Artigo
| Dimensioning of vinylsulfonic supports from cashew apple bagasse biomass in the immobilization of lipases |
|
Paula J. M. LimaI; Jouciane de S. SilvaI; Rafael L. F. MeloII; Francisco S. NetoI; Pierre B. A. FechineIII; Maria V. P. RochaI; Luciana R. B. GonçalvesI; José C. S. dos SantosIV,*
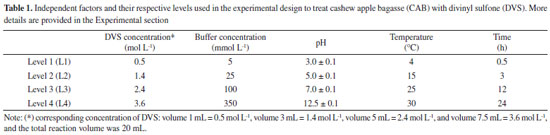
I. Departamento de Engenharia Química, Universidade Federal do Ceará, 60455-760 Fortaleza - CE, Brasil Recebido em: 27/04/2023 *e-mail: jcs@unilab.edu.br In this work, the support, cashew apple bagasse (CAB), was chemically modified with divinyl sulfone (DVS), and it was evaluated to immobilize Candida antarctica lipase A (CAL-A). The best activation conditions of CAB support were defined by an advanced experimental design using the Taguchi method, assessing five factors at four levels (concentration of DVS, ionic strength, pH, temperature, and time). The support and biocatalyst (CAB-DVS-CAL-A) were characterized by Fourier transform infrared spectroscopy (FTIR), elemental analysis, thermogravimetry (TGA), scanning microscopy (SEM), fluorescence spectroscopy (XRF) and electrophoresis. The optimal conditions to activate the support were DVS concentration of 1.4 mol L-1 (3 mL of DVS in 20 mL of reaction volume), a concentration of sodium bicarbonate buffer at 5 mmol L-1, pH 3.0 at 30 °C for 12 h. The immobilization on CAB-DVS promoted increased thermal stability at 70 °C and different pHs of CAL-A. Therefore, the selected conditions allowed for a catalyst with a catalytic activity of 6.8 U g-1 and more stable than the free enzyme (CAL-A). This demonstrates that pretreated and DVS-activated CAB is a promising support for enzyme immobilization. INTRODUCTION Lipases are the most widely used enzymes in biocatalysis in academic and industrial research. Lipases are highly stable, with high substrate and region specificity, chemoselectivity, and stereoselectivity.1,2 They are used in various reaction media (e.g., organic solvents, ionic liquids, and supercritical fluids). They can catalyze reactions (e.g., transesterification hydrolysis, ester bond synthesis, and amination).3,4 Within the lipases, lipase A from Candida antarctica (CAL-A) presents unique features: high thermostability (optimal temperature above 90 °C) and stability in the acidic pH range.5 The CAL-A has some capability to attack the sn-2 position of triglycerides5 and its particular recognition of the trans-trans-fatty acids and it is also considered to be an excellent biocatalyst for the asymmetric synthesis of amino acids/amino esters due to its chemoselectivity towards amine groups.5,6 Free enzymes generally have limited use because of their lower stability, catalytic activity, selectivity, and reduced inhibition. Also, there are difficulties in recovering and reusing.7,8 These limitations influence the cost of the process, a relevant factor for industrial-scale applications.9,10 One of the ways to overcome these disadvantages is enzyme immobilization, which enables enzyme recovery and reuse, reducing these problems caused by the use of free enzymes. Also, recent developments in materials science, bioprocess engineering, and protein science make it possible to boost the use of enzymes on an industrial scale.9,10 Enzyme immobilization can be achieved through physical and chemical methods (e.g., adsorption, covalent binding, crosslinking, and encapsulation).11-13 Immobilization methods influence the enzymes specificity, activity, and stability.14,15 Among the known techniques, the covalent bond immobilization method is widely used because it provides a robust covalent interaction between the support and the enzyme. This interaction can make the biocatalyst more stable in the face of changes in reaction conditions (e.g., variations in pH, temperature, and using organic solvents).16,17 Thus, developing new supports with enhanced properties that can form covalent bonds is relevant and will be addressed in this study. Several supports may be used for enzyme immobilization.18,19 Natural supports are being investigated in this context because they are more environmentally compatible, biodegradable, and less toxic than synthetic supports. Also, natural support can reduce the costs involved in the immobilization process, facilitating their application on an industrial scale.20 Among the natural supports, the wastes generated from methods developed in agroindustries are an excellent option for enzyme immobilization because they are considered renewable sources and can have low cost and high availability. For example, the support evaluated in this study, cashew apple bagasse (CAB), is regarded as one of the sources of waste in Brazilian agribusiness,21 is an alternative for obtaining value-added products and may be used as a support for immobilizing enzymes. This raw material has low commercial value, approximately US$ 30.00 per ton.21 However, CAB compounds form a rigid and unreactive structure, requiring modification of the chemical system of the support by pretreatment to obtain a material that allows immobilization for subsequent application.22 Pretreatments reduce the crystallinity of the biomass, can generally separate the lignin of the cellulose, and can hydrolyze the hemicellulose.23 Chemical, physical and biological processes, or combinations, can accomplish these. Studies show that divinyl sulfone (DVS) promotes alterations on the surface of the support or promotes an intense multipoint covalent bond.24,25 DVS is the most popular cross-linker reagent among the different bisvinyl sulfones because of its reactivity, stability, solubility in water, and affordable price.26 The DVS reactive groups are inserted into the support by the chemical reaction with the amino, phenol, imidazole groups or thiol of proteins, residues commonly present on the surface of lipases and, therefore, essential in the realization of countless connections between the support and the enzyme.27 Also, DVS is applied as a reagent for single-point protein modification.24 The immobilization process using DVS-activated support generally follows three steps: (i) immobilization, (ii) incubation, and (iii) blocker.27,28 The incubation process is due to the multipoint covalent bonding requires a long time in the process of immobilization since the support and the enzyme are very rigid structures that require a specific time to obtain a correct alignment for a favorable multipoint covalent bond under conditions in which the support has good fixation and reactivity.15,17 To prevent this reaction for continuing to occur, it is necessary to carry out the blocking step.27 The literature does not report the modification of cashew apple bagasse with vinyl sulfone groups aimed at enzyme immobilization. Thus, this research assessed the treatment of CAB with DVS, intending to obtain support to immobilize CAL-A. In this paper, optimization via the Taguchi method (a method that provides an efficient and systematic way to optimize a process) was adopted to analyze the influence of the variables of chemical modification of CAB treated with DVS on the production of CAB DVS-CAL-A biocatalysts. FTIR technique, elemental analysis, TGA, SEM, XRF and electrophoresis were used to characterize the support and the biocatalyst. Finally, the biocatalyst was evaluated for its maximum capacity to load CAL-A and thermal and pH stability.
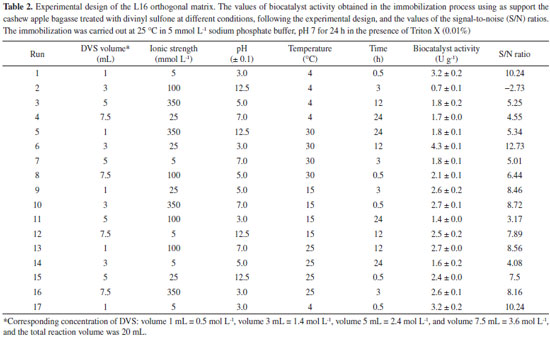
EXPERIMENTAL Materials The CAL-A (20.88 mg of protein per mL) was provided by Novozymes (Alcobendas, Spain). The CAB (Anacardium occidentalis L.) was kindly donated by the Jandaia Processing Industry Juice (Ceará, Brazil). The divinyl sulfone (DVS), ethylenediamine (EDA), Triton X-100, and p-nitrophenyl butyrate (p-NPB) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemical reagents used were of analytical grade. The Taguchi method prepared the experimental design using Statistica® (StatSoft, USA).29 All experiments were performed at least in triplicate, and the results are presented as the mean of these values and standard deviation (usually less than 5%). Support preparation: preparation and chemical modification of CAB with DVS The preparation of CAB was performed according to a previous study.22 The CAB was washed with distilled water, dried at 60 °C for 24 h, and milled in a ball mill to obtain an average particle size of less than 80 mesh (0.177 mm) and then used in the experiments. The CAB was stored at room temperature (20 to 30 °C). The main components (cellulose, hemicellulose and lignin) of the CAB were determined following the protocol.30 Afterwards, DVS treated the CAB, and an experimental design was used to maximize the treatment conditions, aiming to obtain a material with better properties to be used as support in the immobilization process. Experimental planning advanced by the Taguchi method, with a standard orthogonal L16 matrix (where the "L" represents the Latin square and "16" is the number of experiments), was used to evaluate five factors at four levels to optimize the chemical modification process of CAB. The values of factors were determined according to previous studies of our research group27,31 and the evaluated parameters were based on the analysis.9 For this, 1 g of CAB was suspended in sodium bicarbonate buffer, varying the buffer concentration (5, 25, 100, and 350 mmol L-1) and pH (3, 5, 7, and 12.5, with the accuracy of ± 0.1 and measured by pHmeter), added, then, DVS varying the concentration (0.5 mol L-1 (volume 1 mL), 1.4 mol L-1 (volume 3 mL), 2.4 mol L-1 (volume 5 mL) and 3.6 mol L-1 (volume 7.5 mL)) and the total reaction volume was 20 mL for all experiments. After, the treatment occurred at different temperatures (4, 15, 25, and 30 °C) and times (0.5, 3, 12, and 24 h) under constant stirring. After, the CABs treated at different conditions were used as support to immobilize the CAL-A enzyme. The best needs of the treatment were identified by reading the enzyme activity obtained after immobilization. The software Statistica® (StatSoft, USA) was used for experimental design and statistical analysis. Given the goal of this study to maximize the response (biocatalyst activity), the signal-to-noise (S/N) ratio values for biocatalyst activities were calculated using the "the higher, the better" function. The Taguchi experiments often use a two-step optimization process: (i) in step 1, use the signal-to-noise ratio to identify those control factors that reduce variability, and (ii) in step 2, identify control factors that move the mean to target and have a small or no effect on the signal-to-noise ratio, i.e., this method uses the signal-to-noise ratio (S/N) to measure the variability in performance, while ANOVA evaluates the relative importance of each factor.32 The program calculated the S/N ratio value for each experiment according to Equation 1. 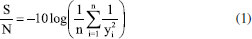 where y is the biocatalyst activity for the corresponding experiment, i is the number of repetitions, and n is the number of responses for the combination of factor levels at any specific parametric combination (Table 1). The ratio predicted under optimal process conditions to obtain the biocatalyst activity was estimated by Equation 2.33 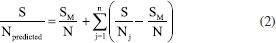
SM/N is the arithmetic mean of all the S/N ratios, S/N is the S/N ratio at the optimal point for each factor, n is the number of factors significantly affecting the process, and j is the number of repetitions. Determination of enzyme activity and protein concentration The hydrolytic activity of free and immobilized CAL-A was determined following the method described by a previous study.31 The p-nitrophenyl butyrate (50 mmol L-1 p-NPB in acetonitrile) was used as the substrate solution. Thus, 50 μL of the p-NPB solution was added to 2.5 mL of sodium phosphate buffer pH 7.0 (25 mmol L-1) at 25 °C, along with 50 μL of enzyme solution or 30 mg of immobilized enzyme. The reaction product, p-nitrophenol, was quantified spectrophotometrically at 348 nm (ε = 5.236 mol-1 cm-1).31 A unit of activity (U) is the amount of enzyme that hydrolyses 1 μmol of p-NPB per minute under the conditions described above. The protein concentration was estimated using the strategy described in another study34 with bovine serum albumin as a reference. Immobilization of CAL-A CAL-A was immobilized on treated CABs (material obtained in each experiment of Taguhi design), following the three steps. The first stage is the immobilization step, occurred in sodium phosphate buffer with a concentration of 5 mmol L-1 at pH 7.0, and 25 °C under constant stirring for 24 h using 1 mg protein per gram support and in the presence of 0.01% (v/v) Triton-X.33 After, the biocatalysts were incubated in 100 mmol L-1 sodium bicarbonate buffer, pH 10 at 25 °C in the ratio of 1:10 (m/v) of biocatalysts per buffer. The incubation step increases the interactions between the enzyme and the activated support. Then, the biocatalysts were incubated in 1 mol L-1 EDA solution at pH 10 and 4 °C for 24 h. This last step is named blocking and is performed to eliminate the chemical reactivity of the support. Finally, the biocatalysts were vacuum filtered, washed with 25 mmol L-1 sodium phosphate buffer, pH 7, and stored at 6 °C. The control assay was also performed, in which an enzyme solution was prepared under the same immobilization conditions but without support (CAB-DVS).33 After the immobilization processes, immobilization parameters were determined according to the method presented in a previous study.33 The immobilization yield (Y) describes the percentage of enzyme activity that was successfully immobilized on the support. The theoretical activity (AtT) was calculated using the immobilization yield (Y) and the enzyme load. The recovery activity (AtR) was determined as the ratio of the biocatalyst activity (At) to the theoretical activity (AtT). With the support that made it possible to obtain a biocatalyst with more significant activities, the treatment of cashew bagasse was defined, and the material obtained by this was named CAB-DVS. Also, to verify the influence of the DVS groups in the CAB in the immobilization, the CAB was treated under the optimal conditions to be achieved by experimental design without DVS. The material obtained for this treatment was named CAB-TRAT, which was used to support immobilize CAL-A. Also, an immobilization process using only CAB as support was performed as a control. The biocatalysts obtained using CAB and CAB-TRAT as support were named CAB CAL-A and CAB-TRAT-CAL-A, respectively. The enzymatic desorption procedure was performed to verify the type of immobilization that was carried out. Samples of the biocatalysts (CAB-CAL-A; CAB-TRAT-CAL-A; and CAB DVS CAL-A) were suspended in 1 mol L-1 NaCl or 1 mol L-1 NaCl + 0.2 % (v/v) Triton X-100 at 25 °C and pH 7.0. The biocatalysts were incubated under continuous and gentle agitation for 1 h on a rotary shaker. Afterwards, they were separated from the supernatant and washed with 25 mmoL-1 sodium phosphate buffer (pH 7.0). The supernatants were subjected to electrophoresis analysis. Electrophoresis was performed according to a previous study.31 The gels were prepared at a concentration of 12% polyacrylamide for the separating gel and 5% for the concentrating gel. Enzyme samples were incubated in the rupture buffer (2% sodium dodecyl sulfate and 10% mercaptoethanol) and heated to boiling temperature for 10 min. A low-molecular-weight (14.4-97 kDa) marker (GE - Healthcare Life Sciences) was used for identification purposes. The protein bands were detected by the silver nitrate method.34 Effect of pH in the immobilization step and the load enzymatic After selecting the best conditions to treat/activate cashew apple bagasse with DVS, a study of the effect of pH and the load capacity of CAB-DVS was evaluated in the immobilization step. In the assays to evaluate the pH effect, the processes occurred in sodium acetate buffer (5 mmol L-1; pH 5), sodium phosphate buffer (5 mmol L-1, pH 7), and sodium carbonate buffer (5 mmol L-1, pH 9) for 24 h and 25 °C. Samples of the supernatant and the suspension (or biocatalyst) were collected periodically, and the enzyme activity was measured. Also, the control assay was performed. The remaining steps (incubation and blocking) were maintained under the same conditions. The support load capacity was investigated by offering different amounts of protein per gram of support, from 1 to 15 mg of enzyme per gram of support. The immobilization conditions were the best obtained in the study of the course of immobilization and pH effect. Thermal and pH stability of biocatalyst The biocatalyst with the highest activity obtained in the previous step was incubated in sodium acetate buffer (5 mmol L-1, pH 5), sodium phosphate buffer (5 mmol L-1, pH 7), and sodium carbonate buffer (5 mmol L-1, pH 9) at 70 °C. The activity was measured periodically using the p-NPB assay. Residual activity was calculated over the initial percentage of activity (hydrolytic activity before thermal incubation). Half-lives were calculated according to the model of Sadana and Henley.35 Support characterization before and after optimization of pretreatment conditions Fourier transform infrared spectroscopy (FTIR) analysis was performed to investigate the structure of CAL-A and CAB and the activation in CAB-DVS-CAL-A. We completed the studies in a Fourier transform spectrophotometer (VERTEX 70-Bruker Optics), attenuated total reflectance with (FTIR-ATR) under ZnSe crystal. We conducted the investigations at the Department of Physics, Federal University of Ceará. Thermogravimetric analyses were performed using a Q50 V20 Thermogravimetric analyzer (TA Instruments) with 20 mg of each sample, monitoring the mass loss of the materials under heating from 25 to 800 °C at a rate of 10 °C min-1 under nitrogen flow (50 cm3 min-1). The elemental analysis used an elemental analyzer - PerkinElmer 2400 series, in CHNS mode. SEM imaging and XRF spectroscopy were performed to assess morphology and chemical composition. SEM was performed on the QUANTA 450 FEG microscope. The samples were fixed on carbon tape and metalized with silver using the Quorum QT150ES metallization equipment. An electron beam with 20 kV was applied. The XRF was performed using a Shimadzu model EDX-7000 equipment equipped with a rhodium tube, involving a power of 4 kV to the powder samples.
RESULTS AND DISCUSSIONS Optimization of CAL-A immobilization onto CAB-DVS In the present study, optimizing the activation of the cashew bagasse support to immobilize enzymes involves the manipulation of several parameters, such as volume or concentration of divinylsulfone (DVS), ionic strength, pH, temperature and time. Each of these parameters plays a crucial role in the effectiveness of activation and successful immobilization of enzymes. Increasing the volume or concentration of DVS may result in greater exposure of the cashew bagasse support to DVS reactive groups. This can lead to more efficient immobilization of the enzymes as long as excess DVS does not cause denaturation or inactivation. Accurate volume or concentration control is crucial to avoid reagent waste. The ionic strength of the solution can affect the interaction between DVS and cashew bagasse. Generally, moderate ionic strength is desirable to maintain the necessary chemical interactions, but very high or low levels may impair activation effectiveness. Fine-tuning the ionic strength can improve the binding of the DVS to the support. The pH of the activation solution is critical as it affects the reactivity of the DVS functional groups and the stability of the enzymes. An inadequate pH can result in the denaturation of the enzymes or failure to activate the support. The ideal pH varies according to the enzyme and support, and is generally close to neutral pH. Temperature influences the kinetics of the activation reaction. Increasing the temperature can speed up the response but must be controlled to avoid denaturation of the enzymes or excessive heating of the support. A suitable temperature should be selected based on the thermostability of the enzymes. The reaction time affects the extent of activation and, consequently, the number of reactive groups available on the support. A longer time may lead to more complete activation, but there may be a saturation point beyond which no significant increase in efficacy occurs. The ideal time may vary depending on the system and must be determined experimentally. In summary, optimizing the activation of cashew pomace scaffold to immobilize enzymes requires a careful balance between these parameters. This work aims to maximize the effective immobilization of enzymes, maintaining their activity and stability. Systematic optimization experiments are necessary to determine the ideal values of each parameter in a specific context, considering the characteristics of the enzymes and the cashew bagasse support in question. Table 2 shows the biocatalysts' activity in the immobilization process using CABs treated with DVS according to the experimental design by the Taguchi method, in which the S/N ratio was determined by the "the higher, the better" function. In the control assay of the immobilization process, the enzyme maintained its enzymatic activity, indicating that the conditions of this process did not denature the enzyme.
The optimized conditions for the treatment of CAB, to be used as a support in immobilization to obtain the catalyst with the highest enzymatic activity, were defined by the highest S/N ratio, and it shows the best level for each parameter. The optimal S/N ratio predicted for the optimized conditions was calculated from Equation 1, and for this study, the best ratio reached to maximize the conditions for the treatment was 15.18. Figure 1 shows the S/N ratio for each evaluated parameter. The best levels for each parameter were L2 (3 mL) for DVS concentration, L1 (5 mmol L-1) for ionic strength, L1 (3.0 ± 0.1) for pH, L4 (30 °C) for temperature, and L3 (12 h) for time (Figure 1). For this combination of parameters, the theoretical activity of biocatalyst (named CAB-DVS-CAL-A), immobilized enzyme in the CAB treated in the optimized conditions, determined by the program, was 6.5 U g-1. The enzymatic activity obtained in the validation experiments was 6.83 ± 0.55 U g-1, confirming the values obtained by the Taguchi method. The optimized conditions provided changes in the chemical structure of CAB, which favored the immobilization of CAL-A.
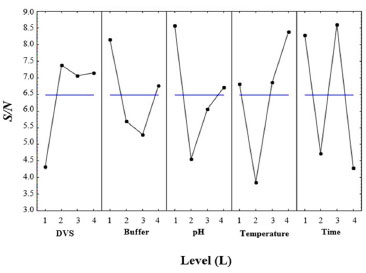 Figure 1. Signal-to-noise (S/N) ratio plots for pH, ionic strength, temperature, and time for optimization of the treatment of CAB with DVS to apply it as support in the-CAL-A immobilization
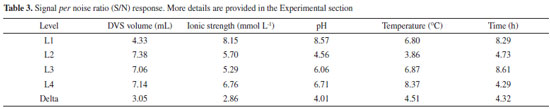
According to the S/N ratio response (Table 3), based on the difference in the S/N ratio values between the highest and lowest levels of the process factors (delta values), the temperature was the factor that had the most influence on the treatment of CAB, favoring the CAL-A immobilization step on this support. On the other hand, ionic strength was the factor that least influenced immobilization.
Temperature increases molecular collisions and causes the energy barrier between reagent molecules to decrease, increasing the reaction speed of the process.36 The increase in temperature increases the mobility of the polymers that make up the cell wall of cashew bagasse, causing disorder in cellulose, hemicellulose and lignin.37 This increase in temperature causes exposure of the functional groups and increases the material's porosity, which may facilitate the immobilization of the CAL-A enzyme on the support. The second most influential factor in this study was time (Table 3). Time is considered an essential indicator of process efficiency and economic performance, as a biotechnological process used on a large scale should combine high conversion rates with relatively short reaction times.38 However, among the times studied (0.5, 3, 12, and 24 h), the 12 h time was presented as an optimal condition (Figure 1). The amount of DVS used in the treatment of CAB was also investigated in this study. As shown in Figure 1, 3 mL of DVS per gram of support was determined as an optimal condition among the evaluated values (1, 3, 5 and 7.5 mL). This result suggests that high concentrations of DVS disfavor its function as an activating agent to form the link between the enzyme and the support. Considering the characteristics of DVS, the literature explains this phenomenon by the fact that at higher concentrations of DVS, the extent of crosslinking is also high enough to form a compact structure, capable of excluding water molecules and thus avoiding enzymatic immobilization.39 However, the pH had a more significant influence on the results obtained than the amount of DVS added, as shown in Table 3. As the DVS groups are stable in a pH range of 5 to 10, extreme pH values may not favor the introduction of DVS as support.40,41 However, DVS had the expected effects as an activating agent, as demonstrated in our discussions. Although the ionic strength had less influence on the maximization of the results (Table 3), this is a relevant parameter to be considered during the preparation and activation of the enzymatic support immobilization.42 As shown in Figure 1, the lowest value studied for the ionic strength (5 mmol L-1) was determined as an ideal condition to obtain the best results for biocatalyst activity. Regarding the biocatalyst activity (Table 2, run 6), the ionic strength that most influenced the treatment process of the CAB using DVS was 5 mmol L-1. A high number of ions in the solution can decrease the solubility of the enzyme, leading to its precipitation.42 Depending on the ion, this precipitation can reduce the catalytic capacity and enzyme deactivation.40,43 Therefore, bagasse prepared with low ionic strength favored enzymatic immobilization. Figure 2 shows the activation of cashew apple bagasse (CAB) and its activation with divinyl sulfone (DVS) forming the CAB DVS support that was subsequently used (under conditions) for the immobilization of Candida antarctica lipase A (CAL-A), including the CAB-DVS-CAL-A biocatalyst.
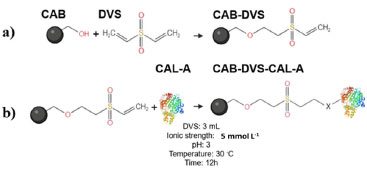 Figure 2. Activation of cashew apple bagasse with DVS and the reaction of DVS-activated supports with proteins (adapted from Santos et al.),27 (a) CAB activation mechanism with DVS; (b) CAL-A immobilization mechanism by the CAB-DVS support, where X represents the frequently reactive groups on the surface of enzymes
The pretreatment with DVS on the material aims to change the structure of the lignocellulosic biomass and thus make the OH groups of the cellulose more accessible (Figure 2). The activation of CAB with the DVS molecule should generate S=O groups in its structure. Figure 2 shows the binding mechanisms between the CAB constituent groups and the DVS. The main functional groups present in cashew apples are hydroxyl (OH), carboxyl (COOH), and carbonyl (C=O) groups,27 and the hydroxyl (OH) groups present in the structure of cellulose and lignin are the main ones that react with DVS, as illustrated in Figure 2. Then, CAB treated under the optimized conditions was named CAB-DVS, characterized and used as a support in the other immobilization studies of the CAL-A enzyme carried out in this study. Characterization of support The CAB composition used in this study was 19.5 ± 0.8% m/m of cellulose, 18.2 ± 0.5% m/m of hemicellulose, 31.5 ± 1.2% m/m of total lignin, results similar to reported for previous reports.21,22 The FTIR analyses were carried to evaluate the modification of the CAB structure because of the activation reaction with DVS under the optimal conditions obtained from the experimental design, forming a material called CAB-DVS; after the immobilization of CAL-A, in which the biocatalyst was named CAB-DVS-CAL-A. The spectra obtained for CAB, CAB-DVS and CAB-DVS-CAL-A are shown in Figures 3a, 3b and 3c. The bands at 3375, 2927, and 2852 cm-1 in all ranges are associated with O-H stretching and asymmetric and symmetric C-H vibrations, respectively. A band around 1739 cm-1 was also observed, related to the C=O stretching associated with acetyl groups and carboxylic acids in the hemicellulose molecule.44,45
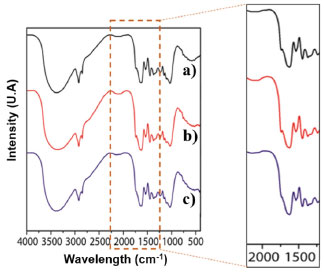 Figure 3. Infrared spectra of the samples: (a) cashew apple bagasse (CAB), (b) bagasse treated in the presence of divinyl sulfone (CAB-DVS), obtained under optimized conditions, and (c) of CAB- DVS-CAL-A biocatalyst. More details are provided in the Experimental section
We observed the bands related to the asymmetric stretching of carboxylates at approximately 1376 cm-1. Still, skeletal vibrations characteristic of the C-C bonding of aromatic rings were followed between 1560 and 1500 cm-1.27,44 Characteristic lignin bands in the samples were observed at 1516 and 1448 cm-1 and are associated with C=C stretching and angular deformation of the C-H bond in phenolic rings, respectively.27,44 We also observed other bands characteristic of lignocellulosic materials in 1375, 1236, 1153, and 1031 cm-1. They may be associated with the C-H bond stretching vibration, C-O of acetyl groups in hemicellulose, asymmetric C-O bond stretching, and C-O-C connection stretching, respectively.27,44 About the groups originating from DVS, this characterization was not sensitive enough to show the reaction or interaction of CAB with DVS because of the complex structure of these feedstocks. The band at 1230 cm-1 showed an increase in intensity given an increased O-H contribution, besides the band at 1017 cm-1, which is related to the aliphatic O-H group.27,44 The spectra obtained for the synthesized samples are very similar. Activation of CAB with the DVS molecule should generate S=O groups in its structure (Figure 2), with absorption close to 1300 cm 1.27,44 However, it was not possible to observe changes in the relative intensities of these bands since lignocellulosic materials are pretty complex, as well as the presence of many functional groups and the fact that this method is qualitative; it was not possible to observe changes in the chemical structure of CAB with DVS by FTIR analysis. The same occurred with the analysis of the spectrum of the CAB DVS-CAL-A biocatalyst, in which the complex chemical structure of the support made it difficult to observe bands that could suggest the insertion of the CAL-A enzyme. It was possible to identify only a subtle change around 1700 to 1750 cm-1, attributed to C=O stretches of ketones, carboxylic acids and non-conjugated esters, groups that are part of the bagasse.46 Table 4 shows the results of the elemental analysis (percentage of C, H, N, and S) of the feedstock (CAB), support (CAB-DVS), and biocatalyst (CAB-DVS-CAL-A). The carbon, hydrogen, and sulfur percentage in CAB was not significantly changed compared with nitrogen. Regarding the amount of sulfur, it did not observe a high increase in the CAB treated per activated with divinyl sulfone (CAB-DVS) through elemental analysis because, in this analysis, the amount of C, H and N is the reference. However, when evaluating the components in smaller amounts by XRF analyses, an increase in the percentage of sulfur is observed when comparing CAB and CAB DVS (Figure 4). However, the considerable change in the amount of nitrogen in the biocatalyst suggests that the enzyme was immobilized.
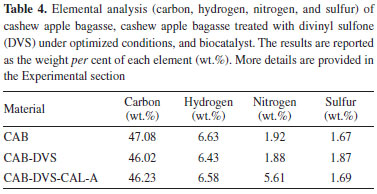
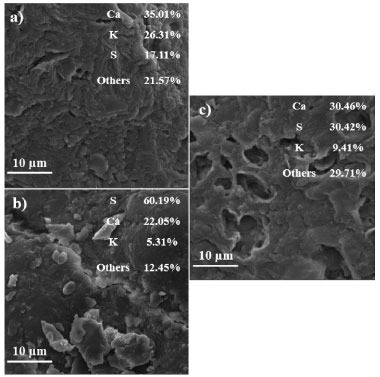 Figure 4. SEM micrographs/XRF maps and elemental spectra: (a) cashew apple bagasse (CAB), (b) bagasse treated in the presence of divinyl sulfone (CAB-DVS), obtained under optimized conditions, and (c) of CAB-DVS-CAL-A biocatalyst
Due to the pH range, DVS favored reacting with the OH groups of the support. DVS groups activated the support in the acidic pH region, i.e. pH 3.0. Possibly, the connection between the support and the DVS occurred at the ends of the molecule (Figure 2). The scanning micrographs (SEM) of the CAB, CAB-DVS and CAB-DVS-CAL-A and the fluorescence spectroscopy (XRF) maps are shown in Figures 4a, 4b and 4c. The morphology of the three supports shows a lamellar structure. The texture of CAB in natura appeared irregular and probably with a layer of wax or fatty acids commonly found in its composition.47 Activation of CAB with DVS showed an increase in sulfur composition (35% S for 60% S), shown by XRF analysis, evidencing that DVS was incorporated by CAB. CAB-DVS-CAL-A showed a decrease in the sulfur composition, which the immobilization of CAL-A could explain. After the immobilization of CAL-A, the formation of large agglomerates, fine bundles and a rougher surface compared to the CAB-DVS sample occurred.46 SEM and XRF served as complementary analyzes to determine whether CAL-A was immobilized on the surface of the support, thus forming the CAB-DVS-CAL-A biocatalyst. Figures 5a and 5b show the TGA and DTG curves obtained for CAB, CAB-DVS, and CAB-DVS-CAL-A. The first event occurs from 25 to 168 °C and may be attributed to the evaporation of water in the samples.47 At this stage, CAB, DVS, and CAB-DVS-CAL-A had a mass loss of 14.3, 17.2, and 16.1%, which may be attributed to better water retention with functionalization and immobilization. The degradation of the main CAB components occurred in the second thermal event, which started at 187 °C and continued up to 500 °C. At this stage, the mass loss was 51.9, 49.9, and 55.7% for CAB, CAB-DVS, and CAB-DVS-CAL-A, respectively. We observed the onset of degradation of cellulose, hemicellulose, and lignin, which are the main CAB components. The DTG analysis can indicate the temperature that contributes most to the degradation. It reveals that the second thermal event had three degradation maxima concerning its main components for the pure CAB. Studied separately the pyrolysis of the main features of lignocellulosic materials and reported that hemicellulose is the component that decomposes easiest, followed by cellulose and lignin.47 The first maximum occurring at 257 °C may be related to the degradation of hemicellulose; the second and third happening at 306 and 335 °C and may be related to cellulose and lignin degradation, respectively.47
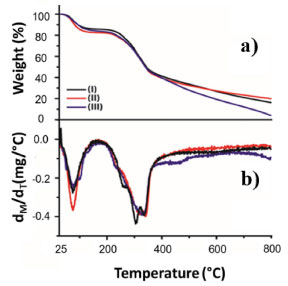 Figure 5. (a) Thermogravimetric analysis, and (b) derivative thermogravimetry. (I) cashew apple bagasse (CAB), (II) bagasse treated in the presence of divinyl sulfone (CAB-DVS), and (III) CAB-DVS-CAL-A biocatalyst. Further details are provided in the Experimental section
Interestingly, only two absorption maxima for the treated CAB (CAB-DVS) were observed at 320 and 340 °C, whereas for the biocatalyst (CAB-DVS-CAL-A), only one degradation maximum at 329 °C was observed. For all materials, the degradation continued up to 800 °C. However, although the CAB and CAB-DVS samples have a total mass loss below 85%, possibly because of the depolymerization of lignin-forming coke,47 the model with the CAL-A enzyme obtained a total loss above 90%, proving to be less thermally stable. However, the application of the enzyme in industrial processes is usually at temperatures below 100 °C. In this range, the mass loss is less than 20%. Also, the percentage of moisture of cashew apple bagasse before (CAB) and after (CAB-DVS) treatment was measured. The CAB before treatment showed 8.5 ± 0.06% moisture; after treatment, the CAB-DVS (material dried only by vacuum filtration) showed 49.0 ± 0.54%. This material (CAB-DVS) was used as the support for immobilization. Thus, a drying step was not considered before the immobilization of CAL-A, avoiding a new step in the process. Influence of pH in the CAL-A immobilization After defining the best conditions to prepare the support, got by the Taguchi method, we evaluated the effect of pH on the immobilization procedure. The CAL-A lipase was immobilized in sodium acetate buffer (pH 5.0), sodium phosphate buffer (pH 7.0), and sodium carbonate buffer (pH 9.0). The results are shown in Table 5.
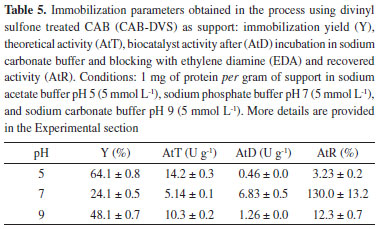
In the immobilization at pH 5.0, the most significant decrease of the supernatant activity was observed, and 68% of the enzyme was immobilized with a yield of 64.1 ± 0.8%. In this range, the reactive residues probably present more significant interaction between the enzyme and the enzyme support. As the CAL-A isoelectric point is 7.5, it has an overall positive charge at this pH, facilitating the ionic interaction with the hydroxyl groups in the CAB-DVS. However, at pH 5.0, the biocatalyst presented the lowest activity. It recovered activity, 0.46 ± 0.0 and 3.23 ± 0.2 U g-1, respectively, which may be related to better interaction of the reactive residues of the enzyme with the support, distorting the structure of the enzyme and resulting in lower activity. In the immobilization at pH 9.0, the lowest values of CAL-A immobilization yield (48.1 ± 0.7%) were achieved, as the supernatant activity showed a slight decrease, possibly because of the repulsion of negative supports and enzyme charges that may have occurred. The biocatalyst activity was 1.26 ± 0.0 U g-1. The biocatalyst with the highest activity was obtained at pH 7.0 (6.83 ± 0.5 U g-1), despite the low immobilization yield (24.1 ± 0.5%). This result can be related to the conformation of CAL-A by immobilizing the most reactive residues in this pH range, lysine, aspartate, and arginine.48,49 To verify the influence of the DVS groups in the CAB in the immobilization, the CAB was treated under the same optimal conditions reported by the Taguchi method (5 mmol L-1 ionic strength, pH 3.0, 30 °C, 12 h) without DVS. The material obtained for this treatment was named CAB-TRAT, and it was used as support to immobilize CAL-A. Also, an immobilization trial using only CAB as support was performed. The results of the immobilization parameters are shown in Table 6. The biocatalysts obtained in the processes used CAB and CAB-TRAT as supports were named CAB-CAL-A and CAB-TRAT-CAL-A.
The CAB and CAB-TRAT supports presented superior performance in the immobilization yield compared to CAB-DVS (Table 6), with values of 66.0 ± 0.9 and 40.2 ± 1.6%, respectively. The difference in immobilization yields using CAB, CAB-TRAT, and CAB-DVS support may be related to the cross-linking formed with the addition of DVS that causes coating of the support surface, increasing the diffusive effects and reducing the immobilization capacity. However, the biocatalyst obtained using the CAB-DVS presented the highest activity. These results may be related to enzyme-support interactions, which are very weak in immobilization by physical adsorption, and probably after washing and vacuum filtration of the derivative, indicating that it occurred adsorption immobilization using CAB and CAB-TRAT as support, in which the enzyme was more easily desorbed from the support, and that it occurred covalent immobilization using CAB-DVS as support. For CAB-TRAT-CAL-A, the biocatalyst activity was 6.11 ± 0.8 U g-1. This result is close to that found for the CAB treated with DVS (6.83 ± 0.5 U g-1), indicating that the chemical structure of CAB may have occurred because of the thermal treatment, favoring the enzyme immobilization process. Then, to understand the type of immobilization that occurs, desorption assays were performed, and the result of the electrophoresis analysis is shown in Figure 6.
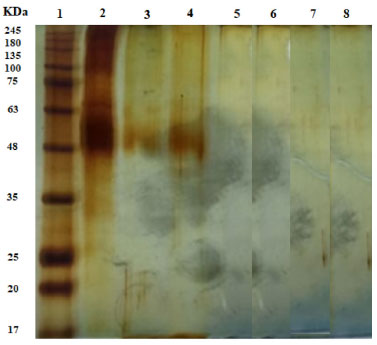 Figure 6. CAL-A electrophoresis gels in samples of supernatant obtained after desorption assays in 1 mol L-1 NaCl or 1 mol L-1 NaCl + Triton at 25 °C for 1 h. (1) Highlighter; (2) Free enzyme; (3) CAB-CALA (NaCl + Triton); (4) CAB TRAT-CALA (NaCl + Triton); (5) CAB-DVS-CALA (NaCl + Triton); (6) CAB-CALA (NaCl); (7) CAB-TRAT-CALA (NaCl) and (8) CAB DVS CALA (NaCl)
Figure 6 shows the enzyme band (45-48 kDa) in the supernatants of the desorption assays carried out in the presence of NaCl and Triton for the CAB-CAL-A and CAB-TRAT-CAL-A catalysts (wells 3 and 4, respectively), indicating that a hydrophobic interaction occurred between the enzyme and the CAB and CAB-TRAT supports. In the desorption process, in the presence of NaCl alone, the enzyme band was not identified (wells 6 and 7), indicating no ionic interaction between the enzyme and these supports. Thus, besides immobilization by adsorption (hydrophobic interaction), immobilization by covalent bonding may have also occurred. However, in the two desorption assays carried out on the CAB-DVS-CAL-A catalyst, the enzyme band was not identified (wells 5 and 8), indicating that there was no immobilization by adsorption (ionic or hydrophobic interaction), and that occurred the covalent immobilization. This result also proves that treating this lignocellulosic material with DVS promotes functional groups that favor bonds with the amino acid residues of CAL-A. The CAB-DVS-CAL-A biocatalyst showed more excellent enzymatic activity, so it was selected for further characterization studies. Thermal and pH stability of biocatalyst The free enzyme and biocatalyst were evaluated for thermal inactivation at 70 °C at different pHs. The stability of CAL-A and CAB-DVS-CAL-A was investigated by incubating them in sodium acetate buffer at pH 5, sodium phosphate buffer at pH 7, and sodium carbonate buffer at pH 9, and the results are presented in Table 7.
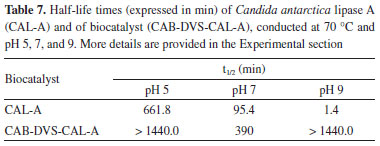
The half-life of the free enzyme (Table 7) was lower than that of the immobilized enzyme at all studied pHs. Better thermal stability was obtained at pH 5, as the half-life of the biocatalyst was not reached at 70 °C after 1440 min (24 h), with a decrease in its initial activity of only around 25%. This is because CAL-A has high stability at acidic pH.50 At pH 9, the free enzyme reached its half-life in less than 2 min. Therefore, the inactivation of the biocatalyst becomes quite interesting for pH 9, because at 1440 min of analysis, the initial activity of CAB DVS-CAL-A decreased by approximately 35%. Studies report that CAL-A can tolerate a relatively wide pH range (5-9), and, when used in its immobilized form, presents good stability, which allows its use at elevated temperatures for thousands of hours without significant loss of activity.51,52 The stability of the CAB-DVS-CAL-A biocatalyst at pH 7.0 (5 mmol L-1 sodium phosphate) was much lower than the strength at 5.0 and 9.0 in the presence of the other two buffers (acetate and carbonate). The authors confirmed a very negative effect of sodium phosphate at pH 7.0 for the stability of lipases from Candida antarctica (A and B), Candida rugosa, and Rhizomucor miehei.53 Generally, the phosphate ion negatively affects the stability of the lipases immobilized via interfacial activation.53 These authors immobilized the lipases on octyl agarose while using glutaraldehyde support. The effect is minor (still very significant using CAL-A), and in some cases, the effect disappeared (for example, in CAL-B). Despite being stabilized at pH 7.0, the immobilized enzyme was more stable than the free enzyme, with a half-life four times longer. These results indicate two things: first, the immobilization protected the enzyme against pH variation in the reaction medium, achieving one of the advantages of this process; second, the incubation of the biocatalyst at a higher temperature did not contribute to modify the conformational structure of the enzyme that impaired its hydrolytic action. CAL-A can maintain its hydrolytic activity at higher temperatures (above 90 °C) and over a wide pH range (5-9).54 Loading capacity of the CAB-DVS support To make the most of the outer surface of the cashew apple bagasse, the maximum enzyme load was evaluated to analyze the amount of the enzyme that can be immobilized on a given support particle. Figure 7 shows the immobilization performance and theoretical activity at different loads of CAL-A (0.5 to 15 mg of protein offered per gram of support).
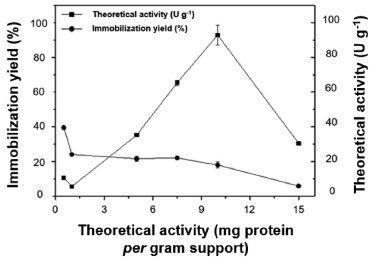 Figure 7. Loading capacity (0.5 to 15 mg of protein) is offered per gram of CAB-DVS support. More details are provided in the Experimental section
The highest immobilization yield (40%) was obtained at 0.5 mg g-1 loading, decreasing with increasing CAL-A loading. The theoretical activity increased when the process was conducted at 10 mg g-1; the highest theoretical activity (96 U g-1) was achieved at a loading of 10 mg g-1; from this load on, the theoretical activity is the lowest. The higher enzyme loading may have increased the diffusion effect, thus improving access to the substrate. Further, high loadings may cause enzyme-enzyme interactions and inhibit the flexible elongation of the enzyme conformation, thus resulting in steric hindrance and enzyme inactivation.40 Previous studies evaluated the maximum protein loading in the immobilization process of Candida antarctica lipase B using cashew apple bagasse pretreated with alkaline hydrogen peroxide as support. The authors activated the support with glutaraldehyde, and 0.5 to 30 mg of protein was offered per gram of support. The highest immobilization yields were observed using low protein concentrations, with the maximum loading capacity of the support being approximately 10 to 11 mg per gram of support. The authors evaluated the immobilization of lipase in lignin from cashew apple bagasse conjugated with magnetic nanoparticles, and they reported immobilization yields similar to those obtained in the present study.55 However, the load offered is lower than the evaluation in the present study, and the lipase is B from C. antarctica. Closing remarks Considering the results of the present study, as well as previous work carried out by our research group using agarose activated with divinyl sulfone (DVS) as a support for enzyme immobilization, we infer that the use of residual biomass, such as cashew bagasse, to immobilize enzymes presents several advantages compared to the use of agarose or other conventional supports. The benefits of using residual cashew bagasse as a support to immobilize enzymes include environmental sustainability. In other words, when using residual biomass, such as cashew bagasse, materials that would otherwise be discarded are used, reducing waste and contributing to environmental sustainability. Taking into account the low cost, in many cases, residual biomass can be obtained at even free of charge, making it an economical option compared to commercially available materials such as agarose. Furthermore, residual biomass is generally biocompatible and non-toxic, essential for enzyme immobilization as it does not interfere with enzyme activity. Moreover, some studies have demonstrated that residual biomass can provide more excellent stability to immobilized enzymes than conventional supports such as agarose. Finally, residual biomass often has a greater enzyme loading capacity, making it possible to immobilize more enzymes per support unit. The choice between residual biomass such as cashew bagasse and other materials as support to immobilize enzymes depends on the system's specific characteristics and the experiment's objectives. Each support has advantages and disadvantages, and the choice must consider factors such as cost, raw material availability, enzymatic stability, loading capacity and specific requirements of the application in question. In some cases, exploring both options may be beneficial to determine which is best suited to the situation. The present study sought to evaluate the feasibility of using cashew bagasse as residual biomass in the immobilization of enzymes. However, we consider that new studies with this material should be carried out for subsequent comparison with conventional materials, such as agarose. Further studies should address critical issues, such as immobilization efficiency, enzymatic stability, carrying capacity, enzymatic recovery after reactions and reuse in catalysis cycles. Furthermore, it is essential to investigate the ideal divinylsulfone (DVS) activation conditions to avoid denaturation of the enzymes. In this context, new studies should examine the advantages and disadvantages of materials as enzyme support in terms of cost, availability, catalytic performance, reusability, effect on enzyme activity and applicability in different industrial applications. Direct comparison between these supports will help determine which one is most suitable for a given purpose, taking into account economic, environmental and efficiency aspects. Therefore, further comprehensive experimental research is essential to establish the effectiveness of cashew bagasse as a waste biomass for enzyme immobilization and its comparison with traditional supports such as agarose in various biotechnological and industrial applications.
CONCLUSIONS The optimization of the conditions applied to the treatment of cashew apple bagasse, using the advanced experimental planning by the Taguchi method as a tool for analysis, has obtained an active and stable biocatalyst. The optimization of fundamental parameters for producing a biocatalyst was of great importance because the process costs are reduced, especially in the treatment step of the cashew apple bagasse, which is necessary for using these raw materials as support. This strategy of obtaining a biocatalyst from agroindustrial waste may be environmentally benign and reusable, used in reactions to industrial interest. The immobilization of CAL-A on CAB-DVS at pH 7.0 presented more relevant immobilization parameters than those performed at pH 5.0 and 9.0, getting a biocatalyst activity of 6.83 U g-1. The CAB-DVS-CAL-A showed a high thermal stability at 70 °C at all pHs studied (5.0, 7.0, and 9.0) compared to the thermal stability of the free enzyme. At pH 7.0, the biocatalyst was approximately four times more stable than the free enzyme. The findings of this study allow us to conclude that CAB, after chemical and physical alterations in its structure, can be support for immobilizing lipases.
ACKNOWLEDGMENT The authors would like to thank the Brazilian research funding agencies Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico - FUNCAP, Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq, Coordenação de Aperfeiçoamento de Ensino Superior - CAPES.
REFERENCES 1. Marcucci, S. M. P.; Zanin, G. M.; Arroyo, P. A.; Microporous Mesoporous Mater. 2022, 337, 111951. [Crossref] 2. Marrone, A.; Fish, R. H.; J. Organomet. Chem. 2021, 943, 121810. [Crossref] 3. da Silva, J. L.; Sales, M. B.; Bizerra, V. C.; Mara, M.; Nobre, R.; Braz, A. K. S.; Sousa, P. S.; Cavalcante, A. L. G.; Melo, R. L. F.; Sousa Junior, P. G.; Simão Neto, F.; Fonseca, A. M.; dos Santos, J. C. S.; Fermentation 2023, 9, 581. [Crossref] 4. Melo, R. L. F.; Sales, M. B.; Bizerra, V. C.; Sousa Junior, P. G.; Cavalcante, A. L. G.; Freire, T. M.; Simão Neto, F.; Bilal, M.; Jesionowski, T.; Soares, J. M.; Fechine, P. B. A.; dos Santos, J. C. S.; Int. J. Biol. Macromol. 2023, 253, 126709. [Crossref] 5. Monteiro, R. R. C.; Virgen-Ortiz, J. J.; Berenguer-Murcia, A.; da Rocha, T. N.; dos Santos, J. C. S.; Alcantara, A. R.; Fernandez-Lafuente, R.; Catal. Today 2021, 362, 141. [Crossref] 6. de María, P. D.; Carboni-Oerlemans, C.; Tuin, B.; Bargeman, G.; der Meer, A. V.; Gemert, R. V.; J. Mol. Catal. B: Enzym. 2005, 37, 36. [Crossref] 7. Sales, M. B.; Lima Neto, J. G.; Braz, A. K. S.; de Sousa Junior, P. G.; Melo, R. L. F.; Valério, R. B. R.; Serpa, J. F.; Lima, A. M. S.; de Lima, R. K. C.; Guimarães, A. P.; de Souza, M. C. M.; Lopes, A. A. S.; Rios, M. A. S.; Serafim, L. F.; dos Santos, J. C. S.; Electrochem 2023, 4, 181. [Crossref] 8. Yu, Y.; Raganati, F.; Simão Neto, F.; Mello Neta, M.; Sousa, A.; Damasceno, L.; Sousa, B.; Medeiros, S.; Melo, R.; Lopes, A.; Santos, J.; Rios, M.; Processes 2023, 11, 2442. [Crossref] 9. Boudrant, J.; Woodley, J. M.; Fernandez-Lafuente, R.; Process Biochem. 2020, 90, 66. [Crossref] 10. Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R.; Process Biochem. 2012, 47, 1220. [Crossref] 11. Feng, J.; Huang, Q. Y.; Zhang, C.; Ramakrishna, S.; Dong, Y. B.; Int. J. Biol. Macromol. 2023, 248, 125729. [Crossref] 12. Nobre, M. M. R.; da Silva, A. F.; Menezes, A. M.; da Silva, F. L. B.; Lima, I. M.; Colares, R. P.; de Souza, M. C. M.; Marinho, E. S.; Melo, R. L. F.; dos Santos, J. C. S.; da Fonseca, A. M.; Compounds 2023, 3, 411. [Crossref] 13. Mittersteiner, M.; Machado, T. M.; De Jesus, P. C.; Brondani, P. B.; Scharf, D. R.; Wendhausen, R.; J. Braz. Chem. Soc. 2017, 28, 1185. [Crossref] 14. Liu, D. M.; Chen, J.; Shi, Y. P.; TrAC, Trends Anal. Chem. 2018, 102, 332. [Crossref] 15. Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F.; Biotechnol. Adv. 2010, 28, 628. [Crossref] 16. Castillo, P.; Cutiño-Avila, B. V.; González-Bacerio, J.; Planes, M. C.; Brito, J. D.; Seijas, J. M. G.; del Monte-Martínez, A.; Enzyme Microb. Technol. 2023, 171, 110323. [Crossref] 17. Battiston, C. S. Z.; Ficanha, A. M. M.; Levandoski, K. L. D.; da Silva, B. A.; Battiston, S.; Dallago, R. M.; Mignoni, M. L.; Quim. Nova 2017, 40, 293. [Crossref] 18. Zahirinejad, S.; Hemmati, R.; Homaei, A.; Dinari, A.; Hosseinkhani, S.; Mohammadi, S.; Vianello, F.; Colloids Surf., B 2021, 204, 111774. [Crossref] 19. Popkov, A.; Su, Z.; Sigurdardóttir, S. B.; Luo, J.; Malankowska, M.; Pinelo, M.; J. Membr. Sci. 2023, 688, 122049. [Crossref] 20. de Souza, T. C.; Fonseca, T. S.; Silva, J. S.; Lima, P. J.; Girão Neto, C. A. C.; Monteiro, R. R.; Rocha, M. V. P.; dos Santos, J. C. S.; Gonçalves, L. R.; de Mattos, M. C.; Bioprocess Biosyst. Eng. 2020, 43, 2253. [Crossref] 21. Girão Neto, C. A. C.; Prasilde, I. C. M.; da Silva, A. S.; Silva, L. M. A.; Canuto, K. M.; Fontenelle, R. O. S.; Rodrigues, T. H. S.; Rocha, M. V. P.; Process Biochem. 2023, 131, 244. [Crossref] 22. Rodrigues, T. H. S.; de Barros, E. M.; Brígido, J. S.; da Silva, W. M.; Rocha, M. V. P.; Gonçalves, L. R. B.; Appl. Biochem. Biotechnol. 2016, 178, 1167. [Crossref] 23. Banu, R.; Sugitha, S.; Kavitha, S.; Kannah, R. Y.; Merrylin, J.; Kumar, G.; Frontiers in Energy Research 2021, 9, 646057. [Crossref] 24. Morales-Sanfrutos, J.; Lopez-Jaramillo, F. J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F.; J. Org. Chem. 2010, 75, 4039. [Crossref] 25. Isidoro, D.; Córdova, C.; Borges, R. M.; Guadalupe, G.; Arizaga, C.; Wypych, F.; Krieger, N.; Quim. Nova 2009, 32, 1495. [Crossref] 26. Hirata, D. B.; Albuquerque, T. L.; Rueda, N.; Virgen-Ortíz, J. J.; Tacias Pascacio, V. G.; Fernandez-Lafuente, R.; J. Mol. Catal. B: Enzym. 2016, 133, 117. [Crossref] 27. dos Santos, J. C. S.; Rueda, N.; Barbosa, O.; Fernández-Sánchez, J. F.; Medina-Castillo, A. L.; Ramón-Márquez, T.; Arias-Martos, M. C.; Linares, M. C. M.; Pedroche, J.; del Mar Iusti, M.; Gonçalves, L. R. B.; Fernandez-Lafuente, R.; RSC Adv. 2015, 5, 20639. [Crossref] 28. Guimarães, J. R.; Carballares, D.; Rocha-Martin, J.; Alcántara, A. R.; Tardioli, P. W.; Lafuente, R. F.; Catalysts 2023, 13, 108. [Crossref] 29. Statistica Software for Windows, version 2018/13.4; StatSoft Inc., Tulsa, OK, USA, 2018. 30. National Renewable Energy Laboratory, Determination of Structural Carbohydrates and Lignin in Biomass, 2012. [Link] accessed in November 2023 31. Pinheiro, B. B.; Rios, N. S.; Aguado, E. R.; Fernandez-Lafuente, R.; Freire, T. M.; Fechine, P. B. A.; dos Santos, J. C. S.; Gonçalves, L. R. B.; Int. J. Biol. Macromol. 2019, 130, 798. [Crossref] 32. Alavi-Borazjani, S. A.; Tarelho, L. A. C.; Capela, M. I.; Int. J. Hydrogen Energy 2021, 46, 21372. [Crossref] 33. Chakraborty, R.; RoyChowdhury, D.; J. Chem. Eng. 2013, 215, 491. [Crossref] 34. Bradford, M. M.; Anal. Biochem. 1976, 72, 248. [Crossref] 35. Henley, J. P.; Sadana, A.; Enzyme Microb. Technol. 1985, 7, 50. [Crossref] 36. Rueda, N.; dos Santos, J. C. S.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R.; Torres, R.; Catal. Today 2016, 259, 107. [Crossref] 37. Albuquerque, T. L. D.; Rueda, N.; dos Santos, J. C. S.; Barbosa, O.; Ortiz, C.; Binay, B.; Özdemir, E.; Gonçalves, L. R. B.; Fernandez-Lafuente, R.; Process Biochem. 2016, 51, 865. [Crossref] 38. Verdasco-Martín, C. M.; Villalba, M.; dos Santos, J. C. S.; Tobajas, M.; Fernandez-Lafuente, R.; Otero, C.; Biochem. Eng. J. 2016, 111, 75. [Crossref] 39. Rueda, N.; Albuquerque, T. L.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Torres, R.; Ortiz, C.; dos Santos, J. C. S.; Barbosa, O.; Fernandez-Lafuente, R.; Molecules 2016, 21, 646. [Crossref] 40. Rios, N. S.; Andrade Neto, D. M.; dos Santos, J. C. S.; Fechine, P. B. A.; Fernández-Lafuente, R.; Gonçalves, L. R. B.; Int. J. Biol. Macromol. 2019, 134, 936. [Crossref] 41. Rios, N. S.; Pinheiro, M. P.; dos Santos, J. C. S.; Fonseca, T. S.; Lima, L. D.; de Mattos, M. C.; Freire, D. M. G.; da Silva, I. J.; Aguado, E. R.; Gonçalves, L. R. B.; J. Mol. Catal. B: Enzym. 2016, 133, 246. [Crossref] 42. dos Santos, J. C. S.; Rueda, N.; Gonçalves, L. R. B.; Fernandez-Lafuente, R.; Enzyme Microb. Technol. 2015, 77, 1. [Crossref] 43. dos Santos, J. C. S.; Rueda, N.; Barbosa, O.; Millán-Linares, M. D. C.; Pedroche, J.; Yuste, M. D. M.; Gonçalves, L. R. B.; Fernandez-Lafuente, R.; J. Mol. Catal. B: Enzym. 2015, 117, 38. [Crossref] 44. dos Santos, J. C. S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L. R. B.; Fernandez-Lafuente, R.; RSC Adv. 2015, 5, 35801. [Crossref] 45. Silva, A. R. M.; Alexandre, J. Y. N. H.; Souza, J. E. S.; Lima Neto, J. G.; de Sousa Júnior, P. G.; Rocha, M. V. P.; dos Santos, J. C. S.; Molecules 2022, 27, 4529. [Crossref] 46. Correia, J. A. C.; Silva, J. S.; Gonçalves, L. R. B.; Rocha, M. V. P.; Biomass Convers. Biorefin. 2022, 12, 2767. [Crossref] 47. Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S.; J. Mater. Res. Technol. 2015, 4, 26. [Crossref] 48. Danait-Nabar, S.; Singhal, R. S.; Process Biochem. 2022, 122, 181. [Crossref] 49. Sharma, P.; Gaur, V. K.; Sirohi, R.; Larroche, C.; Kim, S. H.; Pandey, A.; Ind. Crops Prod. 2020, 152, 112550. [Crossref] 50. Yang, H.; Yan, R.; Chen, H.; Lee, D. H.; Zheng, C.; Fuel 2007, 86, 1781. [Crossref] 51. Souza, T. C.; Fonseca, T. S.; Costa, J. A.; Rocha, M. V. P.; Mattos, M. C.; Fernandez-Lafuente, R.; Gonçalves, L. R. B.; dos Santos, J. C. S.; J. Mol. Catal. B: Enzym. 2016, 130, 58. [Crossref] 52. Monteiro, R. R. C.; Oliveira, B.; Menezes, F. L.; Souza, M. C. M.; Fechine, P. B. A.; dos Santos, J. C. S.; Appl. Clay Sci. 2022, 228, 106634. [Crossref] 53. Kornecki, J. F.; Carballares, D.; Morellon-Sterling, R.; Siar, E. H.; Kashefi, S.; Chafiaa, M.; Arana-Peña, S.; Rios, N. S.; Gonçalves, L. R. B.; Fernandez-Lafuente, R.; Process Biochem. 2020, 95, 288. [Crossref] 54. Monteiro, R. R. C.; Andrade Neto, D. M.; Fechine, P. B. A.; Lopes, A. A. S.; Gonçalves, L. R. B.; dos Santos, J. C. S.; de Souza, M. C. M.; Fernandez-Lafuente, R.; Int. J. Mol. Sci. 2019, 20, 5807. [Crossref] 55. Serpa, J. F.; Matias, G. A. B.; Fechine, P. B. A.; da Costa, V. M.; Freire, R. M.; Denardin, J. C.; Gonçalves, L. R. B.; de Macedo, A. C.; Rocha, M. V. P.; J. Chem. Technol. Biotechnol. 2021, 96, 2472. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access