Artigo
| Microwave assisted lactide-glycolide homo and copolymerization initiated by magnesium lactate |
|
Matheus Alves Coelho; Marcos L. Dias* Instituto de Macromoléculas Professora Eloisa Mano (IMA), Universidade Federal do Rio de Janeiro, 21941-598 Rio de Janeiro - RJ, Brasil Received: 10/19/2023 *e-mail: mldias@ima.ufrj.br In this work, microwave-assisted ring opening polymerization (ROP) of lactide (LA) and glycolide (GA) initiated by magnesium lactate was investigated. The influence of microwave power, temperature, and reaction time on homopolymers and copolymer's structure and thermal properties is discussed. Polymerization of L-lactide (LLA) initiated by the magnesium compound resulted in yields up to 78% and poly(L-lactic acid) (PLLA) with average molar mass of up to 30,600 g mol-1. Homopolymerization of glycolide generate poly(glycolic acid) (PGA) in yields up to 90% and in L-lactide/glycolide copolymerizations yields between 34-82%. PLLA prepared with the initiator presented microstructure with significant amount of stereo errors as the result of a racemization process during polymerization as well as low glass transition temperature (Tg) and melting temperature (Tm), while the PGAs presented high Tg, due to high crystallinity, and Tm in accordance with the literature. LLA and GA copolymerization result in poly(lactic acid-co-glycolic acid) (PLGA) with composition close to polymerization feed composition. INTRODUCTION In recent times, interest in biodegradable materials has intensified, that is, the interest in materials that undergo degradation through the action of microorganisms.1 The most widely studied class of biodegradable polymers are aliphatic polyesters, as they belong to a group of polymers that, in addition to biodegradability, they have no toxicity and, when disposed in an aqueous environment, undergo hydrolytic degradation. These properties justify the fact that biodegradable aliphatic polyesters have gained great prominence in recent years, especially in the health area.2 The technique of ring opening polymerization (ROP) of cyclic esters (lactones) is widely applied in the synthesis of polymers, mainly in the synthesis of aliphatic polyesters. The use of ROP can provide high control over molar mass, also allowing the achievement of high molar masses. In this sense, polymerization via ROP enhances the search for new biomaterials.3-5 In recent decades, there has been a growing interest in synthesizing biomaterials through microwave irradiation, which optimizes the reactions, providing a more homogeneous heating and higher yields in shorter reaction times, when compared to conventional heating. This way of heating is particularly interesting for monomers containing polar groups in its structure, which in turn favors the absorption of microwaves.6 In addition, microwave irradiation can be considered a green method of heating.7 Microwave heating is favored in monomers that contain polar groups, as the large difference in electronegativity between atoms which constitutes the molecules triggers the displacement of the electron cloud towards the more electronegative atoms, forming dipoles, which in turn, favor microwave heating.8,9 Studies show that ROP significantly benefits from microwave-assisted heating, enabling excellent control over polymerizations, combined with a tremendous acceleration of polymerization rates.7 In the literature, the efficiency of tin octoate as an initiator of lactone polymerizations via ring opening is widely known, allowing polymers with high molar masses to be obtained.10 However, the toxicity and the difficulty of removing tin residues from the polymer can limited its applications, mainly in biomedicine. Therefore, there is a scientific community effort in the search for alternatives to tin-based compounds to find less toxic and more biocompatible catalysts.11 Magnesium is a mineral compound present in our metabolism, and its compounds has been presented as a viable alternative to tin catalyst in lactone polymerization, particularly the cyclic dimer of lactic acid (lactides) and glycolic acid (glycolide).12 In the literature, the first report on microwave-induced polymerization of D,L-lactide (DLLA) was performed by Li et al.13 In just 15 min of reaction, the authors obtained polymers with a weight average molar mass (Mw) of 6.7 × 104 g mol-1 with yield of 56.7%.13 Subsequently, Zhang et al.14 investigated the influence of microwave power on yield and Mw of poly(D,L-lactide) (PDLLA) obtained at different time intervals. Such results indicated that at low powers (170 and 255 W) of microwave irradiation, PDLLA remains stable, and at high powers (340 and 510 W) it is degraded. Reactions carried out under a power of 255 W generate polymers with Mw greater than 4 × 105 g mol-1 in 30 min of reaction. Two years later, Jing et al.15 analyzed the effect of monomer purity on microwave irradiated DLLA polymerizations. The researchers observed that the molar mass of PDLLA increased with increasing degree of purity of DLLA, obtaining Mw of 2.19 × 105 g mol-1 in 30 min of reaction. Nikolic et al.16 brought their contributions in the study of microwave-induced and tin octoate-catalyzed polymerization via ROP of DLLA. The researchers observed that in just 10 min of reaction, a polymer with Mw greater than 100,000 g mol-1 was obtained, reaching Mw greater than 200,000 g mol-1 with 30 min of reaction, with a yield of 89%. Interest in bioabsorbable polymers has increased in the last decades, being one of the most studied biomaterials the copolymer of lactide and glycolide, poly(lactide-co-glycolide) (PLGA). This copolymer has several applications in medicine, such as sutures, devices for controlled drug release and guided regeneration of tissues.17 Copolymerization is an important process since it can generate materials with great versatility of properties and performance. This variety of properties can be attained by manipulation of the relationship of comonomers and in the case of PLGA, depending on the proportion between lactide and glycolide, it is possible to control the biodegradation time in the body and, therefore, direct the material towards more specific applications.17 A previous work on the copolymerization of lactide and glycolide using microwave heating was reported by Li et al.18 The authors studied the ideal conditions for PLGA synthesis by microwave irradiation using tin octoate and concluded that reaction is successful when carried out at 120 ºC and a power of 300 W. It is worth mentioning that no investigation of PGA synthesis using microwaves was found in the literature. In addition, only the report of lactide-glycolide microwave assisted polymerization with standard tin octoate catalyst was found in the literature.18 So, this work presents as main novelty the microwave assisted bulk lactide-glycolide homo and copolymerization with magnesium lactate as initiator. It presents results and discusses the PLA microstructure, copolymer composition, and thermal properties of the polymers obtained from this initiator. As far as we are concerned, no similar investigation was previously reported.
EXPERIMENTAL Materials Preparation of homopolymers and copolymers were carried out using L-lactide (purity > 99%) and glycolide (purity > 99%) supplied from Corbion Purac (Netherlands). Magnesium lactate was synthesized from the reaction of 85% lactic acid supplied from Vetec (Brazil) and magnesium oxide supplied from Mallinckrodt-Quimis (Brazil). Preparation of homopolymers and copolymers Bulk lactide/glycolide homopolymerizations and copolymerizations catalyzed by magnesium lactate were carried out in a CEM Mars 6 model microwave reactor using 8 mL capacity glass vials under nitrogen inert environment. Polymers characterization 1H and 13C NMR spectra were recorded at 25 ºC using a Varian Mercury VX-300 NMR spectrometer and CDCl3 (D-chloroform) or CF3COOD (trifluoroacetic acid-D) as solvent. Gel permeation chromatography (GPC) was carried out on a Shimadzu LC 20 instrument equipped with a set of Shim-pack GPC-803 e Phenogel 5 µm linear and refractive index detector using chloroform as a solvent at a flow rate of 1.0 mL min-1. A calibration curve constructed of monodisperse polystyrene standards was used. Thermal properties were determined by differential scanning calorimetry (DSC) using a Hitashi 7020 calorimeter with a heating and cooling rate of 10 ºC min-1 from –10 to 220 ºC under N2 flow. Glass transition (Tg) and melting temperature (Tm) were obtained from a second heating after a quenching run. The degree of crystallinity (Xc) was determined using the following Equation:  where ∆Hm is the melting enthalpy and ∆Hmo is the standard melting enthalpy for 100% crystalline poly(lactic acid) (PLA) (106 J g-1). Thermogravimetric analyses (TGA) were performed on a TA TGA Q500 thermoanalyzer. Measurements were carried out under N2 at a heating rate of 10 ºC min-1 up to 700 ºC, with a gas flow rate of 20 mL min-1.
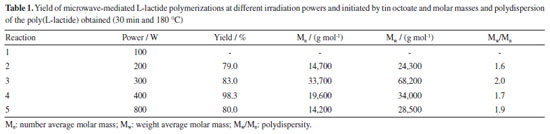
RESULTS AND DISCUSSION Determination of power of microwave irradiation for L-lactide polymerization For the determination of the adequate microwave power for the polymerization of L-lactide, the standard catalyst tin octoate was used at 180 ºC for 30 min. The reactions were performed at 4 different powers: 100, 200, 300, 400 and 800 W. According to the data presented in Table 1, it is possible to observe that, the higher the power, the greater the molar mass of the polymer, reaching a weight average molar mass (Mw) of 68,200 g mol-1 at 300 W. At higher powers (400 and 800 W), a decrease in molar mass begins. This decrease of Mw may be associated with the fact that when the polymer is subjected to high powers, a degradation process begins during the course of the reaction. In reaction 1, it was not possible to obtain a polymer using the power of 100 W for 30 min of microwave irradiation.
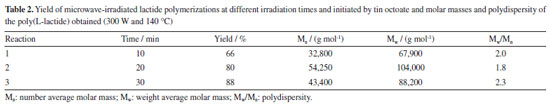
Determination of microwave irradiation time for L-lactide polymerization at 140 ºC Subsequently, reactions with the tin standard catalyst were carried out using a power of 300 W, since at this power the best results indicated in Table 1 were obtained. In the attempt to improve polymer molar mass, the reaction temperature was decreased to 140 ºC, varying the reaction time (10, 20 and 30 min). The results are shown in Table 2.
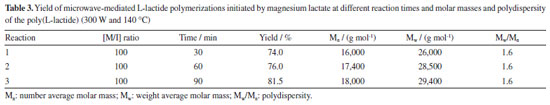
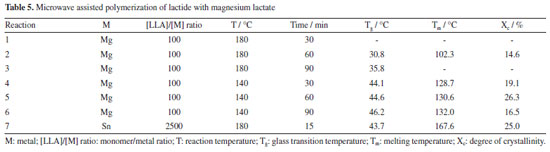
The results of Table 2 show that the molar mass of the polymer increases as the reaction time increases, reaching Mw equal to 104,000 g mol-1 in reactions with a time of 20 min. For the reaction time of 30 min, the molar mass of the polymer starts to decrease probably due to polymer degradation competition with polymerization process. Regarding the yields obtained in the polymerizations, it is possible to state that the increase in the polymerization time, increases the yield, reaching 88% in 30 min of reaction. Lactide polymerization initiated with magnesium lactate From the results presented in the previous experiments with the standard tin initiator, it was considered that the best conditions for polymerization of L-lactide with microwave irradiation are the power of 300 W at 140 ºC since the highest molar mass was attained. Thus, the microwave assisted synthesis of poly(L-lactide) (or poly(L-lactic acid)) from L-lactide (LLA) initiated by magnesium lactate was first carried out at molar ratio LA/Mg = 100. The results presented in Table 3 show that the reaction yield and molar mass increase with increasing reaction time. At 90 min reaction time and 140 ºC, 81.5% yield and Mw = 29,400 g mol-1 was attained. It is noteworthy that polydispersity remained unchanged.
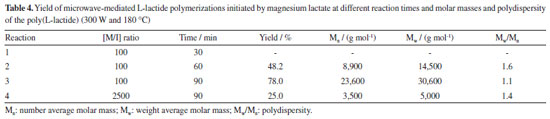
We also performed the reaction at high temperature (180 ºC) (Table 4). It is observed that with 90 min of reaction, yield was 78% and Mw 30,600 g mol-1, which is practically the same yield and Mw.
It is possible to note that for polymers synthetized in both temperatures, the higher the reaction time, the higher is the yield. When the polymerization was carried out at 180 ºC, the polymers presented liquid viscous aspect after precipitation and drying. The longer the reaction time, the polymers presented darker coloration. Polymers synthesized at 140 ºC presented solid structure and white coloration. Reactions initiated with magnesium lactate were also studied at the molar ratio monomer/initiator [M/I] = 2500 (standard ratio in tin-based catalysts) at both temperatures. When comparing the two [M/I] ratios (100 and 2500), it was possible to observe that the presence of highest amount of catalyst improved the yield and increases the resulting PLLA molar mass. The main transition temperature of PLLAs obtained at 140 and 180 ºC was attained by DSC analysis (Table 5). In general, the lactide homopolymers synthesized by microwave irradiation and magnesium lactate initiator showed low glass transition temperature (Tg) and crystalline melting temperature (Tm). This result may be related not only to low molar mass, but also to the less stereoregular structure of the polymers. This lower stereoregularity of the polymer chains can cause the formation of smaller or more imperfect crystals of lower melting temperature.19 From DSC analysis, in addition to the transitions, it was also possible to observe the occurrence of some endothermic events near Tg, which may be related to enthalpic relaxation phenomena, or to the evaporation of residual solvent resulted from reaction product recovery, still present in the polymer.
It is noteworthy that polymers produced at 140 ºC showed a significant increase in thermal transition temperatures (Tg and Tm), when compared to polymers synthesized at 180 ºC. According to the results of the thermogravimetric analysis (TGA), the lactide homopolymers showed low initial temperatures of degradation (Tonset), varying between 247 to 260 ºC. These low values observed may be related to low molar mass and structural irregularities caused by the racemization process that probably occurred during polymerization. In general, polymers synthesized at 140 ºC showed lower Tonset when compared to those of polymers produced at 180 ºC. The structure of the polylactides was evaluated by nuclear magnetic resonance (13C and 1H NMR), being possible to identify all the main signals that should appear for the poly(L-lactic acid) (PLLA). The 13C NMR spectra showed peaks with chemical shifts located at d 16.0, 69.0 and 169.0 ppm, which correspond to methyl (CH3), methyne (CH) and carbonyl (C=O), respectively. The characteristic signal of the solvent (deuterated chloroform) appears with high intensity in the spectrum at around 77.0 ppm. The 1H NMR spectra of all lactide homopolymers show characteristic signals of PLLA attributed to methyne (CH) and methyl (CH3) groups, located at d 5.15 and 1.5 ppm, respectively, in addition to the typical solvent peak located around d 7.3 ppm. We have used the region of the carbonyl group signal in the 13C NMR spectra at around 169.5 ppm to investigate the differences in the microstructure of PLLA prepared by these polymerizations initiated by magnesium lactate at different reaction condition. At this region of the spectrum (Figure 1), the intense signal is attributed to the expected isotactic configurational meso tetrad sequences (mmm) since polymerization of L-lactide isomer as monomer would produce a completely isotactic polymer.12
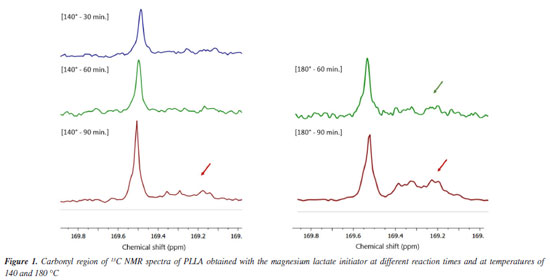 Figure 1. Carbonyl region of 13C NMR spectra of PLLA obtained with the magnesium lactate initiator at different reaction times and at temperatures of 140 and 180 °C
However, depending on the reaction condition, the spectra showed side peaks. Presence of these side peaks indicates stereo errors in the PLLA chemical structure, which may have been originated from racemization processes during the Mg-catalyzed polymerization. These processes cause an inversion of the L-configuration and introduces units with D-configuration, decreasing the structural regularity of these polymers.10,12 Table 6 summarizes the polymerization conditions and the percentage of tetrads mmm (%mmm) of the polymers. The percentage of mmm sequences was determined by the ratio between the signal at d 169.5 ppm and the total area of signals corresponding to the carbonyl group, which represents the sum of all tetrad sequences. Considering the values of %mmm, 100% would correspond to a completely stereoregular isotactic polymer. Lower values mean loss of chain stereoregularity caused by racemization effects.
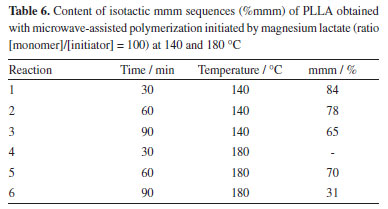
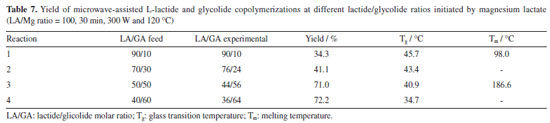
The results show that, at both temperatures (140 and 180 ºC), the increase in the reaction time, increases the racemization effects, since the %mmm decreases. It is noteworthy that, comparatively, polymers synthesized at 140 ºC showed highest %mmm, what mean a lower content of stereo errors, probably due to a lower intensity of the racemization reactions at this temperature. Glycolide polymerization Microwave-assisted synthesis of poly(glycolic acid) (PGA) by ROP using glycolide (GA) as monomer and magnesium lactate as initiator was also performed. Polymerization was carried out at 120 ºC using a reaction time of 30 min. A yield of 90.1% was obtained. DSC curves of the glycolide homopolymer synthesized by the initiation with magnesium lactate showed a high glass transition temperature (Tg = 43.1 ºC), indicating that the material has high crystallinity, since it can increase the temperature of relaxation of the amorphous phase close to the crystals.20 An exothermic peak at 101.1 ºC was also observed and attributed to crystallization during heating which can take place after the glass transition temperature, called cold crystallization (Tcc). It also shows an endothermic peak of crystalline melting (Tm) at 220 ºC, in agreement with the mentioned authors. Thermogravimetric analysis (TGA) of the PGA showed a low initial degradation temperature (Tonset = 171 ºC), which may be probably related to the low molar mass of the polymer. 1H NMR spectrum of this PGA obtained with the magnesium lactate initiator presented the expected signal of PGA methylenes at d 5.5 ppm and other signals, one around 2.0 ppm and several one in the range d 4.9-5.40 ppm.21 These signals may be related to glycolic acid formed by the decomposition of the glycolide monomer and polymer, as well as residues of the initiator and may indicate that the solvent used in the NMR analyses (CF3COOD) may have degraded part of the PGA before analysis due to the high acidity. The 1H NMR spectrum of PGA is available in supplementary material. Lactide-glycolide copolymerization Synthesis of poly(lactic acid-co-glycolic acid) (PLGA) from magnesium lactate-initiated microwave assisted ROP in different LLA-GA molar ratio was carried at 120 ºC and a reaction time of 20 min. Copolymers with yields varying between 24 and 72.2% were successful obtained. Table 7 presents the results of copolymer composition and copolymer's thermal transitions (Tg and Tm) and shows that the copolymer's composition is near to the values of monomer's feed ratio.
It is interesting to note that, in this reaction conditions, yield increases as the glycolide content increases in the feed. This behavior may be attributed to the higher reactivity of glycolide in comparison with lactide. It is also important to mention that the L-lactide-glycolide copolymers presented Tg values in agreement with Li et al.18 Tg slightly decrease as the glycolide content increase in the copolymer. From DSC analyses, we could also observe that the copolymers presented a crystalline melting event, suggesting that LLA and GA blocks may be present in the copolymer structure. These blocks can crystallize, with crystals presenting different Tm, depending on the predominance of these two different blocks. In fact, only in the composition LA/GA 90/10 and 50/50 was possible to observe such a transition. For comparison purposes, the literature reports that the Tg of PLLA and PGA are 55 and 35 ºC, respectively.22 The 1H NMR and 13C NMR spectra of PLGA 50/50 and 70:30 are available in supplementary material. We observed that the increase in the glycolide content in the copolymer increases it coloration to dark brown indicating oxidation. Although the presence of higher levels of glycolide in the feed increases the conversion, it makes the reaction much more exothermic, initiating degradation processes. It is noteworthy the impossibility of carrying out the reaction at lower temperatures, since the melting point of the initiator is 118 ºC, and any reaction temperature below that used (120 ºC) could result in a reaction in a heterogeneous medium. Copolymerizations of L-lactide and glycolide were also carried out at higher temperatures (140, 160 and 180 ºC). However, the reactions resulted in very low yields and the PLGA copolymers was recovered as a viscous liquid with dark coloration, indicating degradation. In almost all attempts of polymerizing the monomers at higher temperatures, the reactions became so exothermic that they caused cracking and/or breaking of the vials used in the reactions.
CONCLUSIONS According to the results discussed, we can conclude that it is possible to obtain PLLA, PGA and PLGA by microwave irradiation using magnesium lactate as initiator. The ideal conditions for polymerization of L-lactide irradiated by microwaves are: power of 300 W, reaction temperature of 140 ºC, with a reaction time of 20 min for reactions initiated by tin octoate and 30 min for reactions initiated by magnesium lactate. Microwave-assisted polymerizations have high efficiency when compared to thermal heating polymerization. Magnesium lactate initiator proved to be an efficient initiator in LA polymerizations irradiated by microwaves, producing polymers with Mw in the order of 30,000 g mol-1 and 70% yield. Polyglycolides can also be produced in high yields (90%) in 20 min of reaction. Copolymers of L-lactide/glycolide with yields ranging from 34 and 72% was also obtained. Studies on the microstructures of PLLAs obtained from microwave irradiation and initiated by magnesium lactate showed that the PLA homopolymers presented an intense racemization process.
SUPPLEMENTARY MATERIAL Some images used in this work are available in http://quimicanova.sbq.org.br, as PDF file, with free access.
ACKNOWLEDGMENTS Authors thank the Brazilian Agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (Grant 307364/2018-6) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, FAPERJ (Grants E-26/202.538/2019 and E-26/200.359/2023).
REFERENCES 1. Song, J. H.; Murphy, R. J.; Narayan, R.; Davies, G. B. H.; Philos. Trans. R. Soc., B 2009, 365, 1526. [Crossref] 2. Idris, S. B.; Arvidson, K.; Plikk, P.; Finne-Wistrand, A.; Albertsson, A. C.; Bolstad, A. I.; Mustafa, K.; J. Biomed. Mater. Res., Part A 2010, 94, 631. [Crossref] 3. Nuyken, O.; Pask, S. D.; Polymers 2013, 5, 361. [Crossref] 4. Xia, Y.; Zhao, J.; Polymer 2018, 143, 43. [Crossref] 5. Zhang, C.; Liao, L.; Gong, S.; J. Appl. Polym. Sci. 2008, 110, 1236. [Crossref] 6. Gotelli, G. A.; Bonelli, P.; Abraham, G. A.; Sosnik, A.; J. Appl. Polym. Sci. 2011, 12, 1321. [Crossref] 7. Kempe, K.; Becer, C.; Schubert, U.; Macromolecules 2011, 44, 5825. [Crossref] 8. Saxena, K.; Chandra, U. In MicrowaveHaeting; Chandra, U., ed.; IntechOpen: London, 2011, ch. 1. [Crossref] 9. Sanseverino, A. M.; Quim. Nova 2002, 25, 660. [Crossref] 10. Kricheldorf, H. R.; Lee, S. R.; Polymer 1995, 36, 2995. [Crossref] 11. Wei, Z.; Liu, L.; Qu, C.; Qi, M.; Polymer 2009, 50, 1423. [Crossref] 12. Silvino, A. C.; Correia, P. S.; Dias, M. L.; J. Appl. Polym. Sci. 2014, 131, 40771. [Crossref] 13. Li, L.; Zhang, C.; Liao, L.; Wang, X.; Zhuo, R.; Chin. Chem. Lett. 2001, 12, 663. [Link] accessed in March 2024 14. Zhang, C.; Liao, L. Q.; Liu, L. J.; Macromol. Rapid Commun. 2004, 25, 1402. [Crossref] 15. Jing, S.; Peng, W.; Tong, Z.; Baoxiu, Z.; J. Appl. Polym. Sci. 2006, 100, 2244. [Crossref] 16. Nikolic, L.; Ristic, I.; Adnadjevic, B.; Nikolic, V.; Jovanovic, J.; Stankovic, M.; Sensors 2010, 10, 5063. [Crossref] 17. Makadia, K.; Siegel, S.; Polymers 2011, 3, 1377. [Crossref] 18. Li, G.; Zhao, N.; Bai, W.; Chen, D.; Xiong, C.; e-Polym. 2010, 10, 1. [Crossref] 19. Dias, M. L.; Palermo, L. C.; Silvino, A. S.; Macromol. Symp. 2011, 299, 156. [Crossref] 20. Gilding, K.; Reed, M.; Polymer 1979, 20, 1459. [Crossref] 21. Erbetta, C.; Alves, R.; Resende, J.; Freitas, R.; Sousa, R.; J. Biomater. Nanobiotechnol. 2012, 3, 208. [Crossref] 22. Velde, K.; Kiekens, P.; Polym. Test. 2011, 21, 433. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access