INTRODUCTION
Wound healing is a complex biological process for skin tissue regeneration that requires quick and non-scarring treatments, which makes a big obstacle for healthcare systems globally.1,2 The skin (and its appendages: nails, hair, and glands) forms the largest organ in the human body and can reach more than 2 m2 of surface area. This structure acts as a barrier between the external environment and the body's interior and protects against mechanical, chemical, osmotic, thermal, and microbial damage. In addition, the skin participates in the synthesis of vitamin D, regulation of body temperature, psychosexual communication, and sensory stimuli recognition.3
The body automatically activates a biological phenomenon called epithelial repair - or external skin repair - when the epidermis is injured, this process starts in a cascade mechanism: (i) the detection of the injuries by cells occurs; (ii) recruitment and migration of immune cells; (iii) blood stagnation; (iv) wound protection; (v) cleaning and (vi) closure.4,5 Repairing a skin lesion may occur by complete replacement of the affected layer, or by scarring: when the injured tissues are not able to rebuild completely.6 Extensive trauma (such as second and third-degree burns) in deep tissues may expose the individual to complex medical treatments and regenerating them may be hard to achieve.7,8
In the last half-century, the advance of technology provided the development of therapies and products for repair, replacement, and systemic integration of damaged tissue.9 Among all technologies, tissue engineering surged as an alternative in the regeneration of organs/tissues through basically three strategical tools: cells, growth factors, and scaffolds. Scaffolds are 3D biostructures formed mainly by polymeric materials that present biological properties, they may have a variety of architectures and must be capable of mimicking natural tissue, also they must have interconnected pores that favor cell interaction.10-13
Scaffolds may be formed by biomolecules such as chitosan - a semisynthetic biopolymer derived from chitin with excellent properties as biocompatibility (when the formulated material biologically co-exists with the tissue without damaging), antimicrobial and hemostatic. Chitosan is used in the fabrication of scaffolds and covers several applications including epithelial, neural, and cartilaginous tissue culture; however, chitosan scaffolds exhibit degradation by hydrolysis. So, chitosan scaffolds with collagen are quite observed.14-17
Collagen is the most abundant protein in the human body, it is also dominant in the extracellular matrix of almost all tissues. This protein has great biodegradability and participates in the formation of cells' structural support, playing a significant role in the cellular behavior direction, and chymosin - an enzyme with strong clotting activity - aids in storage and release. Collagen scaffolds have been used for the cultivation of dermal, ophthalmic, vascular, and orthopedic tissues,18,19 and the combination of chitosan/collagen may promote better cell proliferation.20
Another alternative to construct a great scaffold is through plant extracts such as Jatropha molissima - popularly known as "Pinhão-bravo" and predominantly has flavonoids, and tannin compounds able to act in the regulation of cellular signaling.21 Chitosan/gelatin/Jatropha molissima latex scaffolds presented no cytotoxicity and were indicated for dressing applications.22 Nonetheless, the use of collagen in this composition is not seen yet. In this sense, this work aimed to synthesize scaffolds of chitosan, collagen, and Jatropha mollissima for the manufacture of dressings. The scaffolds were obtained by lyophilization (we froze the biomaterial, reduced pressure, and added heat to remove water) and characterized according to their chemical and morphological properties.
EXPERIMENTAL
Materials
Medium molecular weight chitosan (260 kDa) with a deacetylation degree of ~ 90% and powdered Jatropha mollissima sap were provided by the Laboratory of Evaluation and Development of Biomaterials in the Northeast - CERTBIO (Campina Grande, PB, Brazil). Collagen from bovine flexor tendon, phosphate-buffered saline (PBS) and lysozyme were purchased from Sigma-Aldrich®, Merck Group (Darmstadt, Germany). Lactic acid and sodium hydroxide (P.A.) were obtained from Dinâmica® (Indaiatuba, SP, Brazil).
Methods
This work was challenging due to the limited information on the development of Jatropha mollisima extract scaffolds with chitosan and collagen. It was observed a lack of standardization of natural extracts such as Jatropha mollisima, which can vary in composition depending on factors: geographic location, harvest time, and extraction methods.
Initially, an aqueous lactic acid solution was prepared at a 1% (v/v) concentration. Then, chitosan (Q) and collagen (C) powder were added, in the mass proportions chitosan:collagen of 9:1, totalizing a 2% solution (w/v) of these biopolymers. This solution was mechanically stirred at room temperature (25 °C) for 4 h, and a homogeneous gel was obtained and divided into two aliquots.
The incorporation of Jatropha mollissima sap in the chitosan/collagen solution was performed by adding the lyophilized powder of the plant at concentrations of 5, 10 and 15% (w/w), under the same conditions described above. The solutions prepared to produce scaffolds are illustrated in Figure 1. Lyophilization is a method quite common in the construction of these membranes for dressing applications.23 Furthermore, the samples developed are demonstrated in Table 1.
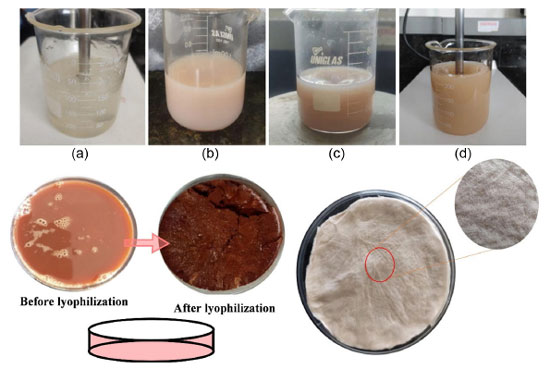 Figure 1.
Figure 1. Chitosan and collagen solution (a); chitosan, collagen, and the Jatropha mollissima sap, in the mass proportions of 5% (b); 10% (c), and 15% (d). Before and after lyophilization of Jatropha mollissima, and dried solution of chitosan/collagen and Jatropha mollissima scaffolds
After obtaining the gels without and with the sap, the materials were poured into Petri plates, frozen in an ultra-freezer for 24 h, and lyophilized. Then, they were neutralized in 0.5 mol L-1 of sodium hydroxide (NaOH) for 1 h, and thoroughly washed in distilled water to remove the excess NaOH salts. Finally, the scaffolds were again lyophilized and characterized by optical microscopy, infrared spectroscopy, degree of swelling, and biodegradation. Optical microscopy characterization was used to evaluate the surface morphology of the samples.
The analysis was performed by using the Advanced 3D Digital KH7700 (Hirox) reflection and transmission microscope with extended depth of field with 2D and 3D measurements, docked to a station image analysis which has magnification of up to 3500×, the images were obtained at magnifications of 20, 50 and 100×. Also, the chemical evaluation of the scaffolds produced was performed by Fourier transform infrared spectroscopy, and the spectra were collected from Spectrum 400 model equipment by Perkin Elmer, in the wavelength range of 4000 to 650 cm-1.
The degree of swelling was measured by weighing the samples twice: after immersing the samples in a phosphate-buffer solution (PBS) saline for 24 h, and after placing the samples on filter paper to remove the excess solution. In this way, all samples were weighed (before and after swelling) to verify the system variation, and the swelling degree (SD) was calculated according to Equation 1.
where: Wt: weight of the sample after 24 h of immersion and W0: initial weight of the sample. Finally, the scaffolds' biodegradation was analyzed according to ASTM F1635-11 - Standard Test Method for In Vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants (2011).24 The materials obtained were weighed on a digital scale before and after being submitted to the biodegradation test to verify their mass loss. The samples were divided into two groups, with and without phosphate buffer solution (PBS) in a concentration of 1 mg mL-1.
RESULTS AND DISCUSSION
The images obtained from the scaffolds' production for all compositions (QC, QCS5, QCS10, and QCS15) are shown in Figure 2. All samples demonstrated a homogeneous, smooth external surface with few stretch marks. Furthermore, the cross-sections of the scaffolds were analyzed, and a spongy internal structure was observed with predominantly lamellar interconnected pores and varied sizes. It was also verified a less regular pore intrinsically correlated to the sap concentration. These results were promising as pores of varied dimensions could facilitate the formation of complex tissues during scarring, akin to those found naturally in the human body.25
 Figure 2.
Figure 2. Scaffolds of chitosan/collagen (QC), and chitosan/collagen/Jatropha mollisima sap (QCS5, QCS10 and QCS15)
Infrared spectra are illustrated in Figure 3 and obtained from samples containing only chitosan/collagen (QC) and chitosan/collagen/sap (QCS5, QCS10, and QCS15). The infrared spectra obtained showed similar characteristic bands for all studied compositions. No new absorption bands were observed for samples containing sap extract (when compared to chitosan and collagen), indicating that the addition of Jatropha mollissima to the medium was by physical interaction.
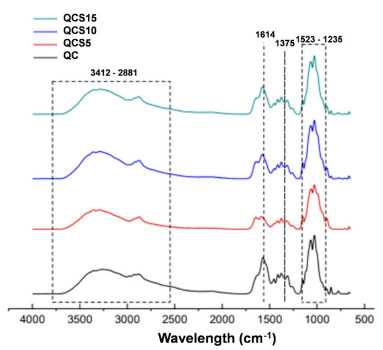 Figure 3.
Figure 3. Infrared spectra of chitosan/collagen (QC) and chitosan/collagen/Jatropha mollissima sap scaffolds (QCS5, QCS10 and QCS15)
For chitosan, there is a peak in 2881 cm-1 characterized by symmetrical and asymmetrical stretching of -CH-.26 Another peak is in 1375 cm-1 characterized by -CH3 symmetrical deformation,27 and in 1076 cm-1 is located the peak of axial deformation and C-O-C of glycosidic acid.28 For collagen, there are peaks in 1628 characterized by amide I (C=O stretching), and 1545 cm-1 for amide II (N-H bending).29
The powdered extract proportion seems to increase in the regions of 1604 and 1522 cm-1 (-C=C-C-) peaks of aromatic compounds, indicating a correlation between the quantity of sap in the samples and the intensity of previous collagen peaks.30 Some other main characteristic bands of chitosan, collagen, Jatropha mollissima, and their associated vibrations are presented in Table 2.
Swelling is an essential property for scaffolds in tissue engineering because of their impact on cell migration and nutrient diffusion. A wound dressing material must absorb the exudates from the wound surface and supply a wet wound environment for skin cell growth.22,35,36 From the swelling values obtained from the samples in Table 3, it was possible to observe a proportional increase in water absorption capacity characterized by the amount (mass ratio) of the Jatropha mollissima extract used.
However, all the compositions presented higher values than pure collagen/chitosan scaffolds, indicating Jatropha mollissima/collagen/chitosan scaffolds may be a potential candidate for skin wound dressing. These results corroborate with the microscopy images, and a possible interaction of water (through hydrogen bonds) with the material's reactive points (-NH3+, -NH2, and -OH) may cause the penetration of the liquid into the scaffolds.37
A limiting factor is the rate of biodegradation, as scaffolds are designed to degrade over time as new tissues form, their rate of degradation must match the rate of tissue regeneration, making it a challenge to achieve a desired degradation profile when combining various materials of natural origin. For tissue engineering, scaffolds that gradually degrade may be the key to enhance dressing quality as a good structural integrity and physical stimuli.38 The results of the biodegradation test obtained in this study are shown in Figure 4, and it was possible to verify that a gradual increase in the percentage degraded overall, being proportional to the amount of Jatropha mollissima extract, and all the scaffolds with sap presented degradation values lower than chitosan/collagen.
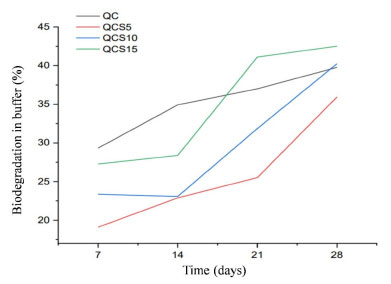 Figure 4.
Figure 4. Biodegradation of QC, QCS5, QCS10 and QCS15 samples
Similar behaviors were found by Gomes,39 who produced chitosan and gelatin scaffolds for the release of fenticonazole. Also, Jatropha mollissima extract/gelatin/chitosan presented similar proportional results.20 These results corroborate with the potential dressing application of the QCS materials.
The introduction of new materials, especially those derived from natural sources, may require extensive testing and regulatory approval before they can be used in clinical applications. Therefore, it is necessary to carry out biocompatibility and cytotoxicity tests, for example, to assess their safety and efficacy. Before using any natural extract in medical applications, it is essential to assess its biocompatibility and potential cytotoxic effects on human cells. Some natural extracts may cause adverse reactions, inflammation, or toxicity, which may limit their use in tissue engineering and regenerative medicine. As well as the evaluation of the mechanical properties, since the combination of natural extracts with chitosan and collagen can alter the mechanical strength and stability of the scaffold, potentially affecting its suitability for specific applications.40
CONCLUSION
Scaffolds constructed by biopolymers are highly scientifically used because of their properties such as biocompatibility, swelling capacity, and biodegradation. In this work, hybrid scaffolds constituted by collagen/chitosan/Jatropha mollissima extract were obtained by lyophilization and analyzed. Through the images obtained by the optical microscope, the formation of a three-dimensional network of interconnected pores was attested with predominantly lamellar shapes and different sizes.
Moreover, the scaffolds' chemical analyses were observed by spectrophotometers, and it was possible to identify the characteristic bands of the materials and indicate a physical mixture. The swelling degree and biodegradation of all samples were proportional to the amount of Jatropha mollissima extract, QSC15 with the highest swelling degree value, and QSC5 with the lowest percentage of degradation. Based on the results achieved, we obtained porous structures (scaffolds) of chitosan/collagen/Jatropha mollissima sap extract scaffolds with possible potential in tissue engineering, particularly as dressings.
REFERENCES
1. Mao, G.; Tian, S.; Shi, Y.; Yang, J.; Li, H.; Tang, H.; Yang, W.; Carbohydr. Polym. 2023, 311, 120757. [Crossref]
2. Xu, Y.; Chen, H.; Fang, Y.; Wu, J.; Adv. Healthcare Mater. 2022, 11, 2200494. [Crossref]
3. Greenwood, J. E.; Damkat-Thomas, L.; Schmitt, B.; Dearman, B.; Burns Open 2020, 4, 121. [Crossref]
4. Enyedi, B.; Niethammer, P.; Trends Cell Biol. 2015, 25, 398. [Crossref]
5. Crosby, L. M.; Waters, C. M.; Am. J. Physiol. 2010, 298, 715. [Crossref]
6. Boyce, S. T.; Lalley, A. L.; Int. J. Burns Trauma 2018, 6, 234. [Crossref]
7. Biswal, T.; Mater. Today: Proc. 2021, 41, 397. [Crossref]
8. He, J. J.; Mccarthy, C.; Camci-Unal, G.; Adv. Biomed. Res. 2021, 1, 210. [Crossref]
9. Ikada, Y.; J. R. Soc., Interface 2006, 3, 589. [Crossref]
10. Melchels, F. P.; Barradas, A. M.; Van Blitterswijk, C. A.; De Boer, J.; Feijen, J.; Grijpma, D. W.; Acta Biomater. 2010, 6, 4208. [Crossref]
11. Przekora, A. A.; Cells 2020, 9, 70. [Crossref]
12. Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Liu, Q.; Zhao, Y.; Chem. Rev. 2022, 122, 5604. [Crossref]
13. Zhang, Z.; Feng, Y.; Wang, L.; Liu, D.; Qin, C.; Shi, Y.; Mater. Today Commun. 2022, 5, 104. [Crossref]
14. Fernandes, L. L.; Resende, C. X.; Tavares, D. S.; Soares, G. A.; Castro, L. O.; Granjeiro, J. M.; Polimeros 2011, 21, 1. [Crossref]
15. Cai, H.; Li, G.; Wound Repair and Regeneration 2020, 28, 751. [Crossref]
16. Lima, P. H. C.; Tavares, A. A.; Silva, S. M. L.; Moura, M. R.; Aouada, F. A.; Grillo, R.; Appl. Clay Sci. 2022, 226, 106548. [Crossref]
17. Tavares, A. A.; Macedo, M. D. M.; Lima, P. H. C.; Barbosa, R. C.; Sousa, W. J. B.; Braz, C. J. F.; Silva, S. M, L.; Research, Society and Development 2022, 11, e25911124684. [Crossref]
18. Kappeler, S. R.; Rahbek-Nielsen, H.; Farah, Z.; Puhan, Z.; Hansen, E. B.; Johansen, E.; Biochem. Biophys. Res. Commun. 2006, 342, 647. [Crossref]
19. Blackstone, B. N.; Gallentine, S. C.; Powell, H. M.; Bioengineering 2021, 8, 340. [Crossref]
20. Si, J.; Yang, Y.; Xing, X.; Yang, F.; Shan, P.; Polym. Degrad. Stab. 2019, 166, 73. [Crossref]
21. Subbaraj, G. K.; Kumar, Y. S.; Kulanthaivel, L.; The Egyptian Journal of Internal Medicine 2021, 33, 105. [Crossref]
22. de Souza, M. F.; da Silva, H. N.; Rodrigues, J. F. B.; Macêdo, M. D. M.; de Sousa, W. J. B.; Barbosa, R. C.; Fook, M. V. L.; Polymers 2023, 15, 603. [Crossref]
23. Amal, B.; Veena, B.; Jayachandran, V. P.; Shilpa, J.; J. Mater. Sci.: Mater. Med. 2015, 26, 1. [Crossref]
24. Silva, R. D. N.; Oliveira, T. A. D.; Conceição, I. D. D.; Araque, L. M.; Alves, T. S.; Barbosa, R; Polímeros 2018, 28, 348. [Crossref]
25. Elsayed, Y.; Lekakou, C. In Designing and Modeling Pore Size Distribution in Tissue Scaffolds; Tomlins, P., ed.; Woodhead Publishing: Cambridge, 2016, ch. 4.
26. Gu, Y.; Hummel, M.; Muthukumarappan, K.; Zhao, Z.; Gu, Z.; Sci. Rep. 2019, 9, 327. [Crossref]
27. Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L.; Biomacromolecules 2007, 8, 2533. [Crossref]
28. Agatonovic-Kustrin, S.; Doyle, E.; Gegechkori, V.; Morton, D. W.; J. Pharm. Biomed. Anal. 2020, 184, 113. [Crossref]
29. Andonegi, M.; Las Heras, K.; Santos-Vizcaíno, E.; Igartua, M.; Hernandez, R. M.; de la Caba, K.; Guerrero, P.; Carbohydr. Polym. 2020, 237, 116. [Crossref]
30. Ahmad, T.; Ismail, A.; Ahmad, S. A.; Khalil, K. A.; Kumar, Y.; Adeyemi, K. D.; Sazili, A. Q.; Food Hydrocolloids 2017, 63, 85. [Crossref]
31. Wang, C.; Tang, L.; Jiang, T.; Zhou, Q.; Li, J.; Wang, Y.; Kong, C.; Ind. Crops Prod. 2021, 160, 113090. [Crossref]
32. Ping, L.; Pizzi, A.; Guo, Z. D.; Brosse, N.; Ind. Crops Prod. 2012, 40, 13. [Crossref]
33. Sobreira, T.; Silva, L.; Menezes, F.; França, E.; Aquino, K.; Quim. Nova 2020, 8, 222. [Crossref]
34. Cowen, S.; Al-Abadleh, H. A.; Phys. Chem. Chem. Phys. 2009, 11, 7838. [Crossref]
35. Batool, S.; Hussain, Z.; Niazi, M. B. K.; Liaqat, U.; Afzal, M.; J. Drug Delivery Sci. Technol. 2019, 52, 403. [Crossref]
36. Ghomi, E. R.; Khalili, S.; Khorasani, S. N.; Neisiany, R. E.; Ramakrishna, S.; J. Appl. Polym. Sci. 2019, 136, 47738. [Crossref]
37. Cruz, J. B.; Catão, C. D. S.; Barbosa, R. C.; Fook, M. V. L.; Rev. Mater. 2016, 21, 129. [Crossref]
38. Zhang, F.; King, M. W.; Adv. Healthcare Mater. 2020, 9, 1901358. [Crossref]
39. Gomes, V. V.: Obtenção de Biomaterial para Liberação de Fenticonazol no Tratamento da Vaginite Fúngica; Dissertação de Mestrado, Universidade Federal de Campina Grande, Paraíba, Brasil, 2017. [Link] accessed in September 2023
40. Sheokand, B.; Vats, M.; Kumar, A.; Srivastava, C. M.; Bahadur, I.; Pathak, S. R.; J. Polym. Sci. 2023, 61, 1389. [Crossref]