Artigo
| A charge-assisted hydrogen-bonded organic framework for selective separation of p-xylene |
|
Chundiao ShiI; Ziling LiI; Shengchang XiangI; Chulong LiuI,*; Ximing ZhengII,*
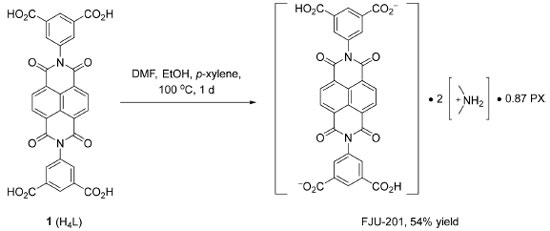
I. Fujian Provincial Key Laboratory of Polymer Materials, College of Chemistry and Materials Science, Fujian Normal University, 350117 Fuzhou, China Recebido em: 12/07/2023 *e-mail: liucl@fjnu.edu.cn; zxm70@wuyiu.edu.cn A charge-assisted hydrogen-bonded organic framework (HOF) FJU-201 is constructed by N,N'-bis(5-isophthalic acid)naphthalimide divalent anion (H2L2-), p-xylene and dimethylammonium. FJU-201 can only crystallize in solutions containing p-xylene, and cannot crystallize in both o-xylene and m-xylene. FJU-201 can also crystallize in xylene mixtures with the content of p-xylene is not less than 50%, which can be used for separating and purifying p-xylene, and it has good recyclability. INTRODUCTION There are three isomers of xylene: ortho-xylene (OX), meta-xylene (MX) and para-xylene (PX). All of them are important chemical raw materials in petrochemical industries and pharmaceutical industries, and also have important applications in organic chemistry, polymer chemistry, etc.1-2 In industry, xylene is usually extracted by catalytic reforming of crude oil or prepared by toluene or benzene methylation. But either solution will result in a mixture of three types of xylenes. Because the xylene isomers have similar physical properties (including boiling point and molecular dynamics size), the separation of three isomers requires high energy consumption (such as azeotropic distillation or extractive distillation) in the existing scheme, and the separation of xylene isomers with low energy consumption and high efficiency is still an industrial topic that needs long-term exploration.3 In the past decades, the selective adsorption of porous materials such as zeolite,4 metal organic frameworks (MOFs),5-6 and covalent organic frameworks (COFs)7 has proved to be a promising industrial separation strategy. It has high selectivity and low energy consumption for various substrates such as gases, drugs, and hydrocarbons. Compared with zeolite, MOFs, COFs, and other porous materials, hydrogen-bonded organic frameworks (HOFs) have the advantages of solution processability, easy purification and characterization, and can be regenerated by simple recrystallization, which make HOFs a promising crystalline materials.8 In recent years, HOFs have been widely used in gas storage and separation,9-13 proton conduction,14-16 photodynamic therapy,17 heterogeneous catalysis,18 fluorescence sensing and other aspects.19-22 However, the relatively weak hydrogen bonding properties also make HOFs have poor stability and design ability. The charge-assisted hydrogen bonding interaction between the negative group (carboxylate, sulfonate, phosphonate, etc.) and positive group (amidinium, animinium, guanidium, etc.) is stronger and easier to design compared with normal H-bonding.23 As a new type of microporous material, hydrogen-bonded organic skeleton has been greatly developed in recent years, but the exploration of xylene separation24 is still much less than that of MOFs25-30 and other porous materials.31-35 In our previous studies, we have reported a charge-assisted HOF FJU-200 crystal constructed from N,N'-bis(5-isophthalic acid)naphthalimide (H4L) and dimethylamine for the detection of aniline.36 Due to its diamond shaped pore structure of appropriate size, we believe that this structure can also be used for the separation of benzene series or related derivatives. Here, we constructed crystal HOF-FJU-201 using H2L2-, dimethylammonium (from DMF), and p-xylene. It has the ability to separate p-xylene from xylene mixtures.
EXPERIMENTAL Synthesis of N,N'-bis(5-isophthalic acid)naphthalimide (1, H4L) The synthesis of 1 (H4L) was followed previous report with some modification (Scheme 1).37 1,4,5,8-tetracarboxydianhydride (2, 1.34 g, 5.0 mmol) was taken into a 250 mL round bottomed flask and suspended in acetic acid (25.0 mL). The mixture was stirred for 10 min. To this solution, 5-aminoisophthalic acid (3, 1.81 g, 10.0 mmol) was added and the solution allowed reflux for 12 h. The reaction was allowed to cool to room temperature and water (20.0 mL) was added to precipitate the product. The product was collected by filtration, washed with ethanol to pH = 7, and dried under vacuum at 80 °C. The product 1 can be used directly without further purification (2.67 g, 90% yield). 1H NMR (400 MHz, DMSO-d6) δ 13.50 (s, 4H), 8.73 (s, 4H), 8.58 (t, J 1.6 Hz, 2H), 8.33 (d, J 1.6 Hz, 4H).
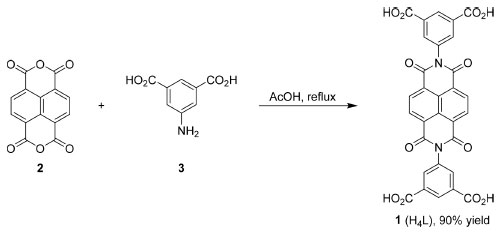 Scheme 1. The synthesis scheme of H4L
Synthesis of FJU-201 The synthesis of FJU-201 is shown in Scheme 2. 1 (H4L) (24.0 mg, 0.04 mmol) was dissolved in DMF (4.0 mL), EtOH (0.5 mL) was added dropwise to the solution, and the powder was dissolved by ultrasonic wave, then p-xylene (3.0 mL) was added to the solution, and the solution was heated at 100 °C for 1 d to obtain FJU-201 ([H4L2-][NH2(CH3)2+]2 0.87PX) as light-yellow crystal (17.1 mg, 54%). 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 4H), 8.57 (t, J 1.6 Hz, 2H), 8.12 (d, J 1.6 Hz, 4H), 7.05 (0.87 eq. PX, 3.5H), 2.55 (s, 12H), 2.24 (0.87 eq. PX, 5.2H) (Figure 3S, supplementary material). TGA curves of FJU-201 in nitrogen atmosphere at a heating rate of 10 °C min-1 (Figure 5S). It can be seen that the weight loss at 200 250 °C is attributed to the weight loss of p-xylene. At 250 450 °C, the weight basically remains stable, and after 450 °C, the mass drops sharply, indicating that the structure begins to collapse.
X-ray crystallography Crystallographic data for FJU-201 were collected on an Agilent Technologies SuperNova single-crystal diffractometer equipped with graphite monochromatic Cu-Kα radiation (λ = 1.54184 Å). The crystal was kept at 285 K during data collection. Using Olex2, the structure was solved with the ShelXT structure solution program using intrinsic phasing and refined with the ShelXL refinement package using least-square minimization. All nonhydrogen atoms were refined with anisotropic displacement parameters. The detailed crystallographic data and structure refinement parameters for FJU-201 are summarized in Table 1S (CCDC 2255993).
RESULTS AND DISCUSSION Single crystal X-ray diffraction analysis reveals that FJU-201 crystallizes in the triclinic space group P-1. It is composed of H2L2-, [NH2(CH3)2]+, and p-xylene (Figure 1a). From the a-axis, the two adjacent H2L2- are connected through the single hydrogen bond (O-H···O/1.76 Å, 154.5°) to form a one-dimensional hydrogen bond band with diamond shaped channels, the size of which is 10.79 × 7.39 Å2 (Figure 1b). Hydrogen bond bands are connected to each other by the hydrogen bond ring formed by two [NH2(CH3)2]+ bridging two H2L2- above and below. And these two [NH2(CH3)2]+ form the same hydrogen bond pattern. Each [NH2(CH3)2]+ interacts with two H2L2- to form hydrogen bonds (N-H···O/1.79 Å, 144.5° and N-H···O/1.83 Å, 151.7°). Through such hydrogen bond rings, the hydrogen bond bands are bridged together to form two dimensional HOFs (Figure 1c). The two-dimensional surface of Figure 1c is seen as a chain of the same color as Figure 1d from the b-axis direction, and then forms a three-dimensional frame through an inclined AA stacking mode.
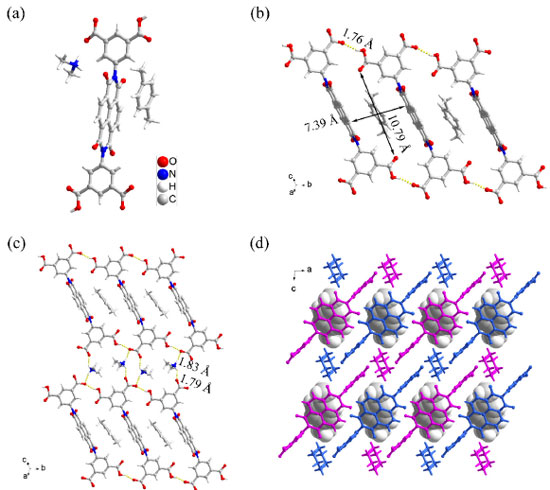 Figure 1. (a) The main components of FJU-201 are H2L2-, [NH2(CH3)2]+, and p-xylene; (b) the one-dimensional structure and the pore size of FJU-201; (c) the connection modes between hydrogen bond bands constitute the two-dimensional structure diagram of FJU-201; (d) schematic diagram of the three-dimensional structure of FJU-201
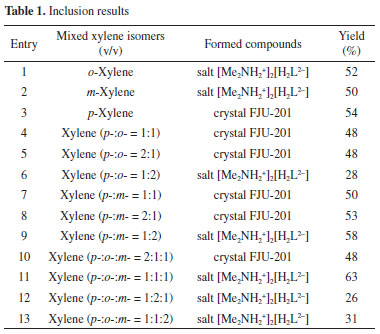
In order to explore the inclusion ability of three xylene isomers by FJU-201, H4L (25.0 mg) was dissolved in DMF (4.0 mL) and EtOH (0.5 mL), then xylene or mixed xylene (3.0 mL) was added to the solution and heated at 100 °C for 1 d. The obtained solids are shown in Table 1. The results showed that crystal FJU-201 crystallized only when p-xylene was added, and salt [Me2NH2+]2[H2L2-] was formed when o-xylene or m-xylene was added (Table 1, entries 1-3; Figure 27S illustrates that salt [Me2NH2+]2[H2L2-] and FJU-201 are different crystals). In further experiments on mixed xylene adding, it was determined through powder X-ray diffraction (PXRD) (Figures 6S, 8S, 11S, 13S and 17S) and 1H NMR (Figures 2, 7S, 9S, 12S, 14S and 18S) that crystal FJU-201 was generated when the content of p-xylene is not less than 50% (Table 1, entries 4, 5, 7, 8 and 10). The 1H NMR spectra of FJU-201 obtained in mixed xylene (Figure 3) are consistent with FJU-201 (Figure 3S), and the molar ratio of H2L2- and p-xylene is approximately 1:0.9 according to the 1H NMR spectra.
 Figure 2. The partially enlarged 1H NMR spectra of crystal obtained in different mixed xylene (from top to bottom: PX:OX = 1:1, PX:MX = 1:1 and PX:OX:MX = 2:1:1 (v/v) (for full spectra see Figures 8S, 12S and 18S, supplementary material)
Furthermore, we explored the recycling of FJU-201 and the extraction of p-xylene. FJU-201 has poor water stability and the crystal framework may collapse in aqueous solutions. After stirring FJU-201 in water for 1 h, 1H NMR analysis was performed on the filtered collected residue, the aqueous phase and the upper organic liquid (extracted with diethyl ether). The results showed that there was only H4L (rate of recovery 99%) in the filter residue and no p-xylene was present (Figure 21S). The upper organic liquid is pure p-xylene (Figure 23S) and only dimethylamine is present in the aqueous phase (Figure 22S). Without any treatment, the H4L recovered from the filter residue was re-prepared into a crystal according to the growth conditions of FJU-201, and the PXRD spectra shows that the crystal is FJU-201 certainly (Figure 24S). This cycle experiment shows that the separation of p-xylene using H4L growth method for FJU-201 can be reused, and the FJU-201 samples grown after two cycles still have good separation ability for p-xylene (Figures 25S, 26S). The well selectivity of FJU-201 towards p-xylene comes from its appropriately sized cavity. FJU-201 has a one-dimensional diamond channel with a size of 10.79 × 7.39 Å2 (Figure 1b), and the molecular size and symmetry of p-xylene perfectly match it (molecular dimension of p-xylene 6.618 × 3.810 × 9.146 Å3, o-xylene 7.269 × 3.834 × 7.826 Å3, and m-xylene 8.994 × 3.949 × 7.315 Å3). In addition, there are multiple weak hydrogen bonds between p-xylene and the inner wall of the pore, as well as weak π.π interactions with H2L2-, further improving its stability in the pore. Due to the inappropriate molecular size and symmetry, we did not obtain crystals containing m-xylene or o-xylene.
CONCLUSIONS In summary, we have successfully constructed a new HOF FJU 201 using H4L with p-xylene and DMF. The construction of FJU-201 can selectively extract p-xylene from xylene mixture with the content of p-xylene is not less than 50%, and the H4L can be recycled. The structural characterization proves that this specific selectivity originates from the one-dimensional diamond shaped pore of FJU 201 and the well matching of the molecular size and symmetry of p-xylene. FJU-201 can provide reference cases for more similar molecular separation and the application of HOF materials.
SUPPLEMENTARY MATERIAL The crystallographic data, PXRD patterns, 1H NMR spectra, and some images of this work are available in http://quimicanova.sbq.org.br, in the form of PDF file, with free access.
ACKNOWLEDGEMENTS This work was supported by the Fujian Provincial Department of Science and Technology (No. 2022H0014), the Fujian Provincial Department of Education (No. JAT200077) and the Nanping Science and Technology Bureau (No. N2020Z011).
REFERENCES 1. Cannella, W. J. In Kirk-Othmer Encyclopedia of Chemical Technology; Ley, C., ed.; Wiley: New Jersey, 2000. [Crossref] 2. Moreira, M. A.; Ferreira, A. F. P.; Santos, J. C.; Loureiro, J. M.; Rodrigues, A. E.; Chem. Eng. Technol. 2014, 37, 1483. [Crossref] 3. Sholl, D. S.; Lively, R. P.; Nature 2016, 532, 435. [Crossref] 4. Yue, B.; Liu, S.; Chai, Y.; Wu, G.; Guan, N.; Li, L.; J. Energy Chem. 2022, 71, 288. [Crossref] 5. Li, L.; Lin, R. B.; Krishna, R.; Li, H.; Xiang, S.; Wu, H.; Li, J.; Zhou, W.; Chen, B.; Science 2018, 362, 443. [Crossref] 6. Zeng, H.; Xie, X. J.; Xie, M.; Huang, Y. L.; Luo, D.; Wang, T.; Zhao, Y.; Lu, W.; Li, D.; J. Am. Chem. Soc. 2019, 141, 20390. [Crossref] 7. Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S.; Chem. Soc. Rev. 2020, 49, 708. [Crossref] 8. Lin, R. B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B.; Chem. Soc. Rev. 2019, 48, 1362. [Crossref] 9. Wang, H.; Li, B.; Wu, H.; Hu, T. L.; Yao, Z.; Zhou, W.; Xiang, W. S.; Chen, B.; J. Am. Chem. Soc. 2015, 137, 9963. [Crossref] 10. He, Y.; Xiang, S.; Chem, B.; J. Am. Chem. Soc. 2011, 133, 14570. [Crossref] 11. Yang, W.; Greenaway, A.; Lin, X.; Matsuda, R.; Blake, A. J.; Wilson, C.; Lewis, W.; Hubberstey, P.; Kitagawa, S.; Champness, N. R.; Schröder, M.; J. Am. Chem. Soc. 2010, 132, 14457. [Crossref] 12. Yang, Y.; Li, L.; Lin, R. B.; Ye, Y.; Yao, Z.; Yang, L.; Xiang, F.; Chen, S.; Zhang, Z.; Xiang, S.; Chen, B.; Nat. Chem. 2021, 13, 933. [Crossref] 13. Yang, Y.; Zhang, H.; Yuan, Z.; Wang, J. Q.; Xiang, F.; Chen, L.; Wei, F.; Xiang, S.; Chen, B.; Zhang, Z.; Angew. Chem., Int. Ed. 2022, 61, e202207579. [Crossref] 14. Yang, W.; Yang, F.; Hu, T. L.; King, S. C.; Wang, H.; Wu, H.; Zhou, W.; Li, J. R.; Arman, H. D.; Chen, B.; Cryst. Growth Des. 2016, 16, 5831. [Crossref] 15. Karmakar, A.; Illathvalappil, R.; Anothumakkool, B.; Sen, A.; Samanta, P.; Desai, A. V.; Kurungot, S.; Ghosh, S. K.; Angew. Chem., Int. Ed. 2016, 55, 10667. [Crossref] 16. Xing, G.; Yan, T.; Das, S.; Ben, T.; Qiu, S.; Angew. Chem., Int. Ed. 2018, 57, 5345. [Crossref] 17. Hu, F.; Liu, C.; Wu, M.; Pang, J.; Jiang, F.; Yuan, D.; Hong, M.; Angew. Chem., Int. Ed. 2018, 57, 7691. [Crossref] 18. Han, B.; Wang, H.; Wang, C.; Wu, H.; Zhou, W.; Chen, B.; Jiang, J.; J. Am. Chem. Soc. 2019, 141, 8737. [Crossref] 19. Hisaki, I.; Suzuki, Y.; Gomez, E.; Ji, Q.; Tohnai, N.; Nakamura, T.; Douhal, A.; J. Am. Chem. Soc. 2019, 141, 2111. [Crossref] 20. Sun, Z.; Li, Y.; Chen, L.; Jing, X.; Xie, Z.; Cryst. Growth Des. 2015, 15, 542. [Crossref] 21. Liu, T.; Wang, B.; He, R.; Arman, H.; Schanze, K. S.; Xiang, S.; Li, D.; Chen, B.; Can. J. Chem. 2020, 98, 352. [Crossref] 22. Lin, Z. J.; Qin, J. Y.; Zhan, X. P.; Wu, K.; Cao, G. J.; Chen, B.; ACS Appl. Mater. Interfaces 2022, 14, 21098. [Crossref] 23. Li, Y.; Handke, M.; Chen, Y. S.; Shtukenberg, A. G.; Hu, C. T.; Ward, M. D.; J. Am. Chem. Soc. 2018, 140, 12915. [Crossref] 24. Ma, L.; Xie, Y.; Khoo, R. S. H.; Arman, H.; Wang, B.; Zhou, W.; Zhang, J.; Lin, R. B.; Chen, B.; Chem. - Eur. J. 2022, 28, e202104269. [Crossref] 25. Vermoortele, F.; Maes, M.; Moghadam, P. Z.; Lennox, M. J.; Ragon, F.; Boulhout, M.; Biswas, S.; Laurier, K. G. M.; Beurroies, I.; Denoyel, R.; Roeffaers, M.; Stock, N.; Düren, T.; Serre, C.; De Vos, D. E.; J. Am. Chem. Soc. 2011, 133, 18526. [Crossref] 26. Jin, Z.; Zhao, H. Y.; Zhao, X. J.; Fang, Q. R.; Long, J. R.; Zhu, G. S.; Chem. Commun. 2010, 46, 8612. [Crossref] 27. Zhang, Z.; Peh, S. B.; Kang, C.; Chai, K.; Zhao, D.; EnergyChem 2021, 3, 100057. [Crossref] 28. Torres-Knoop, A.; Krishna, R.; Dubbeldam, D.; Angew. Chem., Int. Ed. 2014, 53, 7774. [Crossref] 29. Cui, X.; Niu, Z.; Shan, C.; Yang, L.; Hu, J.; Wang, Q.; Lan, P. C.; Li, Y.; Wojtas, L.; Ma, S.; Xing, H.; Nat. Commun. 2020, 11, 5456. [Crossref] 30. Alaerts, L.; Maes, M.; Giebeler, L.; Jacobs, P. A.; Martens, J. A.; Denayer, J. F. M.; Kirschhock, C. E. A.; De Vos, D. E.; J. Am. Chem. Soc. 2008, 130, 14170. [Crossref] 31. Du Plessis, M.; Nikolayenko, V. I.; Barbour, L. J.; J. Am. Chem. Soc. 2020, 142, 4529. [Crossref] 32. Moosa, B.; Alimi, L. O.; Shkurenko, A.; Fakim, A.; Bhatt, P. M.; Zhang, G.; Eddaoudi, M.; Khashab, N. M.; Angew. Chem., Int. Ed. 2020, 59, 21367. [Crossref] 33. Gao, B.; Tan, L. L.; Song, N.; Lia, K.; Yang, Y. W.; Chem. Commun. 2016, 52, 5804. [Crossref] 34. Jie, K.; Liu, M.; Zhou, Y.; Little, M. A.; Pulido, A.; Chong, S. Y.; Stephenson, A.; Hughes, A. R.; Sakakibara, F.; Ogoshi, T.; Blanc, F.; Day, G. M.; Huang, F.; Cooper, A. I.; J. Am. Chem. Soc. 2018, 140, 6921. [Crossref] 35. Sumida, R.; Matsumoto, T.; Yokoi, T.; Yoshizawa, M.; Chem. - Eur. J. 2022, 28, e202202825. [Crossref] 36. Ke, Z.; Chen, K.; Li, Z.; Huang, J.; Yao, Z.; Dai, W.; Wang, X.; Liu, C.; Xiang, S.; Zhang, Z.; Chin. Chem. Lett. 2021, 32, 3109. [Crossref] 37. Mallick, A.; Garai, B.; Addicoat, M. A.; St. Petkov, P.; Heine, T.; Banerjee, R.; Chem. Sci. 2015, 6, 1420. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access