Educação
| The electrostatic force applied to teaching organic chemistry in undergraduate classes |
|
Caio L. Firme
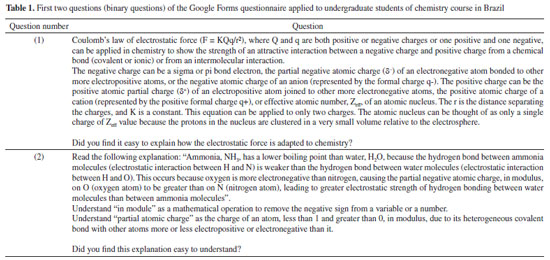
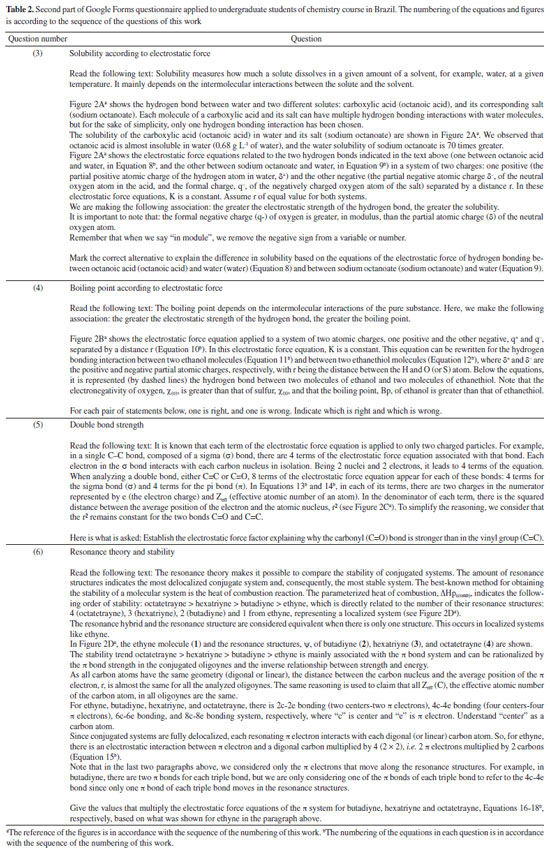
Instituto de Química, Universidade Federal do Rio Grande do Norte (UFRN), 59078-970 Natal - RN, Brasil Recebido em 18/08/2023 *e-mail: caio.firme@ufrn.br; firme.caio@gmail.com The unique fundamental force related to chemical bonds, hydrogen bonds, and van der Waals interactions is the electromagnetic force, where the electrostatic force plays the major role. But, to our knowledge, no paper so far has explored the use of the electrostatic force explicitly to account for any molecular property (boiling point, solubility, etc.), except for the book Introductory Organic Chemistry and Hydrocarbons.1 This work uses the electrostatic force applied to chemistry to explain some molecular properties in organic chemistry. It also evaluates the understanding of Brazilian undergraduate students with respect to some topics of organic chemistry based on the electrostatic force. One questionnaire was applied to chemistry undergraduate students about Coulomb's law and its application to organic chemistry, and the results indicate that most undergraduate students understand the electrostatic force equation applied to chemical education. INTRODUCTION The nature of the covalent bonds (and some related topics) is usually taught using the orbital models (molecular orbital and valence bond theories). There are very few examples in chemical education using Coulomb’s law or electron density: the electrostatic force has been used to account for the membrane chemistry of chitosan smeared with ionic liquid2 as a pre-service chemistry for the teachers of chemistry; and the electron density was depicted as a tool to teach some issues of chemistry.3 There are different models to represent chemical bonds in a molecule,4 but only orbital models are used to describe the nature of covalent bonds.5 However, the transfer or sharing of electrons between atomic centers forming a covalent bond is based on the electrostatic attraction between the protons in nuclei and the electrons in orbitals.6 Literature uses the term “intermolecular forces” with no relation to the fundamental forces in nature.7 Consequently, this term appears to have a loose relation with Coulomb’s law, although it is well-known that all chemical bonds and intermolecular interactions are electrostatic in origin.8,9 Venkataraman10 emphasized the importance of the electrostatic interactions between atoms to help students understand why atoms form chemical bonds, by decreasing the potential energy to a minimum. Some chemistry textbooks,11,12 some internet sources,13,14 and even the IUPAC definition of a covalent bond15 establish the electrostatic nature of the covalent bonds. But many textbooks16-19 and internet sources20-24 disregard the relation between the electrostatic force and the covalent bond. Gillespie,25 well-known by his great contribution to the VSEPR model,26 has already alerted that introductory courses do not mention the electrostatic nature of chemical bonds. He said: “in introductory courses is fraught with many difficulties and at times may even obscure the fundamental reason for the chemical bond - the electrostatic attraction between positive nuclei and negative electrons”. As Taber stated:27,28 “often students learn to “explain” bonds as electron sharing in school science (…), and this becomes a habitual way of talking and thinking by the time they progress to college-level study”. He observed that the “initial (instinctive) response was to explain the bond in terms of electrons shared to fill electrons shells”, indicating one difficulty of the students to associate covalent bonds to its electrostatic nature. In addition, to our knowledge, except for our textbook,1 neither undergraduate organic chemistry textbooks nor papers explicitly explore the electrostatic force equation to explain a molecular property from different substances in organic chemistry. In this work it was developed one Google Forms’ questionnaire whose questions are self-explanatory applied to undergraduate chemistry students. The questionnaire explored some topics in organic chemistry based on the concept of the electrostatic force. The student’s understanding of these topics in the questionnaire was evaluated. This work aims to present a novel pedagogical approach to explain some physical properties in organic chemistry. It also introduced new applications of the electrostatic force, which were not explored in the book Introductory Organic Chemistry and Hydrocarbons.1 Rationale for the electrostatic force in chemistry Schrödinger’s wave equation29 for the hydrogen atom (Equation 1) is: 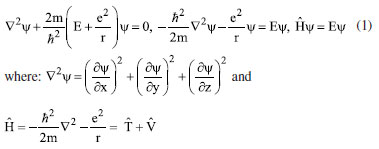 where: and where ψ is the wave function, m is the mass of the electron, e is the charge of the electron, r is the distance between electron and hydrogen nucleus, ћ is the reduced Planck constant, Ĥ is the Hamiltonian operator. From Schrödinger equation (of the hydrogen atom, multi-electron atom, or molecule), the energy of an atom or a molecular system, E, depends on the kinetic and the potential energy operators. However, according to the virial theorem,30 at the equilibrium geometry, T = -0.5V, where T is the kinetic energy, and V is the potential energy. Then, when a chemical bond is formed (for example, in the potential energy surface of the hydrogen molecule as a function of the internuclear distance) where the internuclear distance approaches the equilibrium geometry, the decrease in the potential energy is accompanied by an increase in the kinetic energy by a half. Consequently, the potential energy always dominates, and then it seems appropriate to use a model based on the electrostatic force (which is related to the potential energy). There are four fundamental forces in nature, but the unique fundamental force related to chemical bonds and inter/intramolecular interactions is the electromagnetic force, where the most relevant is the electrostatic force31 based on Coulomb’s law,32 according to Equation 2. 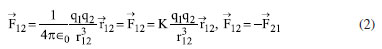 where is the force exerted by q1 on q2 and is the force exerted by q2 on q1, ε0 is the vacuum permittivity (8.854 × 10-12 C2 N-1 m-2), K is the Coulomb’s constant (9 × 109 N m2 C-2). One Coulomb of charge repels equal charge with a force 9 × 109 N when the charges are 1 m apart in a vacuum. Coulomb’s law is limited to the cases which obeys the inverse square law and the potential energy from Coulomb’s law, VC, is (Equation 3):  Nonetheless, other components of the energy associated with the potential energy exist. For example, the effective potential energy of the hydrogen atom33 is the sum of the Coulomb potential (VC) and the centrifugal potential (VL), the potential dependent on the secondary quantum number, l. As Bader8 himself stated: “there are only two forces operative in chemistry, the Feynman force exerted on the nuclei and the Ehrenfest force exerted on the electrons”. Bader34 decomposed the electrostatic force acting on the molecules into two components: the Ehrenfest force (the force acting on the electrons on the entire atom in a molecule) and the Feynman force (the force acting on a nucleus). The sum of all Feynman forces (the summation of the force of all electrons acting on each nucleus and the summation of the force of all nuclei, except for the reference nucleus, acting on each reference nucleus) is zero in a molecule at equilibrium geometry. The Feynman force,35 Fα, on the nucleus A can be expressed as (Equation 4): 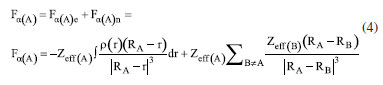 where n represents all nuclei except for A, e represents all the electrons, ρ(r) is the charge density at position r, R is the position of the nucleus A or B, and Zeff is the effective atomic number. The Ehrenfest force acting on all electrons from atom A equals the force exerted on its surface which is similar to the integration of the divergence of the stress tensor, ∇s(r). The stress tensor, s(r), is the quantum mechanical equivalent for the pressure of a force acting on a surface (Equation 5). 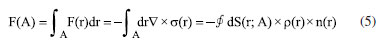 At equilibrium geometry, there are two important facts: (i) the sum of all Feynman forces is zero, and the only force that is non-zero is the Ehrenfest force; and (ii) the virial theorem relates the total kinetic energy to the total potential energy from the equation 2T = -V, where T is the kinetic energy, and V is the potential energy. In the virial theorem, the potential energy decreases, and the kinetic energy increases. Due to a positive charge in the nucleus and a negative charge around the nucleus, all intermolecular/intramolecular interactions and chemical bonds have an electrostatic character. Consequently, they all can be analyzed from the perspective of Coulomb’s law. However, Levine and Head-Gordon36 also showed the importance of constructive quantum interference to rationalize the chemical bond. They stated that “the chemical bond was originally viewed and is still sometimes discussed and taught, as being electrostatic in origin. This was based on the virial theorem: for a (negative) bond energy, the electron potential energy changes (decreases) twice as much as the electron kinetic energy increases in an exact quantum calculation at the equilibrium geometry (…) seminal work by Ruedenberg established for H2+ and H2 that despite the correctness of the virial theorem, roughly 66% of the binding energy can be associated with constructive quantum interference that lowers the kinetic energy”.36 Then, the energetic interpretation of the chemical bond depends on the used theory: (i) from the perspective of the Quantum Theory of Atoms in Molecules, QTAIM,37 the virial theorem based on the electrostatic force prevails; and (ii) from the molecular orbital model, the resonance (or wavefunction interference) origin of the chemical bonding is predominant, although Levine and Head-Gordon36 themselves recognized that the increase or decrease of the kinetic energy depends on the molecular orbital model used and the chosen system. The electrostatic force and QTAIM descriptors were used for developing the local potential energy density, LPE, in Equation 6. The LPE is used to obtain the binding energy density of intra/intermolecular interaction and indirectly the binding energies of the corresponding complexes from its linear relation with the supramolecular energy of the studied complexes.38,39 When analyzing the complexes linked by intermolecular interactions, the electrostatic component is just one of four components in the energy decomposition analysis.39 The LPE equation is an average of the sum of the potential energy from two electrostatic interactions: rbcp interacting with Zeff(I) and rbcp interacting with Zeff(II). 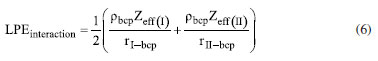 where I and II are the interacting atoms of the intermolecular or intramolecular interaction; rbcp is the charge density of the bond critical point (bcp); rI-bcp and rII-bcp are the distance of the bond path from the bond critical point to the interacting atoms I and II, in Bohr unit, in the corresponding bond path. The electrostatic force applied to organic chemistry In all examples presented in this work, there is a comparison of a specific molecular property (boiling point, solubility, stability, and strength of the double bond) between two (or more) molecular systems where the intermolecular interaction, or chemical bond, or the electron delocalization were evaluated by means of the electrostatic force equation. The generic equation of the electrostatic force is Equation 7 where q+ is an atom or fragment with (partial) positive atomic charge and q- is an atom with (partial) negative atomic charge.  For simplicity purposes, in the examples in the questionnaire questions of this work, the distance between the atomic charges, r, is considered invariant. There are some variations of Equation 7 which are explained in the text of each question and depicted in corresponding figures. Important to emphasize that Coulomb’s law only applies for point charges at rest. When analyzing the charge density of a molecule, it is expected to use the integration of the charge density at r position, ρ(r), interacting with each nucleus over the squared distance as it is depicted in Feynman force Fα(A)e in Equation 4. But, for the sake of simplicity, the charge density distribution was approximated to a local point charge, q-, at the electronegative atom.
METHODOLOGY It was applied one Google Forms questionnaire to the undergraduate students of chemistry course from the Federal University of Rio Grande do Norte, Brazil who were attending the first discipline of theoretical organic chemistry. The questionnaire was applied at the end of the first semester of 2023. There were 34 participants. The questions and answers were in their native language, Portuguese. Students were requested to answer the questions remotely and in asynchronous mode. Students had only one opportunity to answer the questionnaire, i.e., it was not possible to redo the questionnaire after its submission. Soon after the questionnaire was closed, the questions were translated into English by Google Translator following some manual corrections. Some manual corrections were needed, and the numbering of figures and equations in each question was adapted to this paper (Tables 1 and 2). Figures 1 and 2 were in English format in the Google Forms questionnaire, whose corresponding translations were given in the text of each question. The numbering of the equations in Figure 3 was also adapted to this paper. Google Forms automatically generated the statistical analyses, but Google Translator did not translate the data in their images. Then, they were manually edited and translated.
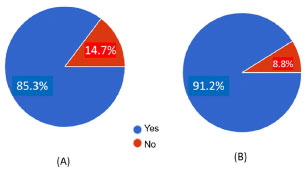 Figure 1. Results of students’ answers to the first two binary questions of the questionnaire to undergraduate students of the Federal University of Rio Grande do Norte, Brazil about (A) Coulomb’s law (question 1) and (B) its application to organic chemistry (question 2)
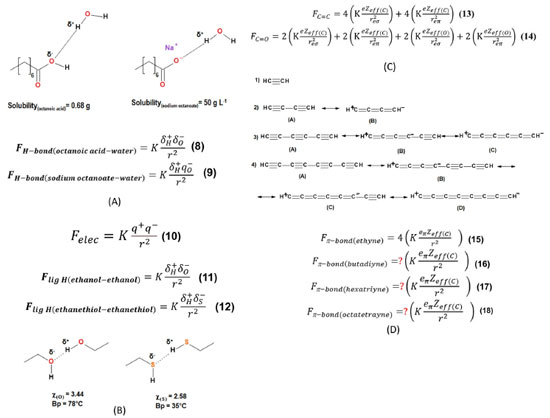 Figure 2. (A) Schematic representation of the hydrogen bond in octanoic acid and sodium octanoate interacting with water along with their solubilities in water and Equations 8 and 9. (B) Equations 10-12 and schematic representation of the hydrogen bond between a pair of ethanol and a pair of ethanethiol molecules of the question 4 from the questionnaire. (C) Equations 13 and 14 associated with the π bond of C=C and C=O groups, respectively. (D) Resonance structures of butadiyne (2), hexatriyne (3) and octatetrayne (4) and the structure of ethyne (1) for comparison reason along with their corresponding Equations (15-18) of the electrostatic force of π bonds. The numbering of the equations was adapted to this work
 Figure 3. (A) Multiple-choice alternatives to question 3 of the questionnaire where the numbering of the equations was adapted to this work; (B) Multiple-choice alternatives from question 4 of the questionnaire in Table 2. (C) Multiple-choice alternatives from question 5 of the questionnaire. (D) Multiple-choice alternatives from question 6 of the questionnaire in Table 2 where the numbering of the equations was adapted to this work
The students received a short introduction to the electrostatic force applied to chemical bonds in one class for five-ten minutes. Assuming that they had insufficient information about Coulomb’s law applied to chemistry, all needed information was provided in each question. The students accessed the Google Forms link and answered the questions remotely. The students had three weeks to complete the questionnaire. No additional supporting material was provided to the students except for the information in the questions and a short presentation on this topic in one class at the beginning of the semester. No individual answer is depicted or analyzed in this work. The responses were only used in the statistical analyses of this work. There were six questions in the questionnaire. The first two questions had only two answers: yes or no (binary questions). The following four questions were at a more cognitive level. All questions were self-explanatory, and there was no need to search for an external source to answer the questions. Then, we survey the students’ understanding of these topics from the perspective of the electrostatic force. Informed consent All participants of this study have consented that their answers were included in the statistical analysis. No individual answer was depicted or analyzed. No identification was requested to fill in the Google Forms, except for their emails. There were two alternatives (yes or no) for the Terms of Consent below and all of them have consented to share the results of their questionnaires. Term of consent: I accept that my responses are used, in aggregate form for statistics and not being treated individually, for the research of Professor Caio Lima Firme. I was informed that my personal data will not be disclosed in the survey, nor will my individual responses be shown separately in the survey.
RESULTS AND DISCUSSION Questionnaires for students The questionnaires were given to the students in their native language, Portuguese. Later, the questions were translated into English by Google Translator and some manual corrections were needed. The students had a very short presentation of the electrostatic force applied to organic chemistry in one single class for five-ten minutes. No further information was given until the questionnaire was available to them. Table 1 depicts the first two introductory questions of the questionnaire. They are binary questions, and the results of the students’ answers are shown in Figure 1. Question 1 explains how Coulomb’s law can be adapted to chemistry involving two atoms of partial or full charges. 85.3% of the students found the explanation easy to understand. Question 2 explains why water has a greater boiling point than ammonia using the resource of the electrostatic force associated with the hydrogen bond. It was necessary only one paragraph to explain the reasoning developed in question 2 and 91.2% of the students found it easy to understand. Both questions were intended to prepare the students for more complex questions ahead in the questionnaire. Table 2 depicts the questions of the second part of the questionnaire. There are four single select multiple choice questions. The reference of the figures and the numbering of the equations from these questions were adapted to this work, following its numbering sequence. Question 3 explains the solubility difference between octanoic acid and sodium octanoate through Coulomb’s law applied to chemistry. From the electrostatic force equations (Equations 8 and 9), the text in Table 2 (supported by Figure 2A) explains that the higher atomic charge of the oxygen atom in sodium octanoate is responsible for greater electrostatic interaction with water through the hydrogen bond in comparison with that between octanoic acid and water. This higher electrostatic interaction of the hydrogen bond between sodium octanoate and water explains the higher affinity between the salt and water than the corresponding acid interacting with water. Question 4 is about the boiling points of ethanol and ethanethiol, which are related to their intermolecular interaction, mainly the hydrogen bond (Figure 2B). This question mentions the partial negative and positive atomic charges, which are the terms of the product in the numerator of the electrostatic force equation (Figure 2B). The alternatives to this question comprise five pairs of right and wrong statements, accounting for the greater boiling point of ethanol with respect to ethanethiol rationalized by electrostatic force applied to their corresponding hydrogen bonds. Question 5 provides, along with Figure 2C, the elements to understand how electrostatic force could explain the stronger force of the double bond in carbonyl group (C=O) compared to that from a vinyl group (C=C). In the end, it is requested to establish which electrostatic force factor specifically could explain this. Only one alternative to this question is correct. Question 6 explains that the order of stability: octatetrayne > hexatriyne > butadiyne > ethyne, can be reasoned through resonance theory according to the number of resonance structures (depicted in Figure 2D) for each oligoyne where ethyne is used as reference. Indeed, the text in question 6 provides a further element to rationalize this order of stability: the strength of the π bond (the one which moves across the resonance structures) in the conjugated oligoynes and the inverse relationship between strength and energy. Equations 15 to 18 in Figure 2D indicate that the only difference in the strength of π bond in the oligoynes is the term multiplying the electrostatic equations, which is the product of the total number of resonating π bond electrons and carbon atoms. There is only one correct alternative providing the right number for the term multiplying the electrostatic equation of the π bond in butadiyne, hexatriyne and octatetrayne. Equations 8 to 18 are all electrostatic force equations applied to different situations in the questionnaire. There are slight differences among them which are related to one or more terms in these equations which are needed for comparison purposes in each question. These equations are important for the correct students’ understanding of the electrostatic force in each situation depicted in the questionnaire. Figure 3A shows four alternatives to question 3. All of them focus on the numerator factor of the electrostatic force, i.e., the charges of the atoms, oxygen, and hydrogen, involved in the hydrogen bond of octanoic acid-water and sodium octanoate-water intermolecular interactions, specifically the atomic charge of the oxygen atom in both cases. The correct alternative is the third one from the top to the bottom. 26 students chose the correct alternative representing 76.5% of all participants (see Figure 1S(A) from Supplementary Material). Figure 3B shows the ten statements to question 4, where each pair has one right and wrong alternative comprising five correct and five wrong answers. The alternatives are statements to explain the higher boiling point of ethanol compared to ethanethiol through the reasoning of the electrostatic force in the hydrogen bond of each pair of molecules of the same substance. Each pair of alternatives deal with the same topic (from the upper left to lower right location in Figure 3B): the partial atomic charge of the oxygen atom, the partial atomic charge of the sulfur atom, the partial atomic charge of the hydrogen atom in ethanol, the partial atomic charge of the hydrogen atom in ethanethiol, respectively. The last pair of alternatives (in the bottom right of Figure 3B) are statements involving both partial atomic charges of the hydrogen bond in ethanol and ethanethiol, respectively, reasoning the higher strength of the hydrogen bond. The correct alternative to each pair of statements is given in Figures 1S(B) to 1S(F) in the Supplementary Material. For the first two pairs of alternatives, 85-88% of the students chose the right option. For the next two pairs of alternatives, 76-79% of the participants marked the right option and only 67.6% chose the right statement for the last pair of alternatives. In general, most of the students answered correctly the five statements associated to question 4. Figure 3C shows the five alternatives to question 5, where only one is correct: the penultimate alternative from the top to the bottom (Zeff of oxygen atom is higher than Zeff of carbon atom). Curiously, only 50% of the students chose the right alternative. An expressive number of participants (47%) marked the first option or the second option which state that the π electron charge, e, in both C=O and C=C has different values. This result demonstrates a serious deficiency of basic knowledge or misinterpretation of terms “electron charge” and “density of the electron charge”. It is suggested to give further information about the aforementioned terms in the text of question 5 in order to evaluate if there will be an increase in the number of correct answers to this question. Figure 3D shows the four alternatives to question 6 related to the number of the term multiplying the electrostatic force of the resonating π bond interacting with carbon atom in the oligoynes. There is only one correct alternative: the second one from the top to the bottom. 58.8% of the students marked the right option, but an expressive number of participants (32.4%) chose the first option (see Figure 1S(H) from Supplementary Material). Probably, the students who chose the first alternative have not correctly understood the idea of the 4c-4e, 6c-6e, and 8c-8e resonating bonding systems for butadiyne, hexatriyne and octatetrayne, respectively, and this relation with Coulomb’s law since the concept of multi-bonding systems is not usually explored in the disciplines of organic chemistry in the undergraduate courses. Figure 4 shows the bar graph of the total score versus the number of participants of the questionnaire. Both median and average are nearly the same (65.5 and 65, respectively). It means that there is a normal distribution of the total score, i.e., the distribution is not distorted, and we can draw accurate conclusions from the data. The average of the total score is moderate. 23 students (67% of all participants) scored 50 or higher. Out of the group of students who scored below 50, 10 out of 11 had total score ranging from 36 to 49, with an average score of 42.
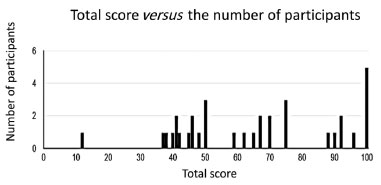 Figure 4. Points scored versus number of participants in the questionnaire
CONCLUSIONS There is a deficiency in teaching organic chemistry focused on Coulomb’s law, which could favor the explanation of some physical properties of organic compounds. To date, some undergraduate organic chemistry textbooks only mention the electrostatic nature of covalent bonds, and almost none of them explore Coulomb’s law in organic chemistry more explicitly, to our knowledge. The electrostatic force applied to chemistry can be used to rationalize several properties of organic chemistry: the strength of hydrogen bond (and its relationship with boiling point and solubility), the strength of a chemical bond, and the stability of resonating molecular systems. This work provides self-explanatory questions that can be used to teach some physical properties in organic chemistry from a novel perspective. We have applied a Google Forms questionnaire to 34 undergraduate students of organic chemistry which had a sparce previous information of the electrostatic force applied to chemistry (ten minutes of only one class). The texts of the 6 questions (two introductory binary questions and four single select multiple choice questions) provided all needed information related to the analyzed chemistry property and the application of the electrostatic force to it, along with figures which assured a better understanding to the questions. Consequently, the median and average of the total score were nearly the same above 60 (65.5 and 65, respectively). 23 participants (67%) scored 50 or above. Therefore, we conclude that the electrostatic force can be successfully applied to explain some properties of organic chemistry and general chemistry.
SUPPLEMENTARY MATERIAL The supplementary material of this work is available in http://quimicanova.sbq.org.br, in the form of PDF file, with free access. It contains the participants’ answers in bar chart for all questions applied in the questionnaire of this work.
ACKNOWLEDGEMENTS We would like to thank all participants of the questionnaire in this work.
REFERENCES 1. Firme, C. L.; Introductory Organic Chemistry and Hydrocarbons: A Physical Chemistry Approach, 1st ed.; CRC Press: Boca Raton, 2020. 2. Hernani; Ulum, L. L.; Mudzakir, A.; J. Phys.: Conf. Ser. 2020, 1521, 042087. [Crossref] 3. Shusterman, A. J.; Shusterman, G. P.; J. Chem. Educ. 1997, 74, 771. [Crossref] 4. van Dulmen, T. H. H.; Visser, T. C.; Coenders, F. G. M.; Pepin, B.; McKenney, S.; Chem. Educ. Res. Pract. 2023, 24, 896. [Crossref] 5. Galbraith, J. M.; Shaik, S.; Danovich, D.; Braïda, B.; Wu, W.; Hiberty, P.; Cooper, D. L.; Karadakov, P. B.; Dunning Junior, T. H.; J. Chem. Educ. 2021, 98, 3617. [Crossref] 6. Jidu, D.; Res. Rev.: J. Chem. 2022, 11, 6. [Link] accessed in December 2023 7. Cooper, M. M.; Williams, L. C.; Underwood, S. M.; J. Chem. Educ. 2015, 92, 1288. [Crossref] 8. Bader, R. F. W.; J. Phys. Chem. A 2009, 113, 10391. [Crossref] 9. Nakatsuji, H.; Koga, T.; J. Am. Chem. Soc. 1974, 96, 6000. [Crossref] 10. Venkataraman, B.; J. Chem. Educ. 2017, 94, 296. [Crossref] 11. Brown, T.; Lemay, H.; Bursten, B.; Murphy, C.; Woodward, P.; Stoltzfus, M.; Chemistry: The Central Science, 4th ed.; Pearson Education United: Harlow, 2017. 12. Morrison, R. T.; Boyd, R. N.; Organic Chemistry, 6th ed.; Prentice Hall: New York, 1992. 13. Western Oregon University, https://wou.edu/chemistry/courses/online-chemistry-textbooks/ch150-preparatory-chemistry/ch150-chapter-4-covalent-bonds-molecular-compounds/, accessed in December 2023. 14. Royal Society of Chemistry, https://edu.rsc.org/resources/chemistry-for-the-gifted-and-talented-book-introduction/616.article, accessed in December 2023. 15. International Union of Pure and Applied Chemistry (IUPAC); Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, 2019. 16. Bruice, P. Y.; Organic Chemistry, 4th ed.; Prentice Hall: New York, 2003. 17. Whitten, K. W.; Davis, R. E.; Peck, L.; Stanley, G. G.; General Chemistry, 7th ed.; Thomson Brooks: Belmont, 2003. 18. Carey, F. A.; Organic Chemistry, 8th ed.; McGraw Hill: New York, 2011. 19. Wade, L. G.; Organic Chemistry, 6th ed.; Pearson Prentice Hall: New York, 2006. 20. Chem Talk, https://chemistrytalk.org/ionic-vs-covalent-bonds/, accessed in December 2023. 21. ThoughCo., https://www.thoughtco.com/definition-of-covalent-bond-604414, accessed in December 2023. 22. Books, https://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/s12-ionic-versus-covalent-bonding.html, accessed in December 2023. 23. Coştu, B.; Niaz, M.; Educ. Quim. 2012, 23, 257. [Crossref] 24. Khan Academy, https://www.khanacademy.org/science/class-11-chemistry-india/xfbb6cb8fc2bd00c8:in-in-chemical-bonding-and-molecular-structure/xfbb6cb8fc2bd00c8:in-in-kossel-lewis-approach-to-chemical-bond/a/single-and-multiple-covalent-bonds, accessed in December 2023. 25. Gillespie, R. J.; Education in Chemistry 1996, 13, 106. 26. Gillespie, R. J.; Spencer, J. N.; Moog, R. S.; J. Chem. Educ. 1996, 73, 622. [Crossref] 27. Science Education, https://science-education-research.com/covalent-bonding-is-sharing-electrons/, accessed in December 2023. 28. Taber, K. S.; Journal of Turkish Science Education 2011, 8, 3. [Link] accessed in December 2023 29. Schrödinger, E.; Ann. Phys. 1926, 79, 361. [Crossref] 30. Slater, J. C.; J. Chem. Phys. 1933, 1, 687. [Crossref] 31. Sanghera, P.; Quantum Physics for Scientists and Technologists: Fundamental Principles and Applications for Biologists, Chemists, Computer Scientists, and Nanotechnologists, 1st ed.; Wiley-Interscience: Hoboken, 2011. 32. Coulomb, C. A.; Histoire de l’Academie Royale des Sciences, 1785. [Link] accessed in December 2023 33. Firme, C. L.; Quantum Mechanics: Detailed Historical, Mathematical and Computational Approaches, 1st ed.; CRC Press: Boca Raton, 2022. 34. Bader, R. F. W.; Fang, D. C.; J. Chem. Theory Comput. 2005, 1, 403. [Crossref] 35. Hernandez-Trujillo, J.; Cortes-Guzman, F.; Fang, D. C.; Bader, R. F. W.; Faraday Discuss. 2007, 135, 79. [Crossref] 36. Levine, D. S.; Head-Gordon, M.; Nat. Commun. 2020, 11, 4893. [Crossref] 37. Matta, C. F.; Boyd, R. J.; In The Quantum Theory of Atoms in Molecules: From Solid to DNA and Drug Design; Matta, C. F.; Boyd, R. J., eds.; Wiley-VCH: Weinheim, 2007. 38. Firme, C. L.; Chem. Phys. Lett. 2020, 754, 137593. [Crossref] 39. Firme, C. L.; Comput. Theor. Chem. 2021, 1197, 113143. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access