Artigo
| Chemical constituents and evaluation of antiproliferative and anti-inflammatory activities from Psychotria schlechtendaliana (Rubiaceae) |
|
José G. de S. CorrêaI; Vagner M. de MouraI; Franciele Q. AmesII; Mirelli BianchinI; Simone B. S. SeboldI; Matheus A. PeixotoI; Armando M. PominiI; João E. de CarvalhoIII; Ana L. T. G. RuizIII; Ciomar A. Bersani-AmadoII; Silvana M. Oliveira SantinI*
I. Departamento de Química, Universidade Estadual de Maringá, 87020-900 Maringá - PR, Brasil Recebido em: 27/04/2023 Phytochemical study of Psychotria schlechtendaliana (Rubiaceae) aerial parts resulted in the isolation of alkaloids 4 N oxide harmane (1) and strictosidinic acid (2), and the terpenoids sitosterol, estigmasterol, α-amyrin, β-amyrin and betulinic acid. The crude extract (CE), its fractions (hexane FH, chloroform FC, ethyl acetate FEA, hydromethanolic FHM, alkaloidal chloroform FCOH, alkaloidal aqueous FAq) and majority alkaloid (1) were investigated for their antiproliferative potential against nine human tumor cells lines and one non-tumoral human cell line (HaCat). CE, FH and FEA fractions exhibited strong growth inhibition for ovary cells (OVCAR-3, GI50 = 5.89; 1.36 and 6.59 µg mL-1, respectively) and FC, FH and FAq fractions showed potent activity on the growth of leukemia cell lines (K562, GI50 = 1.92; 7.23 and 8.81 µg mL-1, respectively). Compound 1 exhibited selective antiproliferative activity to breast cancer (MCF-7, GI50 = 32.7 µg mL-1) and was non-toxic to HaCat cells. To evaluate the anti-inflammatory effect, models of ear edema induced by croton oil and the enzyme myeloperoxidase (MPO) were used. FH and FC fractions exhibited anti-inflammatory effect and reduce ear edema compared to the control group. These fractions showed results superior to those exhibited by indomethacin (75.1%), FH (97.5%) and FC (95.5%) fractions. INTRODUCTION Psychotria is the largest genus within Rubiaceae, comprising approximately 1700 species distributed in the tropical and subtropical regions of the world.1,2 Several South American Psychotria species are used as medicinal plants to treat different diseases, including gastrointestinal disorders, and has antibacterial, antiviral, antifungal, anti-inflammatory, and anticancer action. These different biological activities are associated with substances of the classes of alkaloids, iridoids, terpenoids, and flavonoids recurrently described in the genus.3-6 Psychotria schlechtendaliana (Müll. Arg.) Müll. Arg., synonym of Palicourea divaricata Schltdl and Uragoga schlechtendaliana (Müll. Arg.) Kuntze, is an endemic specie from Brazil Northeast in the caatinga (semi-arid) and Atlantic Forest area.7 As part of our continuing work on the Rubiaceae, in this study we reported the isolation of β-carboline alkaloids and triterpenes and, in addition, antiproliferative, and anti-inflammatory activities of P. schlechtendaliana. This is the first chemical and pharmacological study of this species.
EXPERIMENTAL General experimental procedures The NMR analysis was performed out on a Bruker Avance III HD (500 MHz or 300 MHz for 1H and 125 MHz or 75 MHz for 13C), in CDCl3 and/or CD3OD (Merck, Barueri-SP, Brasil), using TMS as an internal standard. The chemical shifts (δ) were expressed in ppm and coupling constants (J) in Hz. Column chromatography (CC) was performed on silica gel 60 (0.063-0.200 mm, Merck) and Sephadex LH 20 (Sigma-Aldrich, Barueri-SP, Brasil). Thin layer chromatography (TLC) was performed on silica gel 60 (5.0 × 20 cm × 0.20 mm). The organic solvents used in the methodologies were n-hexane (98.5%), chloroform (99.5%), ethyl acetate (99.5%), n-butanol (99.4%), and methanol (99.8%) from Synth (São Paulo, Brazil). Plant material Aerial parts of de P. schlechtendaliana were collected in Private Reserve of the National Patrimony of Serra Bonita (PRNP Serra Bonita), on Camacan, South of Bahia, Brazil (Bapeba Trail: 15º23'30" S, 39º33'55" W), in December of 2013, identified by Prof. Dr. André Márcio Amorim and a voucher specimen (CEPEC 141.213) was deposited in the herbarium of the State University of Santa Cruz. P. schlechtendaliana was registered in the Sistema Nacional de Gestão do Patrimônio Genético e Conhecimento Tradicional Associado (SisGen) at the number A980835. Extraction and isolation The dried and ground aerial parts of P. schlechtendaliana (273 g, 16 mesh) were degreased with n-hexane and extracted with methanol (18 × 500 mL, 72 h), obtaining the crude methanolic extract (CE, 44.52 g) after concentration on a rotary evaporator (40 °C). Part of the CE (19.60 g) was suspended in methanol:water (1:1 v/v) and subjected to acid-base extraction with hydrochloric acid solution (10% v/v) and CHCl3 obtaining the chloroform-acid fraction (FCH, 6.02 g). Acid solution residue was basified with NH4OH to pH 10 and extracted again with CHCl3, providing the alkaloidal chloroform (FCOH, 90.0 mg) and the aqueous fractions (FAq, 10.0 g). Fraction FAq (9.0 g) was suspended in water (100 mL) and partitioned with ethyl acetate and n-butanol, providing the ethyl acetate (FEA1, 95.0 mg), butanol (FB1, 275.7 mg) and aqueous fractions (FAq1, 8.0 g), respectively. Compound 1 (8.0 mg) was obtained from fraction FCOH (80.0 mg) by a preparative TLC, eluting with chloroform:methanol (8:2) and NH4OH drops, followed an extraction of the material with CHCl3:MeOH (1:1). Compound 2 (5.0 mg) was isolated from FB1 fraction (275.7 mg) by successive fractionation by CC on silica gel 60 (14.56 g, Ɵ = 1.5 cm, h = 21 cm), with CHCl3:MeOH in the ratio of 10:0 to 0:10 (with NH4OH drops). Another part of CE (9.62 g) was suspended in MeOH:H2O (1:1) and partitioned with hexane, chloroform and ethyl acetate. This procedure resulted in hexane (FH, 952.9 mg), chloroform (FC, 615.0 mg), ethyl acetate (FEA, 134.0 mg), and hydromethanol fractions (FHM, 6.39 mg). The FH fraction (400 mg) was subjected to chromatographic column silica gel 60 (18.00 g, Ɵ = 1.6 cm, h = 30 cm), eluted in Hex:EtOAc in an increasing polarity gradient, 10 subfractions (FH1-FH10) were obtained and in FH3 (3.0 mg) a white solid was provided which was named by substances 3 and 4. FC fraction (600.0 mg) was treated on silica gel CC (21.00 g, Ɵ = 2.0 cm, h = 25 cm), eluted in hexane, ethyl acetate and methanol in different proportions, in increasing order of polarity. From this process, 27 fractions were obtained (FC1-FC27), and fraction FC15 (141.0 mg) was purified on silica gel CC (9.62 g, Ɵ = 1.45 cm, h = 24 cm) using the CHCl3/MeOH solvent system in a gradient of 10:0 at 0:10, giving a mixture of substances 5, 6 and 7 as white powder. Strictosidinic acid (2) 1H NMR (500 MHz, CD3OD) δ 4.47 (d, 1H, J 11.9 Hz, H-3), 3.72 (m, 2H, H-5), 3.07 (m, 2H, H-6), 7.45 (d, 1H, J 7.9 Hz, H-9), 7.03 (dd, 1H, J 7.6 and 7.9 Hz, H-10), 7.12 (dd, 1H, J 7.6 and 8.1 Hz, H-11), 7.31 (d, 1H, J 8.1 Hz, H-12), 2.12 (ddd, 1H, J 13.8, 11.4 and 4.2 Hz, H-14a), 2.27 (ddd, 1H, J 13.8, 13.0 and 3.2 Hz, H-14b), 2.98 (m, 1H, H-15), 7.62 (brs, 1H, H17), 5.21 (d, 1H, J 10.6 Hz, H-18a), 5.31 (d, 1H, J 17.4 Hz, H-18b), 5.85 (ddd, 1H, J 13.7, 10.6, 7.6 Hz, H-19), 2.71 (m, 1H, H-20), 5.83 (d, 1H, J 9.6 Hz, H-21), 4.82 (d, 1H, J 7.9 Hz, H-1'), 3.24 (dd, 1H, J 10.4, 2.4 Hz, H-2'), 3.45 (dd, 1H, J 7.2, 2.4 Hz, H-3'), 3.25 (dd, 1H, J 10.4, 7.2 Hz, H-4'), 3.41 (t, 1H, J 9.0 Hz, H-5'), 3.68 (dd, 1H, J 11.9, 7.2 Hz, H-6'a), 4.01 (dd, 1H, J 11.9, 1.9 Hz, H-6'b); 13C NMR (125 MHz, CD3OD) δ 130.6 (C 2), 52.6 (C-3), 43.1 (C-5), 19.7 (C-6), 107.4 (C-7), 127.6 (C-8), 119.2 (C-9), 120.7 (C-10), 123.5 (C-11), 112.4 (C-12), 138.3 (C-13), 35.2 (C-14), 33.8 (C-15), 112.8 (C16), 154.2 (C-17), 119.2 (C-18), 136.2 (C-19), 45.8 (C-20), 96.9 (C-21), 175.5 (C-22), 100.5 (C-1'), 74.9 (C-2'), 78.1 (C3'), 72.0 (C-4'), 78.9 (C-5'), 63.2 (C-6'). In vitro assays Cell lines and culture conditions For in vitro antiproliferative screening, the nine human tumor cell lines were tested, being glioma (U-251), breast (MCF-7), ovary, with multiple drug resistance phenotype (NCI-ADR/RES), kidney (786 O), lung, non-small cell type (NCI-H40), prostate (PC-3), ovary (OVCAR-03), colon (HT-29), leukemia (K562). The Frederick Cancer Research and Development Center (National Cancer Institute, Frederick, MA, USA) provided these cell lines. The immortalized keratinocyte non-tumor cell line (HaCat) was donated by Prof. Dr. Ricardo Della Coletta, FOP/UNICAMP. All human cells were cultivated in a 75 cm2 flask (Corning, USA) in complete medium [Roswell Park Memorial Institute (RPMI) medium 1640 (GIBCO, NY, USA) supplemented with 5% fetal bovine serum (FBS) (Vitrocell, SP, BR) and penicillin:streptomycin (1000 U mL-1:1000 µg mL-1, 10 mL L-1 in RPMI 1640, Vitrocell, SP, BR)]. Antiproliferative assay The samples (10 mg; CE, FH, FC, FEA, FHM, FCOH, FAq and compound 1) were diluted in dimethyl sulfoxide (DMSO) followed by successive dilutions in a complete medium to give final concentrations of 0.25; 2.5; 25 and 250 µg mL-1 and incubated at 37 °C in a 5% CO2 atmosphere in a humid environment for 48 h. Doxorubicin hydrochloride 0.4% m/v (Europharma Green Bay, WI) was used as a positive control. Another microplate containing all cell lines was also prepared and use to establish the cell quantities at sample addition (time zero, T0). Subsequently, cells were fixed with 50 µL of 50% trichloroacetic acid (TCA) and cell proliferation was determined by spectrophotometry (540 nm) of the protein content using the sulforhodamine B assay.8 Results were plotted by correlating drug concentration and proliferation for each cell type. The values of GI50 (sample concentration required to 50% inhibition of cell proliferation) by nonlinear regression sigmoid type were established, using Origin 7.5 software.9 In vivo evaluations Animals The anti-inflammatory activity assay was performed using male Swiss mice, weighing 30-40 g kept in collective cages (five animals) in an environment-controlled humidity (40-60%) and temperature (22 ± 2 °C), under a 12/12 h light/dark cycle, with free access to food and water. The protocols for these experiments were approved by the Committee of Ethics in Animal Experimentation of the State University of Maringa (CEUA/UEM 9804/2016). Croton oil-induced ear edema The edema was induced by the application of 20 µL of CO (200 µg) diluted in 70% acetone (acetone/water 7:3 v/v) at the inner surface of the left ear (LE) of mice.10 Crude extract (20 µL, 2.5 mg ear-1) and fractions (FH, FC, FEA and FHM; 20 µL, 2.5 mg ear-1) were applied to the left ear, and the same volume of solvent was applied to the right ear as control. Indomethacin was used as a positive control (1.0 mg ear 1). After 6 h, the animals were anesthetized, euthanized, and each ear was perforated with a metal punch to provide a 6 mm diameter disc. Edema was assessed by subtracting the weight of the disc from the right control ear from the weight of the disc from the left treated ear, thus determining the percentage of edema inhibition. The percentage of edema inhibition was determined according to Bracht et al.11 Myeloperoxidase activity (MPO) The myeloperoxidase activity (MPO) was assessed according to Boller et al.12 Ear tissue was placed in 50 mmol L-1 potassium phosphate buffer, pH 6.0, containing 0.5% hexadecyltrimethylammonium bromide (1 mL 50 mg-1 tissue, Sigma-Aldrich) in Potter homogenizer. The homogenate was centrifuged for 5.0 min at 2500 rpm and 25 °C and supernatant was added to a 96-well microplate, followed by the addition of 200 mL of the buffer solution containing o-dianisidine dihydrochloride (16.7 mg, Sigma Aldrich), distilled water (90 mL), potassium phosphate buffer (10 mL), and H2O2 1% (50 mL). The reaction was stopped by the addition of 30 µL of 1.46 mol L-1 sodium acetate, pH 3.0 and MPO activity was determined colorimetrically using a microplate reader to measure absorbance at 450 nm and expressed as OD per tissue. Statistical analysis For the results of anti-inflammatory activities (n = 6) the data were expressed as mean values ± SEM (standard error of the mean) and tested with analysis of variance (ANOVA) by Tukey test, being considered significant p < 0.05.
RESULTS AND DISCUSSION Chemical characterization The phytochemical investigation of the aerial parts of Psychotria schlechtendaliana afforded seven compounds (Figure 1): the alkaloids 4-N-oxide-harmane (1)13 and strictosidinic acid (2),14 the steroids sitosterol (3) and stigmasterol (4)15 and the triterpenes α amyrin (5), β-amyrin (6) and betulinic acid (7).16 The chemical structures were characterized by 1H, 13C, and 2D NMR analysis, followed by comparison with the literature.
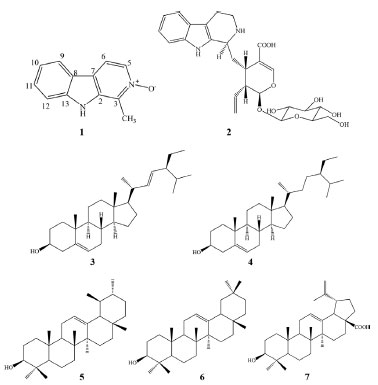 Figure 1. Molecular structure of compounds isolated from aerial parts of P. schlechtendaliana
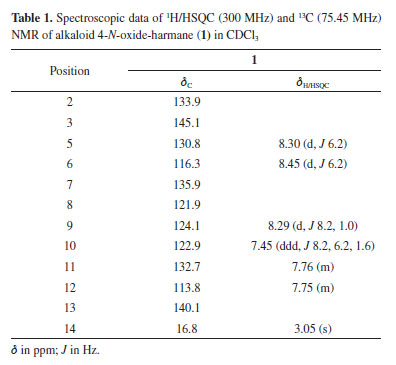
The 1H, 13C and 2D NMR spectra of compound 1 showed six aromatic hydrogens at δH 8.45 (d, J 6.2 Hz, H-6), 8.29 (dt, J 8.1, 1.0 Hz, H-9), 8.30 (d, J 6.2 Hz, H-5), 7.76 (m, H-11), 7.75 (m, H-12) and 7.45 (ddd, J 8.2, 6.2, 1.6 Hz, H-10). The singlet at δH 3.05 (s, CH3) was characterized as a methyl group in the position H-14. By the heteronuclear single quantum correlation (HSQC) experiment, the correlations of all hydrogens with their respective carbons were established (Table 1). The HMBC spectrum showed the correlations between the H-5/C-2/C-6, H-6/C-7, and H-10/C-12 signals belonging to the indolic nucleus. The spectral data were compared with the literature for the harmane alkaloid.17 However, considerable differences in positions H-6/C-6, C-2, and C-14 have been observed, suggesting the oxidation of the nitrogen atom as presented by the 4-N-oxide harmane alkaloid.13 These variations occur due to the protective effect γ of the oxygen atom on C-2, C-6, and methyl carbon, in addition to increasing the electron density in C-3 through the resonance effect. The 4-N-oxide harmane alkaloid (1) was reported in Ophiorrhiza rosacea and this is the first study of isolation this substance in Rubiaceae species.13 The harmane derivatives were described exhibiting anti-depressant, spasmolytic and antiplatelet activity.18-20
The alkaloid 2 was determined as the monoterpene indol alkaloid strictosidinic acid by comparing its data with those of reported values.14 In Psychotria genus, harmane and strictosidinic acid were previously reported only in P. myriantha, P. barbiflora and P. acuminate.3 Studies of Psychotria genus show the presence of other monoterpene indol alkaloids and pyrrolidine indol alkaloids.21-23 Psychotria is a genus taxonomically complex and due to morphological characters and geographical distribution. It is recognized three subgenera: Psychotria (pantropical), Tetramerae (includes some species from Africa and Madagascar) and Heteropsychotria (includes the remainder of the species of Psychotria in the neotropics).24 Several alkaloid types are described on the Psychotria genus and the presence of different profiles of these was observed in the different subgenera. Alkaloids of polyindoline type are frequents in Psychotria subgenus, while indole monoterpene alkaloids are related to the Heteropsychotria subgenus, especially the species collected in Brazil.24 The presence of compounds 1 and 2 in P. schelechtendaliana corroborates this observation. In a recent study, we relate the isolation of strictosidinic acid (2) of Palicourea minutiflora and, in the evaluation of anti-inflammatory activity was observed that this substance presented a low effect of inhibiting the formation of edema in anti-inflammatory activity, however promoted a reduction of 81.9% (1.25 mg ear-1) in the MPO activity. In the assay of antiproliferative activity this alkaloid did not present any effect on the antitumor cell lines at the highest tested concentration.25 Antiproliferative assay The extract and their fractions exhibited activity for different tumor cells (Table 2), according to the parameters established by N'Da and Smith26 for growth inhibition (GI50). Crude extract (CE) exhibited potent activity cytostatic non-selective for ovarian line (OVCAR-3, GI50 = 5.89 µg mL-1) and moderate activity for leukemia (K562, GI50 = 22.22 µg mL-1). Fraction FH showed results against ovary (OVCAR-03, GI50 = 1.36 µg mL-1), leukemia (K562, GI50 = 7.23 µg mL-1), glioma (U251, GI50 = 25.84 µg mL 1), breast (MCF-7, GI50 = 25.72 µg mL-1), ovarian (NCI-ADR/RES, GI50 = 29.37 µg mL-1), kidney (786-O, GI50 = 26.01 µg mL 1), lung (NCI-H40, GI50 = 30.12 µg mL-1) and prostate (PC-3, GI50 = 24.35 µg mL-1) cell lines. In addition, for the non-tumor cell line (HaCat, GI50 = 28.64 µg mL-1). Fraction FC indicated potent activity for leukemia (K562, GI50 = 7.23 µg mL-1), and moderate activity for ovary (OVCAR-03, GI50 = 25.00 µg mL-1). FEA fraction was active for ovary (OVCAR-03, GI50 = 6.59 µg mL-1) and leukemia (K562, GI50 = 24.50 µg mL-1) cell lines. The alkaloidal fraction (FCOH) exhibited moderate activity cytostatic non-selective against cell lines of glioma (U-251, GI50 = 25.92 µg mL-1), lung (NCI-H40, GI50 = 11.61 µg mL-1) and leukemia (K562, GI50 = 12.66 µg mL-1). Fraction FAq presented potent cytostatic activity with selectivity against the leukemia line (K562, GI50 = 8.81 µg mL-1).
Compound 1 isolated of FCOH presented a selective cytostatic activity for breast line (MCF-7) with GI50 = 32.7 µg mL-1 and did not show cytotoxic effect for the non-tumor line (HaCat) up to the maximum concentration evaluated. This is the first pharmacological description for the structure, encouraging further studies. In vitro cytotoxicity assessments of species of the Psychotria genus have shown promising results for different tumor cell lines, especially ovary, prostate, leukemia, glioma, and breast.26-28 These effects may occur due to the chemical composition presented by species of Psychotria, highlighting the alkaloids, terpenoids, and flavonoids.5 Evaluation of the topical anti-inflammatory activity of crude extract and fractions Croton oil-induced ear edema test The application of croton oil to the mouse ear induces an inflammatory response characterized by the formation of edema and leukocyte infiltration, with the participation of inflammatory mediators such as leukotrienes (LT), prostaglandins and (PGs), and cytokines.29 This procedure in the internal tissue of the mice caused inflammation after 6 h (18.7 ± 3.32 mg) in comparison with the vehicle (V) (11.5 ± 0.41 mg). The topical application of the CE, FH, FC and FEA (2.5 mg ear-1) reduced the ear edema in 60.5, 97.5, 95.5 and 57.5%, respectively. On the other hand, the FHM was not effective and generating 38.5% inhibition. Indomethacin (1 mg ear-1) presented reduced the ear edema (75.1%) (Figure 2).
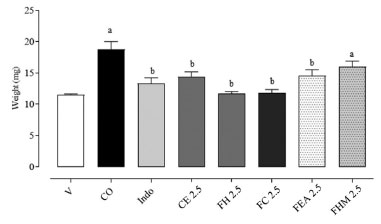 Figure 2. Effect of topical treatment with P. schlechtendaliana extract and fractions. (2.5 mg ear-1) on-ear edema, 6 h after croton oil (CO) application in the inner surface of the ear in mice (n = 6 animals/group). Indo (1 mg ear 1) were use as anti-inflammatory drugs (positive control). V: vehicle (20 µL, acetone 70% v:v); CO: croton oil (inflamed control group); Indo: indomethacin; CE: crude extract; FH: hexane; FC: chloroform; FEA: ethyl acetate; FHM: hydromethanol. The data was express as the mean ± SEM for each group. Different letters indicate the statistical difference between groups (p < 0.05, ANOVA followed by Tukey's test)
Myeloperoxidase activity (MPO) The determination of the number of leukocytes recruited from the tissue of the inflamed ear was performed by indirect quantification of the MPO30 and results show that the MPO was increased in the 6th h after the application of CO (Figure 3). Crude extract reduced MPO activity by 66.2%, while the FH, FC, FEA and FHM fractions yielded a reduction of 51.1, 30.4, 24.4, 17.8%, respectively. Indomethacin presented a reduction of 44.9% in enzymatic activity.
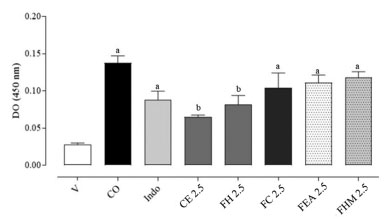 Figure 3. Effect of crude extract and fractions (2.5 mg ear-1) from P. schlechtendaliana on myeloperoxidase (MPO) activity supernatants of homogenates of ear tissue mice (n = 6 animals/group). Indo (1 mg ear-1) were use as anti-inflammatory drugs (positive control). V: vehicle (20 µL, acetone 70% v:v); CO: croton oil (inflamed control group); Indo: indomethacin; CE: crude extract; FH: hexane; FC: chloroform; FEA: ethyl acetate; FHM: hydromethanol. The data was express as the mean ± SEM for each group. Different letters indicate the statistical difference between groups (p < 0.05, ANOVA followed by Tukey's test)
Psychotria species have been use in folk medicine as anti-inflammatory agents, alkaloids and terpenoids are important compounds that lead to these results. Some studies were developed looking for applications such as anti-inflammatory drugs. The inhibition of nitric oxide (NO) caused by compounds isolated from P. prainii,31 P. nuda,32 and P. capensis33 reinforces the responses of the extracts, in addition to indicating the need for further studies with the genus. The evaluation of the in vivo anti-inflammatory capacity carrageenan-induced paw edema in rats indicated that the fruits and stems of P. nilgiriensis showed significant inhibition.34 Triterpenoids, in general, have several pharmacological applications and respond efficiently to various biological assays. α-Amyrin (5), β-amyrin (6), betulinic acid (7) and β-sitosterol (4) are compounds that have numerous descriptions in natural products, in addition to their pharmacological potential been consolidated in the literature. Research reports the anti-inflammatory response of these substances by different models in vitro and in vivo. The significant paw edema inhibitory activity was observed by β-amyrin (6).35,36 β-Sitosterol (4), α-amyrin (5) showed significant anti-edematogenic activity in comparison with that of indomethacin in mouse ear.37 The inhibitory effects against TPA-induced edema as well as against carrageenan-induced edema for 5 and 6 have also been reported. These triterpenoids are also antitumor for different cells.38,39 The compounds 5 and 6 exerts anticancer efficacy by several mechanisms such as proliferation inhibition, enhancing apoptosis and growth receptor modulation, betulinic acid (7) is described as a potent selective inhibitor of human melanoma in vitro and in vivo40 and its anti-inflammatory activity was shown in different models of inflammation. Antitumor activities also have been observed for sitosterol and stigmasterol steroids.41,42 Therefore, the presence of these metabolites could explain the positive results presented by apolar fractions in anti-inflammatory and antiproliferative assays.
CONCLUSION Our findings reveal that Psychotria schlechtendaliana has compounds with antiproliferative and anti-inflammatory activity. Compound 1, the alkaloid 4-N-oxide harmane exhibited selective antiproliferative activity to human breast cancer and was non-toxic to HaCaT cells, showing a promising source in the search for new drugs. The harmane-β-carboline alkaloid and its derivatives have shown several pharmacological activities such as antianxiety, antidepressant, antiplatelet, spasmolytic, antidiabetic, acetylcholinesterase, and myeloperoxidase. On the other hand, adverse effects such as tremorogenic, a symptom of Parkinson's disease, and impairment of learning and memory have also been observed. To the best of our knowledge, this is the first report about the biological proprieties of N-oxide-harmane derivated.43 From the crude extract were also isolated triterpenes with recognized pharmacological proprieties.35,36 Crude extract and fractions showed potent activity against cancer cell lines, particularly to the ovary (OVCAR-3) and leukemia (K562) cells. In addition, they are capable to reduce ear edema, showing robust topical anti-inflammatory activity in the experimental model. Overall, this present contributes significantly to the knowledge of the species and genus.
SUPPLEMENTARY MATERIAL Supplementary data (1H and 13C NMR spectra of compounds 1 and 2) are available free of charge at http://quimicanova.sbq.org.br as PDF file.
ACKNOWLEDGEMENTS The authors would like to thank the Faculty of Pharmaceutical Sciences at the University of Campinas, the Department of Pharmacology and Therapeutics at the State University of Maringá and to CAPES (Coodenação de Aperfeiçoamento de Pessoal de Nível Superior) for financial support.
REFERENCES 1. Porto, D. D.; Henriques, A. T.; Fett-Neto, A. G.; Open Bioact. Compd. J. 2009, 2, 29. [Crossref] 2. Calixto, N. O.; Cordeiro, M. S.; Giorno, T. B. S.; Oliveira, G. G.; Lopes, N. P.; Fernandes, P. D.; Pinto, A. C.; Rezende, C. M.; J. Braz. Chem. Soc. 2017, 28, 707. [Crossref] 3. Peixoto, M. A.; Corrêa, J. G. S.; Moura, V. M.; Silva, J. F.; Ames, F. Q.; Pomini, A. M.; Carvalho, J. E.; Ruiz, A. L. T. G.; Amorim, A. M. A.; Bersani-Amado, C. A.; Santin, S. M. O.; Brazilian Journal of Development 2020, 6, 67217. [Crossref] 4. Soares, P. R. O.; Oliveira, P. L.; Oliveira, C. M. A.; Kato, L.; Guillo, L. A.; Arch. Pharmacal. Res. 2012, 35, 565. [Crossref] 5. Carvalho Junior, A. R.; Vieira, I. J.C.; Carvalho, M. G.; Braz-Filho, R.; Lima, M. A.; Ferreira, R. O.; Maria, E. J.; Oliveira, D. B.; Molecules 2017, 22, 2. [Crossref] 6. Barreto, I. M.; Moreira, P. O. L.; Macedo, G. E. L.; Maia, D. N. B.; Alves, T. M. A.; Oliveira, D. M.; Cota, B. B.; Rev. Bras. Farmacogn. 2021, 31, 709. [Crossref] 7. Campbell, G.; Mielke, M. S.; Rabelo, G. R.; Cunha, M.; Flora 2018, 246, 33. [Crossref] 8. Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; J. Natl. Cancer Inst. 1991, 83, 757. [Crossref] 9. OriginLab® Releases Origin®, version 7.5; OriginLab Corporation, Northampton, MA, USA, 2003. 10. Van Arman, C. G.; Clin. Pharmacol. Ther. 1974, 16, 900. [Crossref] 11. Bracht, L.; Caparroz-Assef, S. M.; Magon, T. F. S.; Ritter, A. M. V.; Cuman, R. K. N.; Bersani-Amado, C. A.; J. Pharm. Pharmacol. 2011, 63, 971. [Crossref] 12. Boller, S.; Soldi, C.; Marques, M. C. A.; Santos, E. P.; Cabrini, D. A.; Pizzolatti, M. G.; Zampronio, A. R.; Otuki, M. F.; J. Ethnopharmacol. 2010, 130, 262. [Crossref] 13. Dachriyanus, H.; Arbain, D.; Putra, D. P.; Sargent, M. V.; Susila, R.; Wahyuni, F. S.; Aust. J. Chem. 2000, 53, 221. [Crossref] 14. Arbain, D.; Putra, D. P.; Sargent, M. V.; Aust. J. Chem. 1993, 46, 977. [Crossref] 15. Erwin; Pusparohmana, W. R.; Safitry, R. D.; Marliana, E.; Usman; Kusuma, I. W.; Rasayan J. Chem. 2020, 13, 2552. [Link] accessed in September 2023 16. Mahato, S. B.; Kundu, A. P.; Phytochemistry 1994, 37, 1517. [Crossref] 17. Seki, H.; Hashinomoto, A.; Hino, T.; Chem. Pharm. Bull. 1993, 41, 1169. [Crossref] 18. Farzin, D.; Mansouri, N.; Int. J. Neuropsychopharmacol. 2008, 11, 126. [Crossref] 19. Shi, C. C.; Liao, J. F.; Chen, C. F.; Pharmacol. Toxicol. 2001, 89, 259. [Crossref] 20. Im, J. H.; Jin, Y. R.; Lee, J. J.; Yu, J. Y.; Han, X. H.; Im, S. H.; Hong, J. T.; Yoo, H. S.; Pyo, M. Y.; Yun, Y. P.; Vasc. Pharmacol. 2009, 50, 147. [Crossref] 21. Fragoso, V.; Nascimento, N. C.; Mourab, D. J.; Silva, A. C. R.; Richter, M. F.; Saffib, J.; Fett-Neto, A. G.; Toxicol. In Vitro 2008, 22, 559. [Crossref] 22. Muhammad, I.; Dunbar, D. C.; Khan, S. I.; Tekwani, B. L.; Bedir, E.; Takamatsu, S.; Ferreira, F.; Walker, L. A.; J. Nat. Prod. 2003, 66, 962. [Crossref] 23. Porto, D. D.; Henriques, A. T.; Fett-Neto, A. G.; The Open Bioactive Compounds Journal 2009, 2, 29. [Crossref] 24. de Oliveira, A. M.; Lemos, R. P. L.; Conserva, L. M.; Biochem. Syst. Ecol. 2013, 50, 339. [Crossref] 25. Moura, V. M.; Ames, F. Q.; Corrêa, J. G. S.; Peixoto, M. A.; Amorim, A. M. A.; Pomini, A. M.; Carvalho, J. E.; Ruiz, A. L. T. G.; Bersani-Amado, C. A.; Santin, S. M. O.; Nat. Prod. Res. 2021, 35, 4715. [Crossref] 26. N'Da, D. D.; Smith, P. J.; Med. Chem. Res. 2014, 23, 1214. [Crossref] 27. Mahmud, Z.; Musa, M.; Ismail, N.; Lajis, N. H.; Int. J. Pharmacogn. 1993, 31, 142. [Crossref] 28. Volobuff, C. R. F.; Oliveira-Junior, P. C.; Santos, S. M.; Pereira, Z. V.; Ferreira, D. C.; Cardoso, C. A. L.; Ruiz, A. L. T. G.; Foglio, M. A.; Carvalho, J. E.; Formagio, A. S. N.; Curr. Pharm. Biotechnol. 2019, 20, 302. [Crossref] 29. Otuki, M. F.; Lima, F. V.; Malheiros, A.; Yunes, R. A.; Calixto, J. B.; Eur. J. Pharmacol. 2005, 507, 253. [Crossref] 30. Bradley, P. P.; Priebat, D. A.; Chiristensen, R. D.; Rothstein, G.; J. Invest. Dermatol. 1982, 78, 206. [Crossref] 31. Tran, P. H.; Le, V. D.; Do, T. H.; Nguyen, T. L.; Nguyen, P. T.; Nguyen, T. T.; Nguyen, T. D.; Nat. Prod. Res. 2019, 33, 695. [Crossref] 32. Carvalho Junior, A. R.; Ferreira, R. O.; Passos, M. S.; Boeno, S. I. S.; Virgens, L. L. G.; Ventura, T. L. B.; Calixto, S. D.; Lassounskaia, E.; Carvalho, M. G.; Braz-Filho, R.; Vieira, I. J. C.; Molecules 2019, 24, 1. [Crossref] 33. Aro, A. O.; Dzoyem, J. P.; Eloff, J. N.; McGaw, L. J.; BMC Complementary Altern. Med. 2016, 16, 1. [Crossref] 34. Iniyavan, M.; Sangeetha, D.; Saravanan, S.; Parimelazhagan, T.; Food Sci. Biotechnol. 2012, 21, 1421. [Crossref] 35. Krishnan, K.; Mathew, L. E.; Vijayalakshmi, N. R.; Helen, A.; Inflammopharmacology 2014, 22, 373. [Crossref] 36. González-Cortazar, M. G.; Ruiz, M. H.; Zamilpa, A.; Ferrer, E. J.; Marquina, S.; Planta Med. 2014, 80, 90. [Crossref] 37. Suarez, M. Z. F.; Christen, J. G.; Taketa, A. T. C.; Villafuerte, M. C. G.; López, V. R.; J. Ethnopharmacol. 2019, 238, 1. [Crossref] 38. Wen, S.; Gu, D.; Zeng, H.; JBUON 2018, 23, 965. [Link] accessed in September 2023 39. Ghante, M. H.; Jamkhande, P. G.; Journal of Pharmacopuncture 2019, 22, 55. [Crossref] 40. Jeong, H. J.; Chai, H. B.; Park, S. Y.; Kim, D. S.; Bioorg. Med. Chem. Lett. 1999, 9, 1201. [Crossref] 41. Rajavel, T.; Mohankumar, R.; Archunan, G.; Ruckmani, K.; Devi, K. P.; Sci. Rep. 2017, 7, 1. [Crossref] 42. Huang, L. J.; Gao, W. Y.; Li, X.; Zhao, W. S.; Huang, L. Q.; Liu, C. X.; J. Agric. Food Chem. 2010, 58, 8983. [Crossref] 43. Khan, H.; Patel, S.; Kamal, M. A.; Curr. Drug Metab. 2017, 18, 853. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access