, C. D.; Hougbeme, A.; Gom, S. N.; Auger, C.; Schini-Kerth, V.; Lobstein, A.; World J. Pharm. Pharm. Sci. 2018, 7, 309. [Link] accessed in September 2023 69. da Silva, E. P.; David, J. M.; David, J. P.; Garcia, G. H. T.; Silva, M. T.; Quim. Nova 2020, 43, 1216. [Crossref] 70. Dang, P. H.; Nguyen, L. T. T.; Nguyen, H. T. T.; Le, T. H.; Do, T. N. V.; Nguyen, H. X.; Le, N. D.; Nguyen, M. T. T.; Nguyen, N. T.; Nat. Prod. Res. 2019, 33, 2883. [Crossref] 71. Razakarivony, A. A.; Lenta, B. N.; Andriamihaja, B.; Michalek, C.; Razanamahefa, B.; Razafimahefa, D. R.; Rakotondramanga, M. F.; Randrianasolo, R.; Lannang, A. M.; Randriamiaramisaina, R.; Boyom, F. F.; Rosenthal, P. J.; Sewald, N.; Zeitschrift für Naturforschung B 2016, 71, 297. [Crossref] 72. Garg, A. N.; Singh, R.; Singh, S. K.; Maharia, R. S.; J. Pharm. Biomed. Anal. 2015, 105, 150. [Crossref] 73. Kinghorn, A. K.; Westenburg, H. E.; Lee, K. J.; Lee, S. K.; Fong, H. H. S.; van Breemen, R. B.; Pezzuto, J. M.; J. Nat. Prod. 2000, 63, 1696. [Crossref] 74. Nguyen, M. T. T.; Nguyen, N. T.; Dang, P. H.; Nguyen, H. X.; Le, T. H.; Van Do, T. N.; Nat. Prod. Res. 2022, 36, 3737. [Crossref] 75. Shen, Y.; Chen, H.; Lang, L. J.; Dong, X.; Xiao, C. J.; Jiang, B.; Phytochem. Lett. 2021, 46, 172. [Crossref] 76. Cheng, Z. Q.; Yang, D.; Ma, Q. Y.; Yi, X. H.; Zhou, J.; Zhao, Y. X.; Chem. Nat. Compd. 2013, 49, 46. [Crossref] 77. Rodriguez-Lopez, V.; Aguirre-Crespo, F.; Salazar, L.; Estrada-Soto, S.; Nat. Prod. Res. 2006, 20, 1. [Crossref] 78. Gu, Q.; Wang, R. R.; Zhang, X. M.; Wang, Y. H.; Zheng, Y. T.; Zhou, J.; Chen, J. J.; Planta Med. 2007, 73, 279. [Crossref] 79. Dang, P. H.; Nguyen, T. T.; Le, T. H.; Nguyen, H. X.; Nguyen, M. T. T.; Nguyen, N. T.; Nat. Prod. Res. 2018, 32, 1745. [Crossref] 80. Ragasa, C. Y.; Vivar, J. L. A.; De Los Reyes, M. M.; van Altena, I. A.; Pharma Chem. 2016, 8, 257. [Link] accessed in August 2023 81. Mori, T.; Chang, C.; Maurtua, D.; Hammond, G. B.; Phytother. Res. 2006, 20, 160. [Crossref] 82. Brice, B. J.; Benson, B. B.; Fernique, K. K.; Mida, K. G. R.; Christian, K. K.; Nathalie, G. K.; Akhanovna, M. B. J.; Alain, B. Y.; Int. J. Pharm. Pharm. Sci. 2018, 10, 64. [Crossref] 83. Pare, D.; N'do, J. Y. P.; Guenne, S.; Nikiema, M.; Hilou, A.; Asian J. Chem. Sci. 2019, 6, 1. [Crossref] 84. Mirghani, M. E. S.; Al-Shwyeh, H. A.; Jamal, P.; Afr. J. Biotechnol. 2011, 10, 18739. [Crossref] 85. Pithayanukul, P.; Leanpolchareanchai, J.; Saparpakorn, P.; Molecules 2009, 14, 3198. [Crossref] 86. Kumar, M.; Saurabh, V.; Tomar, M.; Hasan, M.; Changan, S.; Sasi, M.; Maheshwari, C.; Prajapati, U.; Singh, S.; Prajapat, R. K.; Dhumal, S.; Punia, S.; Amarowicz, R.; Mekhemar, M.; Antioxidants 2021, 10, 299. [Crossref] 87. Jahnavi, C. H.; Jyothsna, K.; Geetika, D. L.; Keerthi, G. S.; Santhosha, D.; Ramesh, A.; J. Pharmacogn. Phytochem. 2020, 9, 1166. [Link] accessed in August 2023 88. Bakka, C.; Hadjadj, M.; Smara, O.; Dendougui, H.; Mahdjar, S.; J. Pharm. Sci. Res. 2019, 11, 3634. [Link] accessed in August 2023 89. Zia, M.; Bibi, Y.; Nisa, S.; Waheed, A.; Ahmed, S.; Chaudhary, M. F.; Indian J. Pharm. Sci. 2012, 74, 375. [Crossref] 90. Taleghani, A.; Eghbali-Feriz, S.; Shokouhnam, P.; Emami, S. A.; Farhadi, F.; Asili, J.; Hasanzadeh, S.; Tayarani-Najaran, Z.; Jundishapur J. Nat. Pharm. Prod. 2021, 16, e69844. [Crossref] 91. Tilkat, E. H.; Kuru, I. S.; Süzerer, V.; Haşimi, N.; Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1885. [Crossref] 92. Kumar, G.; Badoni, P. P.; Int. J. ChemTech Res. 2017, 10, 377. [Link] accessed in August 2023 93. Mutuku, A.; Mwamburi, L.; Keter, L.; Ondicho, J.; Korir, R.; Kuria, J.; Chemweno, T.; Mwitari, P.; BMC Complementary Med. Ther. 2020, 20, 272. [Link] accessed in August 2023 94. Rayne, S.; Mazza, G.; Plant Foods Hum. Nutr. 2007, 62, 165. [Crossref] 95. Al-Naser, Z.; Ibrahim, B.; Int. J. ChemTech Res. 2014, 6, 2799. [Link] accessed in August 2023 96. Morel, A. F.; Gehrke, I. T. S.; Tibursky Neto, A.; Pedroso, M.; Mostardeiro, C. P.; da Cruz, I. B. M.; Silva, U. F.; Ilha, V.; Dalcol, I. I.; J. Ethnopharmacol. 2013, 148, 486. [Crossref] 97. Oliveira, D. B.; Bernardes, N. R.; Heggdorne-Araújo, M.; Borges, I. F. J. C.; Almeida, F. M.; Amaral, E. P.; Lasunskaia, E. B.; Muzitano, M. F.; Rev. Bras. Farmacogn. 2014, 24, 644. [Crossref] 98. Boni, A. N. R.; Ahua, K. M.; Kouassi, K.; Djaman, A. J.; Nguessan, J. D.; Res. J. Pharm. , Biol. Chem. Sci. 2014, 5, 1457. [Link] accessed in August 2023 99. Cordeiro, B. M. P. C.; Santos, N. D. L.; Ferreira, M. R. A.; de Araújo, L. C. C.; Carvalho Júnior, A. R.; Santos, A. D. C.; de Oliveira, A. P.; da Silva, A. G.; Falcão, E. P. S.; Correia, M. T. S.; Almeida, J. R. G. S.; da Silva, L. C. N.; Soares, L. A. L.; Napoleão, T. H.; da Silva, M. V.; Paiva, P. M. G.; BMC Complementary Altern. Med. 2018, 18, 284. [Crossref] 100. Chaudhuri, D.; Ghate, N. B.; Panja, S.; Basu, T.; Shendge, A. K.; Mandal, N.; BMC Complementary Altern. Med. 2016, 16, 262. [Crossref] 101. Madikizela, B.; Aderogba, M. A.; Van Staden, J.; J. Ethnopharmacol. 2013, 150, 609. [Crossref] 102. Vambe, M.; Naidoo, D.; Aremu, A. O.; Finnie, J. F.; Van Staden, J.; Nat. Prod. Res. 2021, 35, 4658. [Crossref] 103. Yang, T. H.; Yan, D. X.; Huang, X. Y.; Hou, B.; Ma, Y. B.; Peng, H.; Zhang, X. M.; Chen, J. J.; Geng, C. A.; Phytochemistry 2019, 164, 228. [Crossref] 104. Silva, F. G.; Curado, F. M. L. M. J.; Gazolla, A. P.; Pedroso, R. C. N.; Pimenta, L. P.; de Oliveira, P. F.; Tavares, D. C.; Andrade-Silva, M. L.; Cunha, W. R.; Pietro, R. C. L. R.; Januário, A. H.; Pauletti, P. M.; Sales, J. F.; J. Med. Plants Res. 2016, 10, 450. [Crossref] 105. Suleiman, M. M.; Elgorashi, E. E.; Samuel, B. B.; Naidoo, V.; Eloff, J. N.; Biochem. Pharmacol. 2013, 2, 1000123. [Crossref] 106. Ahmed, A. S.; McGaw, L. J.; Moodley, N.; Naidoo, V.; Eloff, J. N.; S. Afr. J. Bot. 2014, 95, 9. [Crossref] 107. Martinez-Elizalde, K. S.; Jimenez-Estrada, M.; Flores, C. M.; Hernández, L. B.; Rosas-López, R.; Duran-Diaz, A.; Nieto-Yañez, O. J.; Barbosa, E.; Rodríguez-Monroy, M. A.; Canales-Martinez, M.; BMC Complementary Altern. Med. 2015, 15, 74. [Crossref] 108. Araújo, B. S.; Santos, C. C. S.; Araújo, S. S.; Santos, A. L. L. M.; Almeida, E. C. V.; Dias, A. S.; Damascena, N. P.; Santos, D. M.; Santos, M. I. S.; Júnior, K. A. L. R.; Pereira, C. K. B.; Lima, A. C. B.; Shan, A. Y. K. V.; Sant'ana, A. E. G.; Estevam, C. S.; Rev. Bras. Farmacogn. 2014, 24, 298. [Crossref] 109. Van Giau, V.; Nguyen, N. H.; Thuc-Huy, D.; Nguyen, T. T.; Ma, P. C.; Ta, Q. T. H.; Molecules 2020, 25, 1996. [Crossref] 110. Adesegun, S. A.; Badejo, M. V.; Odebumni, S. O.; Ojobo, P. D.; Coker, H. B.; Trop. J. Nat. Prod. Res. 2019, 3, 58. [Crossref] 111. Sequeda-Castañeda, L. G.; Celis-Zambrano, C. A.; Torrenegra-Guerrero, R. D.; Pharmacology Online 2021, 1, 426. [Link] accessed in August 2023 112. Milella, L.; Russo, D.; Miglionico, R.; Carmosino, M.; Bisaccia, F.; Andrade, P. B.; Valentão, P.; Armentano, M. F.; Int. J. Mol. Sci. 2018, 19, 186. [Crossref] 113. Njume, C.; Afolayan, A. J.; Green, E.; Ndip, R. N.; Int. J. Antimicrob. Agents 2011, 38, 319. [Crossref] 114. Shai, L. J.; Mogale, M. A.; Lebelo, S. L.; Thovhogi, N.; de Freitas, A. N.; Afr. J. Biotechnol. 2011, 10, 15033. [Crossref] 115. Uddin, Z. M.; Rana, S. M.; Hossain, S.; Ferdous, S.; Dutta, E.; Dutta, M.; Bin-Emram, T.; J. Complementary Integr. Med. 2020, 17, 20190102. [Crossref] 116. Al Sayed, E.; Martiskainen, O.; Sinkkonen, J.; Pihlaja, K.; Ayoub, N.; Singab, A. N.; El-Azizi, M.; Nat. Prod. Commun. 2010, 5, 545. [Crossref] 117. Sobeh, M.; Mahmoud, M. F.; Hasan, R. A.; Abdelfattah, M. A. O.; Sabry, O. M.; Ghareeb, M. A.; El-Shazly, A. M.; Wink, M.; Sci. Rep. 2018, 8, 9343. [Link] accessed in August 2023 118. Nitiéma, M.; Belemnaba, L.; Ouedraogo, S.; Ouedraogo, N.; Ouedraogo, S.; Gissou, I. P.; World J. Pharm. Res. 2018, 7, 39. [Link] accessed in September 2023 119. Shah, K. A.; Patel, M. B.; Chauhan, K. N.; Shah, S. S.; Parmar, P. K.; Patel, N. M.; Pharm. Sin. 2010, 1, 156. [Link] accessed in August 2023 120. Ferdous, R. U.; Jami, S. I.; Begum, M.; Begum, T.; Rahman, M. A.; Haque, T.; Khan, A.; Hossain, B.; Tarek, H.; Alam, N. A.; Am. J. BioSci. 2015, 3, 19. [Crossref] 121. Shahid, S.; Taj, S.; 2nd International Conference on Functional Materials and Chemical Engineering (ICFMCE 2018), Lahore, Pakistan, 2019. [Crossref] 122. Youl, E. N. H.; Nassouri, S.; Ilboudo, S.; Ouedraogo, M.; Sombié, C. B.; Ilboudo, S.; Dakuyo, Z. P.; Gissou, I. P.; Afr. J. Pharm. Pharmacol. 2020, 14, 339. [Crossref] 123. Varela-Rodríguez, L.; Sánchez-Ramírez, B.; Rodríguez-Reyna, I. S.; Ordáz-Ortiz, J. J.; Chávez-Flores, D.; Salas-Muñoz, E.; Osorio-Trujillo, J. C.; Ramos-Martínez, E.; Talamás-Rohana, P.; BMC Complementary Altern. Med. 2019, 19, 153. [Crossref] 124. Wang, G.; Li, F.; Du, L.; Wang, J. J.; BioMed Res. Int. 2015, 1, 1. [Crossref] 125. Attanayake, A. P.; Jayatilaka, K. A. P. W.; Pathirana, C.; Mudduwa, L. K. B.; Int. J. Pharmacogn. 2015, 2, 166. [Crossref] 126. Rao, B. G.; Raju, N. J.; Asian J. Chem. 2009, 21, 7416. [Link] accessed in August 2023 127. Panda, B. K.; Patro, V. J.; Mishra, U. S.; Kar, S.; J. Pharm. , Chem. Biol. Sci. 2014, 1, 26. [Link] accessed in August 2023 128. Chaturvedi, B.; Chaturvedi, D. S.; Int. J. Pharm. Sci. Res. 2021, 12, 3744. [Crossref] 129. Stanislaus, N. N.; Ibrahim, S. M.; Usman, P. U.; Sa'adyia, H. H.; Garba, M. M.; Toyin, A. S.; Moji, B. O. T.; Ndifor, A. R.; Osas, E. G.; Oyetunji, S. A.; Salawu, S. M.; Pak. J. Pharm. Sci. 2021, 34, 629. [Link] accessed in September 2023 130. Okoth, D. A.; Koorbanally, N. A.; Chenia, H. Y.; Phytochem. Lett. 2013, 6, 476. [Crossref] 131. Ficoseco, M. E. A.; Sampietro, D. A.; Vattuone, M. A.; Audenaert, K.; Catalán, C. A. N.; J. Appl. Microbiol. 2014, 116, 1262. [Crossref] 132. Beretta, G.; Arlandini, E.; Gelmini, F.; Testa, C.; Angioletti, S.; Nat. Prod. Res. 2021, 35, 4764. [Crossref] 133. Zhang, B.; Fang, J.; Chen, Y.; Zhao, J.; Li, S.; Zeng, L.; Pharm. Biol. 2015, 53, 503. [Crossref] 134. Muñoz-Ramírez, A.; Torrent-Farías, C.; Mascanayo-Collado, C.; Urzúa-Moll, A.; Phytochemistry 2020, 174, 112359. [Crossref] 135. Uddin, G.; Rauf, A.; Al-Othman, A. M.; Collina, S.; Arfan, M.; Ali, G.; Khan, I.; Fitoterapia 2012, 83, 1648. [Crossref] 136. Eaton, A. L.; Rakotondraibe, L.; Harinantenaina, B.; Peggy J.; Goetz, M.; Kingston, D. G. I.; Planta Med. 2015, 78, 1752. [Crossref] 137. Ledoux, A.; St-Gelais, A.; Cieckiewicz, E.; Jansen, O.; Bordignon, A.; Illien, B.; Di Giovanni, N.; Marvilliers, A.; Hoareau, F.; Pendeville, H.; Quetin-Leclercq, J.; Frederich, M.; J. Nat. Prod. 2017, 80, 1750. [Crossref] 138. Roumy, V.; Fabres, N.; Portet, B.; Bourdy, G.; Acebey, L.; Vigor, C.; Valentim, A.; Moules, C.; Phytochemistry 2009, 70, 305. [Crossref] 139. Ledoux, A.; Beriot, D.; Mamede, L.; Desdemoustier, P.; Detroz, F.; Jansen, O.; Frederich, M.; Maquoi, E.; Planta Med. 2021, 87, 1008. [Crossref] 140. Mosa, R. A.; Oyedeji, A. O.; Shode, F. O.; Singh, M.; Opoku, A. R.; Afr. J. Pharm. Pharmacol. 2011, 5, 2698. [Link] accessed in September 2023 141. Nair, P. K. R.; Melnick, S. J.; Wnuk, S. F.; Rapp, M.; Escalon, E.; Ramachandran, C.; J. Ethnopharmacol. 2009, 122, 450. [Crossref] 142. Arimboor, R.; Rangan, M.; Aravind, S. G.; Arumughan, C.; J. Ethnopharmacol. 2011, 133, 1117. [Crossref] 143. Kamkumo, R. G.; Ngoutane, A. M.; Tchokouaha, L. R. Y.; Fokou, P. V. T.; Madiesse, E. A. K.; Legac, J.; Kezetas, J. J. B.; Lenta, B. N.; Boyom, F. F.; Dimo, T.; Mbacham, W. F. M.; Gut, J.; Rosenthal, P. J.; Malar. J. 2012, 11, 382. [Crossref] 144. El Sayed, A. M.; J. Med. Plants Res. 2016, 10, 223. [Crossref] 145. Mosa, R. A.; Cele, N. D.; Mabhida, S. E.; Shabalala, S. C.; Penduka, D.; Opoku, A. R.; Molecules 2015, 20, 13374. [Crossref] 146. Poletti, M.; Marion, C.; FRpat. 2,959,666A1 2010. 147. Dubief, C.; Nadia, K.; Boche, B.; FRpat.2,961,693A1 2010. 148. Ennamany, R.; EP3,250,295B1 2016. 149. Campmany, J. T.; USpat. 8,337,485 B2 2013. 150. Commin, A. R.; WOpat. 2011/01000A1 2011. 151. Buter, K. B.; Buchwald-Werner, S.; US pat. 10,596,212B2 2020. 152. Pushpangadan, P.; Rawat, A. K. S.; Rao, C. V.; Srivastava, S. K.; Govindarajan, R.; WO pat. 2006/061849 A1 2006. 153. Krishnan, G.; EP pat. 2,326,338B1 2009. 154. Quave, C. L.; Lyles, J.; Tang, H.; Porras-Brenes, G.; US pat. 2021/0315906A1 2021. 155. Bogoshi, M. N.; PAT035/TDP2014/03582 2014. 156. Nandeshwar, W. M.; IN pat. 21,007,662, A23K1/18 2017. 157. Elsohly, M.; Gul, W.; Ashfaq, M. K.; US pat. 10,322,103 B2 2019. 158. Rotem, M. O.; Elbaz, M.; Etkes, A. S.; Meihls, L. N.; Ben Naim, N.; Ghosh, D.; US pat. 2021/0171976A1 2021. 159. Mukherjee, B.; Samanta, A.; 61/KOL/2006 2003. 160. Tomioka, T.; US pat. 2006/0008539A1 2006. 161. Fernández Ponce, M. T.; Fernández, E. J. M. O.; Cardoso, L. C.; Gay, D. A.; ES pat. 2,567,530B1 2017. 162. Rieck, H.; Lachaise, H.; Steiger, D.; Labourdette, G.; US pat. 2011/0065580 A1 2011. 163. Bellei, S.; Melis, L.; Peloso, A.; Betti, N.; WO pat. 2021/009379Al 2021. 164. Cyril, N. L.; Raynard, M.; de Chily, P. C.; FR pat.2,883,003 A1 2006. 165. Boucher, C.; Ghislain, H. M.; FR pat. 2,886,547 A1 2006. 166. Falco, G.; WO pat. 2021/124167Al 2021. 167. Miyanaga, A.; Biosci. , Biotechnol. , Biochem. 2017, 81, 2227. [Crossref] 168. Piel, J.; Reiter, S.; Cahn, J. K. B.; Wiebach, V.; Ueoka, R.; ChemBioChem 2020, 21, 564. [Crossref] 169. Nakano, C.; Ohnishi, Y.; Funa, N.; Horinouchi, S.; J. Bacteriol. 2012, 194, 1544. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access