Artigo
| Unveiling the nutritional potential of star fruit (Averrhoa carambola): in vitro bioaccessibility study |
|
Tatiana L. Kamioka; Alexsandra D. S. Xavier; Alana C. B. Coelho; Angerson Nogueira
Department of Chemistry, Federal University of São Paulo (UNIFESP), 09913-030 Diadema - SP, Brazil Recebido em: 28/05/2023 *e-mail: angerson.nogueira@unifesp.br A method was developed for quantifying the concentrations of Ca, Fe, Mg, and Zn in star fruit. An in vitro assay was used to evaluate the bioaccessibility of these elements after a simulated gastrointestinal digestion. The results showed that the total elemental concentration in star fruit were 78-186.2 mg kg-1 for Ca, 92.1-148.1 mg kg-1 for Mg, below the limit of detection for Fe and Zn. The bioaccessibility of Ca and Mg was 40 and 58%, respectively. These bioaccessibility percentages were attributed to the presence of an antinutrient in star fruit that promotes precipitation of insoluble compounds during the in vitro assay. Based on the data from gastrointestinal digestion assay, it is possible to conclude that star fruit intake can supply 1.6-2.04% and 11.2-19.6% of the recommended dietary allowance (RDA) of Ca and Mg, respectively. INTRODUCTION Foods offer an array of nutrients that are essential to the human body, which can be classified into two major groups: (i) macronutrients (carbohydrates, proteins, and fats), which are required in large amounts to provide the energy needed to maintain body functions, build and repair muscle, and insulate and protect our vital organs; and (ii) micronutrients (vitamins and minerals) are necessary in small quantities and are critical to energy metabolism, cellular growth and differentiation, organ function, and the immune system.1,2 Understanding the importance and functions of these elements is essential for comprehending their impact on the body. For instance, calcium, primarily found in bones and teeth, plays a vital role in cell division and growth, metabolic pathways, contraction of muscle fibers, and cell membrane transport.3,4 Similarly, magnesium, an abundant mineral in the body, stabilizes ATP structure in enzymatic reactions, acts as a cofactor in over 300 enzymes involved in various biochemical reactions, and contributes to neuromuscular transmission.2 The absorption of nutrients largely depends on the functioning of the intestinal mucosa.5 However, the digestion of nutrients can be divided into three main stages in the human body: (i) chewing in the mouth reduces food into small particles, while saliva initiates carbohydrates breakdown;6 (ii) gastric digestion, where hydrochloric acid, pepsin, and lipase enzymes are responsible for protein and lipid digestion; and (iii) intestinal digestion: where proteases, amylases, lipases and other enzymes act in the breakdown of foods constituents.6,7 Subsequently, nutrients can be absorbed into the bloodstream and become bioavailable to the human body. Here, the term bioavailability represent the concentration of a nutrient absorbed from the intestine in to the systemic circulation for use in physiological functions or storage, while bioaccessibility denotes the soluble fraction of a nutrient released from food and available for absorption, which can be assessed through in vitro assays.8,9 In fact, a healthy diet is essential for good nutrition, and fruits should be an important part of our daily meals. In this context, Brazil is rich in natural resources and has a wide diversity of fruits in its territory.10,11 However, the nutritional composition, bioaccessibility and bioavailability of Brazilian fruits are still poorly exploited. Despite the advances in understanding the relevance of bioaccessibility and bioavailability in our diet, limited literature exists on the nutritional value of tropical fruits, with star fruit (Averrhoa carambola) being an example of this lack of knowledge.12 Star fruit, also known as carambola, originated from Asia and is extensively cultivated in the southeastern region of Brazil and worldwide.13 Commonly consumed fresh or as a fruit juice, star fruit has been used in some countries as an alternative medication in the treatment of diabetes mellitus due to its potential to promote insulin secretion that helps to control blood sugar levels.14 Additionally, it is used as an appetite stimulant and in the treatment of coughs, sore throats, chronic headaches and eye-related problems.15,16 Moreover, the star fruit is a rich source of nutrients, such as vitamins, calcium, magnesium, dietary fiber, proteins, and amino acids.17 However, despite the diversity of nutrients in star fruit, the total concentration of a substance present in a specific food may not accurately represent the amount of the nutrient that the human body can absorb. Furthermore, the total concentration fails to consider the interactions of dietary compounds within the gastrointestinal tract, which can either enhance or hinder the bioaccessibility or bioavailability of a particular nutrient in the food. Thus, a way to evaluate the absorption of a nutrient from a food can be achieved through in vivo or in vitro assays.6,12,18 In vivo studies, involving animals or human subjects, are generally subjected to long evaluations by ethics committees, a factor which greatly increases the time and costs associated with scientific research.19,20 In contrast, in vitro essay provides a simple and inexpensive way to measure the bioaccessibility of a nutrient present in food, considering variations in the food matrix.21 One such method used in the literature to assess nutrient bioaccessibility is the procedure established by the United States Pharmacopeia (USP), which mimics gastrointestinal digestion that occurs in the human body.1 The simulation employs enzymatic solutions that act on the food, and the bioaccessibility of a nutrient is calculated using the ratio between the concentration of a nutrient in the gastrointestinal solution, obtained after simulated digestion, and the total amount of nutrient in a food. Therefore, further studies are necessary to simulate the impact of bioaccessibility of nutrients in star fruit. In this context, the present study aims to evaluate the bioaccessibility of elements in star fruit using an in vitro essay and calculate their contribution towards meeting the recommended dietary allowance (RDA).
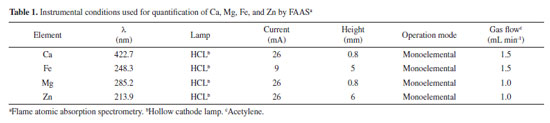
EXPERIMENTAL Instrumental Star fruits were ground in a food processor (Walita, São Paulo, Brazil) fitted with stainless steel discs to slice the samples. The ground fruits were weighed on an analytical balance (Ohaus Adventures, Mettler Toledo, São Paulo, Brazil), and the mass was recorded with an accuracy of four decimal places. For dehydration of the samples, a bench-top freeze dryer (model L108, Liotop, São Carlos, Brazil) equipped with a vacuum pump was employed. The acid digestion of samples and standard reference material (SRM) was carried out using a closed-vessel microwave digestion system. The system allowed precise control of temperature and pressure, and it was equipped with 100 mL perfluoroalkoxy (PFA) vessels. The equipment used for the acid digestion was the Ethos One (Milestone, Sorisole, Italy). The in vitro assay was performed in a water bath (Dubnoff, Quimis, São Paulo, Brazil) with a constant shaking. Following this, a centrifuge (Quimis, São Paulo, Brazil) was used to separate the solid residue from the supernatant. Elemental analysis was performed in a flame atomic absorption spectrometer (SpectrAA 50B, Varian, Australia) equipped with a hollow cathode lamp (HCL: hollow cathode lamp, Australia) as radiation sources. The determinations for Ca, Mg, Fe, and Zn were conducted using an air/acetylene flame. The optimization of the flame composition was based on Ca, Mg, Fe, and Zn absorbance signals. Detailed instrumental conditions for FAAS measurements can be found in Table 1. Analytical signals were measured as peak height during the analysis.
Reagents and samples For the preparation of all solutions, deionized water with a resistivity of 18 MΩ cm was used. The water was obtained from a Milli-Q purification system (Millipore, Bedford, MA, USA). Calcium, iron, magnesium and zinc chloride stock standards were obtained as 1000 mg L-1 solution from Merck (Darmstadt, Denmark). The accuracy of the proposed method for total elemental determination was evaluated by using a SRM obtained from NIST (Peach Leaves, Maryland, USA). The in vitro gastrointestinal digestion protocol followed the guidelines stated in the U.S. Pharmacopeia.22 The digestion was mimicked using a combination of pepsin (Sigma-Aldrich, St. Louis, USA), pancreatin (Sigma Aldrich, St. Louis, USA), bile salts (Sigma Aldrich, St. Louis, USA), sodium bicarbonate, di-potassium hydrogen phosphate, sodium hydroxide and hydrochloric acid (Merck, Darmstadt, Denmark).22 Star fruits from the southeast region of Brazil were acquired from seven local markets, each market providing twelve samples. Prior to analysis, the samples were stored at 5 °C in a freezer. Procedures Total elemental concentration in star fruit To quantify the total elemental concentration in star fruit, a step-wise sample preparation was performed following the procedure outlined in Figure 1.
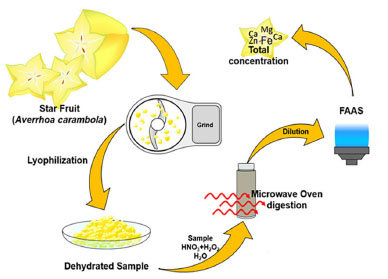 Figure 1. Sample preparation used to quantify Ca, Fe, Mg and Zn concentrations in star fruit
Seven local markets (F1, F2, F3, F4, F5, F6, F7) were selected for the study, and twelve star fruits (n = 12) were purchased from each market. Initially, the fruits were washed with deionized water. Subsequently, a pool of 12 star fruits from one local market was ground and weighed (m = 25 g) in a polyethylene tube. Then, the mixture was dehydrated in a laboratory freeze dryer at -30 °C for 24 h under a total pressure of 1.3 mbar. Microwave-assisted digestion was performed using 200 mg of lyophilized sample, which was weighed into a PFA vessel. To each vessel, 2 mL of HNO3 (65%), 1 mL of H2O2 (30%), and 7 mL of deionized water were added. The heating program used to digest the samples consisted of five steps (temperature (°C), ramp (min), hold (min)): 1 (100, 7, 2), 2 (120, 4, 2), 3 (140, 4, 5), 4 (180, 4, 20) and 5 (20 min of cooldown) using 725 W of power and reaching a maximum pressure of 50 bar. All procedure described before was performed in triplicate for each pool of fruits obtained from the local markets, blank solution, and SRM. The solutions were analyzed in triplicate by FAAS using calibration standards containing Ca, Fe, Mg and Zn prepared in 0.1% HNO3. Method validation for the quantification of elements in star fruit The method for elemental determination was evaluated in terms of the addition/recovery test, linear range, determination of limits of detection and quantification (LOD and LOQ), and analysis of SRM. The addition/recovery test was performed using acid digested samples containing 10 mg L-1 of Ca, Fe, Mg and Zn. The lowest concentrations that could be detected (LOD) were determined according to the Equation 1, where SD(blank) represent the standard deviation of 10 independent analyzes of the blank, and b is the slope of the calibration curve.  The limit of quantification (LOQ) was calculated based on the following definition: "The lowest analyte concentration that can be determined with an acceptable level of uncertainty". The LOQ was determined using Equation 2:23,24  To establish the linear range of the method, solutions containing the elements in the concentration range of 0-20 mg L-1 were analyzed. The SRM analysis was performed using the same sample preparation steps that were applied to the star fruit, and all solutions were analyzed by FAAS. In vitro assay for the evaluation of bioaccessibility in star fruit The gastrointestinal digestion was performed in accordance with the U.S. Pharmacopeia procedure, using three main stages:22
To perform the gastrointestinal digestion of the star fruit, an experimental procedure was established following the scheme depicted in Figure 2.
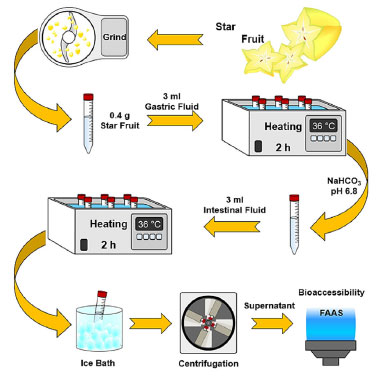 Figure 2. Flowchart of gastrointestinal procedure used to evaluate the bioaccessibility of the elements in star fruit
The sample was ground in food processor and weighed (400 mg) into a polyethylene tube. Subsequently, 3 mL of gastric fluid was added to the tube with a pipette. The mixture was then placed in a thermostatic bath at 36 ºC for 2 h. Afterwards, the pH was adjusted from 1.2 to 6.8 using the inhibitor solution. Following this, 3 mL of intestinal fluid was added to the mixture, and it was again placed in the thermostatic bath under the same conditions as described earlier. The solution obtained from gastrointestinal digestion was placed to an ice bath to inhibit enzymatic activity for 30 min. Later, the mixture was centrifuged at 6500 rpm to separate the residue from the supernatant. The supernatant was collected in a polyethylene tube, filtered, and analyzed by FAAS using the instrumental parameters described in Table 1. Statistical analysis All samples were analyzed in triplicate, and the results were found to be statistically similar based on a paired-samples t-test (p = 0.05). To validate the method, average results obtained from the SRM analysis were compared to certified values using a t-test.
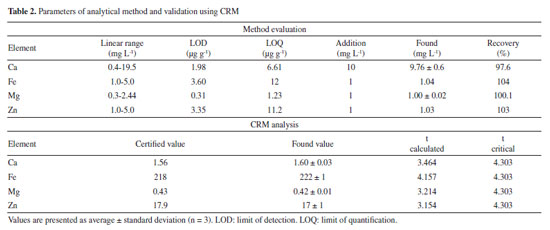
RESULTS AND DISCUSSION Figures of merit The concentrations of elements in star fruits were determined using a method that evaluated several figures of merit, including linear range, LOD, LOQ, and the accuracy of the analysis using a SRM (Table 2).
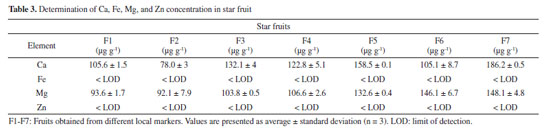
The results indicated a direct proportionality between the analytical signal and the concentration of elements within specific ranges. The concentration ranges were found to be 0.4 19.5 mg L-1 for Ca, 0.3 2.44 mg L-1 for Mg, and 1-5 mg L-1 for Fe and Zn. Notably, magnesium exhibited higher sensitivity compared to calcium, iron, and zinc calibration solutions. Despite the atomization mechanisms of Ca and Mg in acetylene flame occurring via MO (metal oxide) formation, and the MO dissociation energies for CaO (363.3 ± 50 kJ mol-1) and MgO (358.2 ± 7.2 kJ mol-1) being similar, magnesium has a higher sensitivity in an air/acetylene flame due to the more intense analytical line for the element compared to Ca.25,26 The calculated LODs were 1.98 and 0.31 µg g-1 for Ca and Mg, respectively. The values are significantly lower than the LODs for Ca and Mg in some methods published in literature (approximately 45 and 645 orders of magnitude below, respectively).27 The low LODs values demonstrate the high sensitivity of the method for the determination of essential elements in star fruit. However, the LODs for Fe and Zn were comparatively higher, likely due to factors such as the absorption line used during the analysis, burner design, air/acetylene rate, and observation height, which can influence the sensitivity of measurements by FAAS. The experimentally calculated LOQs enable the quantification of elements at concentrations equal to or higher than 6.61 µg g-1 for Ca, 1.23 µg g-1 for Mg, 11.2 µg g-1 for Zn, and 12 µg g-1 for Fe. To verify the presence or absence of matrix interference, a recovery test was performed, where known amounts of the analytes were added into the sample before digestion using a microwave oven. The results demonstrated no matrix interference, as the recoveries were in the range of 97.6-104%. The accuracy of the analytical method was determined through the analysis of an SRM. The results were compared with the certified values shown in Table 2 using a Student's t-test to verify the differences between the population means. The proposed method provided good accuracy, with recoveries in the range of 95-102.5%, and the analyses were in agreement with the certified values, considering a Student's t-test at a 95% confidence level (p < 0.05), which is appropriate for the purpose of this method. Considering all the figures of merit evaluated from Table 2, the proposed method is suitable for quantification of Ca, Fe, Mg, and Zn in star fruits. Determination of Ca, Fe, Mg, and Zn concentrations in star fruit The concentrations of Ca, Fe, Mg, and Zn in star fruits were determined using the FAAS method, and the results are presented in Table 3. The table displays the mean concentrations obtained from three distinct portions of the same fruit. Fe and Zn were found to be below the LOD, while Ca and Mg were present a similar concentration levels in star fruits. The concentration ranges of these elements in all samples are 1.3-3.7 orders of magnitude higher than those reported in the literature.17
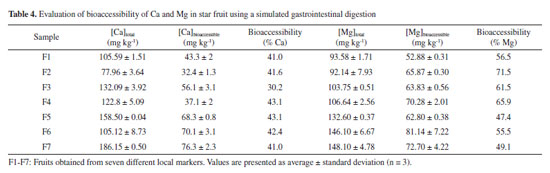
An analysis of variance (ANOVA) of the data revealed a significant difference in mean content of Ca and Mg among different suppliers (p < 0.05). This difference is due to the geographical origin of the samples, type of fertilization, climatic conditions (temperature, light, humidity), which can influence the concentration of essential elements in fruits and vegetables.28 The average concentration of Ca and Mg in star fruit was 126.89 ± 3 mg kg-1 and 117.56 ± 3 mg kg-1, respectively. These values can be compared to the Recommended Dietary Allowance (RDA), defined "as the average daily intake level sufficient to meet the nutrient requirement of nearly all (97-98%) healthy individuals in a particular life stage and gender group".29,30 The RDA established by the National Academies of Sciences (NAS) for men and women aged 19-70 years old is higher for Ca (1000 1300 mg day-1) than for Mg (240-420 mg day-1).30 Based on the RDA values and average concentration of Ca and Mg in star fruit, it can be concluded that 4 units (400 g) of star fruit per day could supply 3.9-5.1% of the RDA for Ca for men and women, considering the FAO/WHO recommendation.31,32 However, it is important to note that the total concentration in a food does not necessarily represent the amount of nutrient that will be absorbed by the human body. Therefore, further evaluation of bioaccessibility using an in vitro assay is necessary. Based on this definition, Cadore and co-authors33 evaluated the bioaccessibility of essential elements in some fruits consumed in Brazil and found that these nutrients showed a moderate bioaccessibility after a gastrointestinal assay. Additionally, some elements values were below the Dietary Reference Intake (DRI), indicating the need for additional consumption of other foods to meet daily requirements of essential elements in a diet. Hence, the bioaccessible assay can provide a more comprehensive evaluation of the elements in a food sample. In vitro assay for evaluation of bioaccessibility of Ca and Mg in star fruit Simulated gastrointestinal digestion was used to assess the bioaccessibility of Ca and Mg in star fruit, aiming to quantify the fraction of the analytes available for absorption by the human body. The bioaccessibility percentages of Ca and Mg were calculated using the Equation 3: 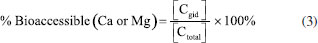 where Cgid represents the concentration of an element in the supernatant obtained after the gastrointestinal digestion of star fruit (Table 4), while Ctotal is the total amount of Ca or Mg determined by FAAS (Table 3).
The obtained values indicated that only 40% of the Ca in star fruit was released from the food matrix following simulated digestion and was available to be absorbed after gastrointestinal digestion. Furthermore, the bioaccessibility of Mg in the same fruit is higher, being 1.45 orders of magnitude higher (58%) than that found for calcium. Considering the RDA of elements for an adult and the results obtained from simulated digestion, the intake of 4 units of star fruit supplies 1.6-2.04% of calcium and 6.5-11.37% of magnesium in a regular diet for adults aged 19-70 years old. After undergoing gastrointestinal digestion, a reduction in the bioaccessibility of Ca and Mg was observed, probably due to the presence of antinutritional compounds in carambola. These compounds, which possess the capability to inhibit nutrient absorption and interfere metabolic processes, act as a natural defense mechanism in plants. Furthermore, they may affect the nutritional content of foods.34-36 Antinutrients, such as oxalate, phytate, tannins, phenolic compounds, and other molecules are found in cereals, legumes, and fruits, including star fruits.36-39 Oxalate, for instance, occurs naturally in star fruit in high levels and can bind with Ca2+ or Mg2+ to form insoluble compounds.40,41 Consequently, the bioaccessibility of Ca and Mg in star fruit is probably reduced due to the formation of calcium or magnesium oxalate during gastrointestinal digestion. These insoluble compounds can pass through the digestive tract without any absorption and be excreted in feces, thereby decreasing the absorption of these essential elements by the human body.41,42
CONCLUSIONS The elemental concentration in star fruit was determined using a validated method, which revealed high concentrations of Ca and Mg in the samples. However, the experimental values for Fe and Zn were below the method's limit of detection. The in vitro assay demonstrated that only 40% of Ca and 58% of Mg were bioaccessible suggesting that only a portion of the elements present in star fruit can be absorbed by the human body. This limited bioaccessibility is probably due to the formation of insoluble compounds with Ca and Mg during gastrointestinal digestion. Additionally, the results highlighted that Mg contributes between 7 to 10 times higher than Ca to the recommended dietary allowance (RDA), making star fruit a potential source of these elements in a balanced diet. However, their contribution to the RDA is modest when incorporated into a regular diet. Therefore, to meet daily requirements of essential elements, it is important to complement star fruit consumption with other foods.
ACKNOWLEDGMENTS We are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, process No. 2016/02603-2; and 18/04957-1) and Financiadora de Estudos e Projetos (FINEP, process No. 0413007800) for financial support.
REFERENCES 1. Ishiguro, E.; Haskey, N.; Campbell, K.; Gut Microbiota: Interactive Effects on Nutrition and Health, 1st ed.; Academic Press: Cambridge, 2018. 2. Raymond, J. L.; Mahan, L. K.; Food & The Nutrition Care Process, 14th ed.; Saunders: Seattle, 2011. 3. Silverthorn, D. U.; Human Physiology: An Integrated Approach, 7th ed.; Pearson Education: Harlow, 2016. 4. Wilhelmi, A. E.; Principles of Biochemistry, vol. 56, 7th ed.; W. H. Freeman: New York, 1955. 5. Mourad, F. H.; Saadé, N. E.; Prog. Neurobiol. 2011, 95, 149. [Crossref] 6. Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S.; Alric, M.; Trends Biotechnol. 2012, 30, 591. [Crossref] 7. Dima, C.; Assadpour, E.; Dima, S.; Jafari, S. M.; Compr. Rev. Food Sci. Food Saf. 2020, 19, 954. [Crossref] 8. Ekmekcioglu, C.; Food Chem. 2002, 76, 225. [Crossref] 9. Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M. E. G.; Grauwet, T.; Crit. Rev. Food Sci. Nutr. 2020, 60, 826. [Crossref] 10. Kelmer, G. A. R.; Nascimento, A. N.; Oliveira, P. V.; J. Braz. Chem. Soc. 2015, 26, 1981. [Crossref] 11. Paz, M.; Gúllon, P.; Barroso, M. F.; Carvalho, A. P.; Domingues, V. F.; Gomes, A. M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C.; Food Chem. 2015, 172, 462. [Crossref] 12. Carbonell-Capella, J. M.; Buniowska, M.; Barba, F. J.; Esteve, M. J.; Frígola, A.; Compr. Rev. Food Sci. Food Saf. 2014, 13, 155. [Crossref] 13. Muthu, N.; Lee, S. Y.; Phua, K. K.; Bhore, S. J.; Bioinformation 2016, 12, 420. [Crossref] 14. Pham, H. T. T.; Huang, W.; Han, C.; Li, J.; Xie, Q.; Wei, J.; Xu, X.; Lai, Z.; Huang, X.; Huang, R.; Wen, Q.; Am. J. Transl. Res. 2017, 9, 36. [Link] accessed in September 2023 15. Wijayaratne, D. R.; Bavanthan, V.; de Silva, M. V. C.; Nazar, A. L. M.; Wijewickrama, E. S.; BMC Nephrol. 2018, 19, 1. [Crossref] 16. Saghir, S. A. M.; Sadikun, A.; Khaw, K. Y.; Murugaiyah, V.; Bol. Latinoam. Caribe Plant. Med. Aromat. 2013, 12, 209. [Link] accessed in September 2023 17. Núcleo de Estudos e Pesquisas em Alimentação (NEPA); Tabela Brasileira de Composição de Alimentos, 4th ed.; Unicamp: Campinas, 2011. 18. Fioroto, A. M.; Nascimento, A. N.; Oliveira, P. V.; J. Agric. Food Chem. 2015, 63, 6331. [Crossref] 19. Epriliati, I.; D'Arcy, B.; Gidley, M.; J. Agric. Food Chem. 2009, 57, 3363. [Crossref] 20. Etcheverry, P.; Grusak, M. A.; Fleige, L. E.; Frontiers in Physiology 2012, 3, 1. [Crossref] 21. Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D.; Food Funct. 2017, 8, 2368. [Crossref] 22. The United States Pharmacopoeia (USP), USP 24-NF 19, The United States Pharmacopeial Convention, Rockville, 2000. 23. Xavier, A. D. S.; Furtado, D. Z. S.; Assunção, N. A.; Nascimento, A. N.; J. Food Compos. Anal. 2019, 83, 103259. [Crossref] 24. Mermet, J. M. In Encyclopedia of Analytical Chemistry; Meyers, R. A., ed.; Wiley: New Jersey, 2006. [Crossref] 25. Agilent Technologies; Flame Atomic Absorption Spectrometry: Method Development, 2017. 26. Komárek, J.; Sommer, L.; Talanta 1982, 29, 159. [Crossref] 27. Mingroni, T. T.; Hamada, J.; Xavier, A. D. S.; Cavalcante, C.; do Nascimento, A. N.; J. Braz. Chem. Soc. 2019, 30, 108. [Crossref] 28. Nour, V.; Trandafir, I.; Ionica, M. E.; Fruits 2011, 66, 353. [Crossref] 29. Barr, S. I.; Appl. Physiol., Nutr., Metab. 2006, 31, 61. [Crossref] 30. National Academies of Sciences, Engineering, and Medicine; Dietary Reference Intakes for Sodium and Potassium, The National Academies Press: Washington, 2019. [Crossref] 31. Salehi, L.; Mohammad, K.; Montazeri, A.; Nutr. J. 2011, 10, 123. [Crossref] 32. World Health Organization (WHO); Technical Report Series 916, Diet, Nutrition and the Prevention of Chronic Diseases, WHO: Geneva, Switzerland, 2003. [Link] accessed in September 2023 33. Pereira, C. C.; Silva, E. N.; Souza, A. O.; Vieira, M. A.; Ribeiro, A. S.; Cadore, S.; J. Food Compos. Anal. 2018, 68, 73. [Crossref] 34. Agbaire, P. O.; Emoyan, O. O.; Afr. J. Food Sci. 2012, 6, 8. [Link] accessed in September 2023 35. Umaru, H. A.; Adamu, R.; Dahiru, D.; Nadro, M. S.; Afr. J. Biotechnol. 2007, 6, 1935. [Crossref] 36. Essack, H.; Odhav, B.; Mellem, J. J.; Food Sci. Technol. 2017, 37, 462. [Crossref] 37. Mubarak, A. E.; Food Chem. 2005, 89, 489. [Crossref] 38. Silva, E. O.; Bracarense, A. P. F. R. L.; J. Food Sci. 2016, 81, R1357. [Crossref] 39. Udomkun, P.; Tirawattanawanich, C.; Ilukor, J.; Sridonpai, P.; Njukwe, E.; Nimbona, P.; Vanlauwe, B.; Food Chem. 2019, 286, 651. [Crossref] 40. Ruiz-Agudo, E.; Burgos-Cara, A.; Ruiz-Agudo, C.; Ibañez-Velasco, A.; Cölfen, H.; Rodriguez-Navarro, C.; Nat. Commun. 2017, 8, 768. [Crossref] 41. Huynh, N. K.; Nguyen, H. V. H.; Plant Foods Hum. Nutr. 2017, 72, 236. [Crossref] 42. Noonan, S. C.; Asia Pac. J. Clin. Nutr. 1999, 8, 64. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access