Artigo
| Survey of tetracyclines residues in brazilian swine muscle |
|
Vivian FeddernI,*; Carolina RucksII; Vanessa GresslerI; Anildo Cunha JuniorI; Carolina Naves AroeiraIII,IV; Alfredo Marcial Montes NiñoV
I. Embrapa Suínos e Aves, 89715-899 Concórdia - SC, Brasil Recebido em: 22/06/2023 *e-mail: vivian.feddern@embrapa.br The use of antibiotics is still intensive in animal production. Among the most used veterinary products in pig farming, tetracyclines can be highlighted. When used in excess, these substances can leave residues in food, which depending on the concentration pose a risk on consumers. Brazil, as one of the world's largest producer and exporter of animal protein, must be attentive to the laws and ensure that their meat products are safe for human health. So, the concentration of veterinary residues eventually detected must be below the maximum residue limits (MRLs) stipulated by the regulatory agencies. To contribute with subsidies for decision making in pork safety to the annual monitoring program, performed by the Ministry of Agriculture and Livestock, this study aimed to evaluate the residues of tetracyclines in swine muscle from the federal inspection system, by validated and officially accepted analytical methodology (HPLC-UV). Any suspect results obtained from the HPLC-UV analysis can be confirmed immediately by injecting the samples onto a LC-MS/MS system. The results showed that all evaluated samples had concentrations of tetracycline residues below the MRL, and therefore, can be considered safe for human consumption. INTRODUCTION The use of antibiotics in animal production is still intensive, mainly due to the established breeding systems to meet the growing demand for meat in the world. The intensive model of animal production brought the need to adopt measures to improve sanitary conditions; one of them is the use of veterinary drugs. Currently, there are four basic ways of using these medications: through growth promoters, and metaphylactic, prophylactic, and therapeutic interventions.1,2 However, frequent consumption of these residues that are present in animal products can cause health problems, being toxic and causing antimicrobial resistance in humans.3,4,5 Brazil as the world's fourth largest pork producer and also exporter in 20226 needs to remain competitive in foreign markets, thereby meeting international requirements, which imply in adopting recognized methodologies of analysis and also monitoring programs that ensure compliance to all trade partners. Brazil follows Codex requirements (CX/MRL 2-2018) to construct its specific legislation as well. In Brazil, the National Residues and Contaminants Control Plan (PNCRC) coordinated by the Ministry of Agriculture and Livestock (MAPA) monitors a wide range of antibiotics since 2010, being the results published annually.7,8 The tetracyclines (TCs) are among the molecules included in PNCRC and are also among the most applied in swine production. Tetracyclines can be classified as natural or semi-synthetic substances produced by bacteria of the genus Streptomyces. All TCs have a broad spectrum of action and are similar to each other, acting on both Gram-positive and Gram-negative bacteria, aerobic and anaerobic, as well as Mycoplasma, Chlamydia, Rickettsiae, Spirochaetes, and some Protozoa.9,10,11,12 In the last 11 annual surveillances of the PNCRC, from 2012 to 2022, from a total amount of 8959 samples analyzed in swine muscle and kidney, 16 samples exceeded the concentrations of the maximum residue limit (MRL) for TCs antimicrobials. Regarding the tetracycline class limits, the Brazilian National Health Surveillance Agency (Anvisa) through its Normative Instruction IN 161 from July 1st 2022 established 100 µg k g -1 and 600 µg kg-1, respectively, for swine muscle and kidney. In order to collaborate with MAPA surveillance program (PNCRC), this study aimed to determine by HPLC the occurrence of residues of tetracycline (TCC), chlortetracycline (CTC), oxytetracycline (OTC) and doxycycline (DXC) in Brazilian swine samples.
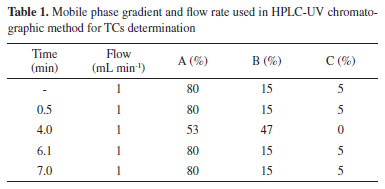
EXPERIMENTAL Chemicals The analytical standards tetracycline hydrochloride, chlortetracycline hydrochloride, oxytetracycline hydrochloride and doxycycline hydrochloride were all purchased from Sigma-Aldrich (St. Louis, USA) with a quality level of 100 (QL 100). All solvents were HPLC grade and acquired from Panreac (Darmstadt, Germany) and J.T. Baker (USA). Purification system provided by Millipore (Advantage A10) was used to obtain ultrapure water. Dichloromethane (P.A.) and petroleum ether (P.A.) were purchased from Êxodo Científica (Sumaré, SP, Brazil), and hydrochloric acid (36.5-38.0% purity) from Panreac (Spain). The filter used was Millex® (Millipore), 33 mm, 0.45 µm. Sampling Twenty-five swine muscle samples received from 7 Brazilian states (Santa Catarina, Rio Grande do Sul, Paraná, Mato Grosso, Minas Gerais, Goiânia and São Paulo) were assessed in order to contribute to the PNCRC of MAPA in 2012. The collected samples were frozen (-20 ºC) and then sent in thermal boxes to Microbiotic Laboratory Analysis (accredited by MAPA). Once in the laboratory, samples were analyzed within 24 h. Sample preparation The procedures followed the methods described in MAPA protocols designated for residue determination.13 A 3.0 ± 0.1 g sample of properly homogenized swine meat was weighed at room temperature in 50 mL Falcon tubes. To evaluate the control sample (with no residue of the analyte under study), 10 portions of 3.0 ± 0.1 g of homogenized sample were weighed into 50 mL Falcon tubes: 1 for blank, 3 for quality controls (QCs) and 6 for the matrix-matched calibration curve. In all samples, including the curve, QCs and blank, 100 µL of HCl (1 mol L-1) was added, and five blank samples were fortified with the standard solution containing 1000 µg L-1 of each tetracycline for preparing a matrix-matched calibration curve (concentration ranging from 25 to 200 µg kg-1). The unfortified blank sample was used as a control. QCs tubes were also fortified at the MRL value of the analyte (200 µg kg-1 for oxytetracycline, tetracycline and chlortetracycline and 100 µg kg-1 for doxycycline). Control and fortified samples were treated as follows: 5 mL of acetonitrile were added, stirred for 1 min, and centrifuged for 10 min at 3000 rpm at 15 ± 2 °C. Next, the supernatant was transferred to another 50 mL Falcon tube and the lower phase discarded. Subsequently, 10 mL petroleum ether and 5 mL dichloromethane were added to the tube with the supernatant, which was then shaken manually for 30 s and centrifuged (5 min, 3000 rpm) at 15 ± 2 °C. The lower (aqueous) phase was transferred to a 15 mL Falcon tube. To the tube with the upper phase, 0.5 mL ultrapure water was added, shaken manually for 30 s, and centrifuged for 5 min at 3000 rpm, 15 ± 2 °C. The lower (aqueous) phase was transferred to the same tube as the previous extraction and the upper phase was discarded. The tubes were vortexed for 15 s and centrifuged for about 5 min at 3000 rpm at 15 ± 2 °C. The upper organic phase was discarded. The remaining organic solvent (dichloromethane and petroleum ether) was evaporated under nitrogen for 20 min at room temperature. The volume of the tube was made up to 2 mL with ultrapure water, passed through the 0.45 µm PTFE filter and injected into the liquid chromatograph. High-performance liquid chromatography coupled to an ultraviolet detector (HPLC-UV) High-performance liquid chromatography (HPLC) coupled to an ultraviolet (UV) detector was used to determine the residues of the different TCs. A C18 4.6 mm × 150 mm (3.5 µm) column was used to separate the compounds. As mobile phase, 0.01 mol L-1 oxalic acid (A), acetonitrile (B) and methanol (C) were used in gradient mode. The injected volume was 100 µL at wavelength λ = 365 nm. Table 1 shows the gradient of the mobile phase.
Liquid chromatography coupled to a mass spectrometer detector (LC-MS/MS) A confirmatory method, by LC-MS/MS was applied to suspected results obtained from the HPLC-UV (data not shown).
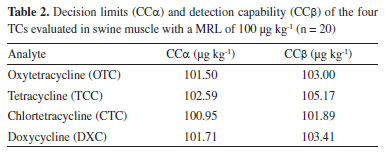
RESULTS AND DISCUSSIONS The PNCRC for TCs in swine comprise analysis in muscle and kidney samples, which are the same selected matrices established by Codex legislation. These target matrices are monitored due to the fact that TCs are well absorbed from the gastrointestinal tract, are widely distributed in the body with highest levels in the kidneys and liver, undergo minimal or no metabolism, and are excreted in urine and feces. Muscle and kidney are complex matrices and usually require an appropriate sample preparation and a robust and sensitive analytical method for determination. To new or modified methodologies, its validation is necessary as it ensures the reliability of the results. Table 2 shows the decision limits (CCα) and detection capability (CCβ) of the different TCs determined in swine muscle samples. The results are in accordance to those reported by Tölgyesi et al.14 for the same evaluated concentration.
The methodology used for the evaluation of TCs showed calibration curves with determination coefficients r2 > 0.99 for all analytes, therefore with satisfactory linearity. Method recovery was also evaluated at the following concentrations: 0.5 MRL (50 µg k g -1), 1.0 MRL (100 µg kg-1) and 1.5 MLR (150 µg kg-1) respectively for each tetracycline in six replicates (Table 3) and the calculated accuracy (Table 4).
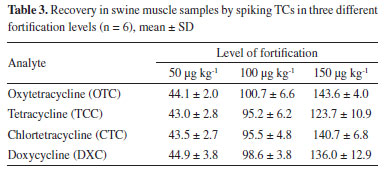
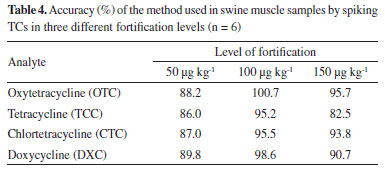
According to the PNCRC, in 2012, from the total amount of 563 samples monitored for TCs residues in swine samples (muscle plus kidney), 25 muscle samples were evaluated by our team in this study and among these samples, no one showed tetracycline residue above the limit of detection (LOD), which was 7 µg kg-1 (LOQ was 25 µg kg-1). To swine muscle recovery rates, our results showed similar or better results when compared with the literature. For the same TCs analyzed herein, Wang et al.15 found recoveries ranging from 86.9 to 102.7% (using molecularly imprinted polymer, MIP and HPLC-UV), and Feng et al.16 observed recoveries of 76.5-92.3% (also using MIP-HPLC-UV). Tölgyesi et al.14 showed recoveries ranging from 103-118% for swine meat using SPE-HPLC-UV. Nguyen et al.17 reported recoveries ranging from 79.2 to 87.2% for TCC, CTC, OTC and DXC with a LL-HPLC-UV method for swine raw muscle. Sokol and Matisova18 using SPE-HPLC-UV reported recovery values of 59.8-91.3% for TCC, CTC and OTC. Although other authors using HPLC-UV have reported lower LOD and LOQ compared to ours14,15,16 in terms of sensitivity, the LOD and LOQ values obtained in this work are suitable for determining TCs in PNCRC samples, once our results showed values below the MRL established by Codex. To a broader overview, MAPA PNCRC annual monitoring data between the years 2012 and 2022 were compiled for swine muscle (Figure 1a) and swine kidney (Figure 1b).
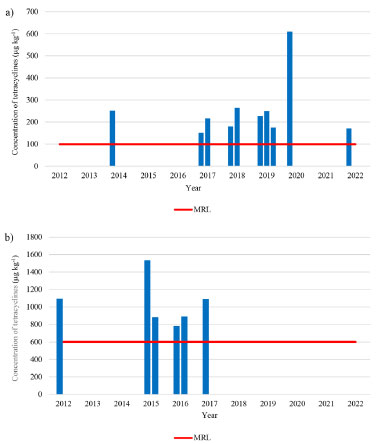 Figure 1. (a) Non-compliant results (concentration of tetracycline residues) in swine muscle in the last 11 years. (b) Non-compliant results (concentration of tetracycline residues) over the last 11 years in swine kidney. Each column represents one sample. Total swine muscle samples analyzed in PNCRC by year: 35 (2012), 48 (2013), 239 (2014), 531 (2015), 602 (2016), 604 (2017), 601 (2018), 609 (2019), 620 (2020), 597 (2021) and 596 (2022). Total swine kidney samples analyzed in PNCRC by year: 528 (2012), 511 (2013), 523 (2014), 521 (2015), 603 (2016), 604 (2017), 587 (2018), 0 (2019), 0 (2020), 0 (2021) and 0 (2022)
In 2012, although no samples exceeded the MRL in swine muscle, in that same year, there was one sample violated for kidney (DXC at 1096.5 µg kg-1). In 2013, there were no violated (values above the MRL) samples, but in 2014 there was one violated sample for muscle (DXC at 251.33 µg kg-1). Two samples above MRL (violated) were verified in 2015 (DXC at 1534.79 and 885.79 µg kg-1) and also two samples violated in 2016 (DXC at 782.17 and 890.89 µg kg-1) for kidney. In 2017, there were three samples that exceeded the MRL, two for muscle (DXC at 151.19 and 217.2 µg kg-1) and one for kidney (DXC at 1092.78 µg kg-1) and in 2018 there were two samples violated for muscle (DXC at 180.2 and 256.67 µg kg-1). However, in 2019 there were three samples violated only for muscle (DXC at 227.98, 248.95 and OXI at 175.9 µg kg-1) and, in 2020, only one sample was violated for muscle (DXC at 610.69 µg kg-1). In 2021, there were no violated samples and in 2022 there was one violated sample for muscle (DXC at 170.54 µg kg-1) (Figure 1). Hence, it is possible to observe that in a period of 11 years (2012-2022) there were few violations. Out of the 8959 muscle and kidney samples analyzed by the PNCRC, only 16 exceeded the MRL (0.18%), highlighting a compliance of more than 99.8% of the samples. Some authors worldwide also investigated chemical residues of tetracyclines in swine tissues. Vragović et al.19 did not detect concentrations above the MRL of tetracycline in swine meat in Croatia, and verified a low (0.45 µg day-1) daily per capita intake of residue in meat. De Almeida et al.20 detected 26 positive samples (n = 501) for tetracycline residues (DXC and OTC), but only 11% of the positive samples were above the Brazilian MRL. Siswanto and Sulabda2 reported that 0.33% of swine meat sold in Denpasar-Indonesia presented tetracycline residue. Huong et al.21 reported that 31 samples (n = 360) of swine meat from 6 provinces in Vietnam showed tetracycline residues and it was the most frequently found antibiotic among all others evaluated by the researchers. In a study carried out by Kyriakides et al.22 in Cyprus, in the Mediterranean, it was found that sulfonamides were the most frequent antimicrobials in swine meat (54.8%), even though tetracyclines were the most used antimicrobials in swine production. Yang et al.23 compared swine meat from Brazil, Russia, Australia, Thailand, and the United States and concluded that Brazilian meat had the lowest risk of residues of these veterinary drugs. This recognition demonstrates the safety of foods of animal origin in Brazil and encourages the ongoing national surveillance plan to continue its efforts to maintain the good reputation of Brazilian meat products and creates the possibility to open new markets for our meat.
CONCLUSION The effectiveness of residue monitoring and control in Brazil supports decision-making by regulatory agencies either within Brazil or in the international level. The use of a recognized methodology in this monitoring program together with the considerably sample compliance (99.8%) regarding tetracycline residues in PNCRC samples (n = 8959), places Brazil in a privileged position in the pork market.
ACKNOWLEDGMENTS The authors thank the Brazilian National Council for Scientific and Technological Development (CNPQ/PIBIC) for providing scholarship to C. R.
REFERÊNCIAS 1. Barcellos, D.; Marques, B. M.; Mores, T. J.; Coelho, C. F.; Borowski, S. M.; Acta Scientiae Veterinariae 2009, 37, s151. [Link] accessed in December 2023 2. Siswanto, S.; Sulabda, I. N.; Kerala J. Vet. Sci. 2019, 2, 79. [Crossref] 3. Beyene, T.; Tesega, B.; J. Vet. Med. Anim. Health 2014, 6, 302. [Crossref] 4. Beyene, T.; J. Vet. Sci. Technol. 2016, 07, 285. [Crossref] 5. Baynes, R. E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J. E.; Food Chem. Toxicol. 2016, 88, 112. [Crossref] 6. ABPA, https://abpa-br.org/abpa-relatorio-anual/, accessed in December 2023. 7. Feddern, V.; Scheuermann, G. N.; Avicultura Industrial 2013, 104, 22. [Link] accessed in December 2023 8. Feddern, V.; Lima, G. J. M. M.; Avicultura Industrial 2015, 106, 18. [Link] accessed in December 2023 9. Klein, N. C.; Cunha, B. A.; Med. Clin. North Am. 1995, 79, 789. [Crossref] 10. Schnappinger, D.; Hillen, W.; Arch. Microbiol. 1996, 165, 359. [Crossref] 11. Nelson, M. L.; Levy, S. B.; Ann. N. Y. Acad. Sci. 2011, 1241, 17. [Crossref] 12. Daghrir, R.; Drogui, P.; Environ. Chem. Lett. 2013, 11, 209. [Crossref] 13. Ministério da Agricultura e Pecuária (MAPA); Guia de Validação e Controle de Qualidade Analítica; Mapa/ACS: Brasília, 2011. [Link] accessed in December 2023 14. Tölgyesi, A.; Tölgyesi, L.; Békési, K.; Sharma, V. K.; Fekete, J.; Meat Sci. 2014, 96, 1332. [Crossref] 15. Wang, G. N.; Zhang, L.; Song, Y. P.; Liu, J. X.; Wang, J. P.; J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2017, 1065-1066, 104. [Crossref] 16. Feng, M. X.; Wang, G. N.; Yang, K.; Liu, H. Z.; Wang, J. P.; Food Control 2016, 69, 171. [Crossref] 17. Nguyen, V.; Li, M.; Khan, M. A.; Li, C.; Zhou, G. H.; Afr. J. Pharm. Pharmacol. 2013, 7, 1448. [Crossref] 18. Sokol, J.; Matisova, E.; J. Chromatogr. A 1994, 669, 75. [Crossref] 19. Vragović, N.; Bazulić, D.; Njari, B.; Food Chem. Toxicol. 2011, 49, 352. [Crossref] 20. de Almeida, M. P.; Rezende, C. P.; Ferreira, F. D.; de Souza, L. F.; de Assis, D. C. S.; de Figueiredo, T. C.; Leite, M. O.; Cançado, S. V. ; Talanta 2015, 144, 922. [Crossref] 21. Huong, L. Q.; Hang, T. T. T.; Ngoc, P. T.; Tuat, C. V.; Erickson, V. I.; Padungtod, P.; J. Food Prot. 2020, 83, 1701. [Crossref] 22. Kyriakides, D.; Panderi, I.; Hadjigeorgiou, M.; Christou, K.; Maou, M.; Kavantzas, N.; Lazaris, A.; J. Food Compos. Anal. 2020, 90, 103512. [Crossref] 23. Yang, Y.; Zhang, H.; Zhou, G.; Zhang, S.; Chen, J.; Deng, X.; Xiaosheng, Q.; Chen, Q.; Niu, B.; J. Food Prot. 2022, 85, 815. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





