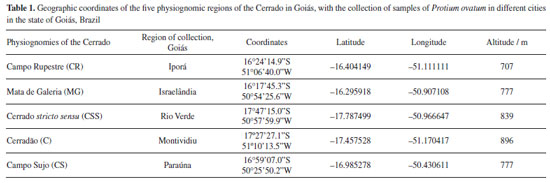
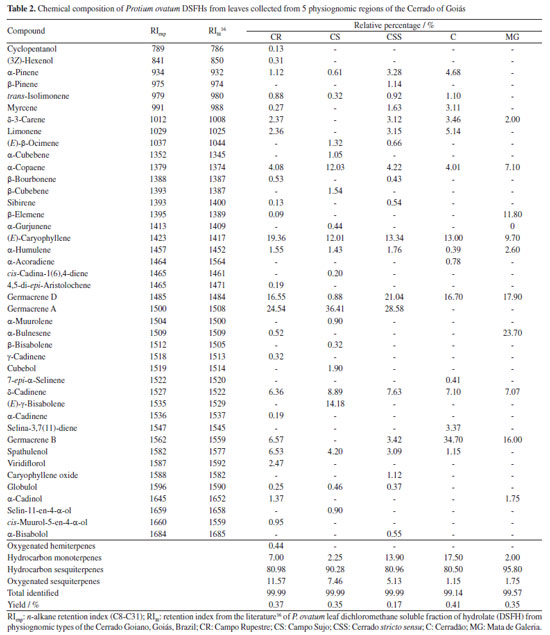
Artigo
| Composition, antioxidant capacity and antimicrobial activity of volatile organic compounds of Protium ovatum Eng. from different regions in Brazilian Savannah |
|
Wendel C. de SousaI; Josemar G. Oliveira FilhoI; Alana K. PereiraII; Samara S. da SilvaIII; Giulia KassabIV; Marcela ChristofoliI; Cassia C. F. AlvesI; Andreia P. MatosV; Moacir Rossi ForimII; Cristiane de M. CazalVI,*
I. Instituto Federal de Educação, Ciência e Tecnologia Goiano, 75901-970 Rio Verde - GO, Brasil Received: 11/02/2023 *e-mail: cristiane.cazal@ifsudestemg.edu.br Protium ovatum Eng. Burseraceae is an endemic species in Brazil used in traditional medicine. This study investigated the chemical variation and the in vitro antioxidant and antimicrobial properties of dichloromethane soluble fraction of hydrolate (DSFH) from leaves of P. ovatum collected from five different vegetation physiognomies within the Cerrado Goiano. The region was characterized based on the DSFH chemical composition by gas chromatography coupled to mass spectrometry (GC-MS) analysis. Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were performed to compare the different DSFHs from five Savannah regions in Brazil. The DSFHs from the Cerrado stricto sensu (CSS), Mata de Galeria (MG) and Cerradão (C) regions were grouped in the negative part of PC1, showing high similarity. Samples from the C and MG regions showed antioxidant activity of IC50 = 0.98 and 0.78 mg mL-1, respectively, and 0.250 mg mL-1 of butylated hydroxytoluene (positive control). DSFH from different regions exhibited antifungal activity against Candida albicans. The results show that the chemical composition and the biological activity of DSFHs can be affected according to the area of collection. INTRODUCTION The Cerrado is the second largest domain in South America. It covers approximately 22% of the Brazilian territory, distributed throughout 15 states. It is comprised of a complex diversity of organisms highly adapted to the climax and its subdivisions, the vegetation physiognomies Cerrado lato sensu and Cerrado strictosensu, Mata de Galeria, Veredas, Cerradão, Campo, Campo Pantanoso, Campo Sujo, Cerrado Rupestre and Campo Limpo.1 The Cerrado vegetation developed a series of adaptive strategies of survival that can change the chemical composition and the concentration of secondary metabolites in vegetative organs.2 Essential oils (EOs) are complex hydrophobic mixtures derived from the secondary metabolism in different plant organs.3 Because of the diversity of volatile organic compounds (VOCs), EOs exhibit such biological properties as antioxidant, antibacterial, antiparasitic, antifungal, anthelmintic, antiseptic and antispasmodic activities.4-6 VOCs can prevent or minimize health problems caused by free radicals and infectious diseases and may be an alternative to synthetic antimicrobials. However, VOCs are susceptible to geological changes that may alter the quality and the quantity of the metabolites produced, depending on the region of collection.7-10 The Burseraceae family is known for its oil-producing potential. It comprises 17 genera, among which Bursera, Commiphora, Protium and Canarium are widely distributed throughout the pantropical regions of the world. In Brazil, 7 genera and 74 species have been described, of which 14 are endemic.11 The genus Protium is the best known of the Burseraceae family. Protium ovatum. Engl., is commonly known as "vick-do-cerrado" and can be found in the Cerrado and the Amazon.12,13 Studies on EOs from P. ovatum leaf have showed potential against Trypanosoma cruzi and Leishmania amazonensis and moderate cell toxicity for the epithelial cell line LLCMK2.6 Previously, Souza et al.14 analyzed the essential oils (EOs) of different shoot organs from P. ovatum including stems, petioles, leaves, flowers and ripe and unripe fruits, evaluating and investigating their antifungal potential against Sclerotinea sclerotiorum. Among the constituents common to all shoot parts, α-pinene (0.80-18.3%), β-pinene (0.58-5.17%), myrcene (0.52-27.3%), and limonene (3.15-59.7%) were most prevalent. The essential oil from ripe fruit showed the strongest antifungal activity, with the highest inhibition of mycelial growth (IMG) (50.11%) at the lowest concentration assayed (18.75 µg mL-1). Therefore, this study aimed to evaluate possible chemical variations of dichloromethane soluble fraction of hydrolate (DSFH) sextracted from the leaves of P. ovatum collected in five different vegetation physiognomies [Cerrado strictosensu (CSS), Mata de Galeria (MG), Cerradão (C), Campo Sujo (CS), Campo Rupestre (CR)] of the Brazilian Cerrado and to analyze their GC-MS (gas chromatography coupled to mass spectrometry) chromatographic profile. The DSFHs were tested against the gram-positive and gram-negative bacteria Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 8739), respectively, and the yeast Candida albicans (ATCC 90028). This is the first chemical monitoring for this species. Chemometric tools were used to determine the specific compounds of each physiognomy of the Brazilian Cerrado and its correlation with antimicrobial and antioxidant activities.
EXPERIMENTAL Plant materials Leaves of P. ovatum were collected from five different regions characterized by the Cerrado physiognomy in the State of Goiás, Brazil, in June 2017 (Table 1). The identification was made by the biologist Erika Virgínia Estefâne de Jesus Amaral. Plant specimens were deposited in the Herbarium of the Instituto Federal de Educação, Ciência e Tecnologia Goiano, Campus Rio Verde, accession number 628.
Extraction of essential oils by hydrodistillation The EOs of P. ovatum were obtained from freshly collected plants from five different regions separately, between 7 and 10 am. Then, the fresh leaves were selected (100 g), mixed in 500 mL of distilled water and hydro-distilled in a Clevenger-type apparatus for 3 h. The hydrolate from each plant was subjected to a liquid-liquid partition with three 10 mL portions of dichloromethane. Anhydrous sodium sulfate (Na2SO4) was used to remove traces of water. The mixture was filtered and the dichloromethane was evaporated using a rotary evaporator. Then, the DSFH was stored in an amber glass bottle protected from light, sealed and kept at an average temperature of 4 ºC until analysis. The average essential oil yield (m/m %) was calculated based on fresh plant matter as described by Zhang et al.15 DSFH analysis A gas chromatography system coupled to a sequential mass spectrometer (GC-MS/MS), sample injector (Combi PAL AOC-5000), fused silica capillary column Restek Rtx-5 ms (30 m × 0.250 mm × 0.25 µm), sequential mass spectrometer (MSTQ8040 Shimadzu) and electronic impact ionization (IE) detector (70 eV) analyzed the chemical constituents of P. ovatum DSFHs. The initial column temperature was maintained at 60 ºC for 3.0 min, programmed to rise to 200 ºC at 3 ºC min-1 and then to rise 15 ºC min-1 to a maximum temperature of 280 ºC, remaining at this temperature for another 1.0 min. Injector and detector temperatures were 230 and 300 ºC, respectively. Helium was used as carrier gas with an injection pressure of 57.4 kPa, splitless ratio of 150, mass spectrometer detection range of m/z 43-550, start time (solvent cut time) of 3.0 min and flow rate of 3 mL min-1. The identification of essential oil components was based on the linear retention index (Kovats index, KI), which was calculated using an n-alkane homologous series retention data (C-07 to C-40), and on the fragmentation pattern observed in the mass spectra, by comparing them with the literature16 and the Nist® 11 spectro library. Multivariate data analysis component (PCA) and hierarchical cluster analysis (HCA) Multivariate data analysis was carried out to test whether there are differences in the chemical constituents of the DSFHs of P. ovatum leaves collected from different physiognomic regions of the Cerrado in the State of Goiás. Initially, the principal component analysis (PCA) was performed to optimize the separation between groups and verify the most relevant characteristics of the DSFHs samples. Partial least squares-discriminant analysis (PLS-DA), hierarchical cluster analysis (HCA) and heatmap were also performed. Data were entered in a Microsoft Excel spreadsheet, csv format, and analyzed using the MetaboAnalyst 5.0 online server.17 The original data were normalized using auto-scaling. DPPH radical scavenging activity The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity was determined according to the method described by Nguyen et al.18 adapted to the microplate reader. A 100 µL aliquot of each sample was mixed with 100 µL of 2.0 × 10-1 mM DPPH solution. The concentrations of DSFH in methanol evaluated were: 14; 7; 3.5; 1.5; 7.5 × 10-1; 3.7 × 10-1; 1.8 × 10-1 and 0.9 × 10-1 mg mL-1. The mixture was incubated at room temperature in the dark for 30 min and the reduction of DPPH radicals was quantified using a UV-Visible spectrophotometer (Versa Max Tunable ™ Molecular Devices, Sunnyvale, USA) at 515 nm. The antioxidant butylated hydroxytoluene (BHT) was purchased from Sigma Inc. (St. Louis, USA) and used as a positive control. BHT is a synthetic antioxidant widely used to preserve the stability of food and pharmaceutical products.19 For this reason, it was chosen as the positive control in this study. The assay was performed in triplicate. The antioxidant activity (AAO) was expressed as percentage (%) of inhibition and calculated with the Equation 1: AAO (%) = [Abassay ctl - (Abssample - Abssample ctl) / Absassay ctl] × 100 (1)
where: AAO is the antioxidant activity, Absassay ctl is the control assay absorbance, Abssample is the sample absorbance and Abssample ctl is the sample control absorbance. The AAO results were expressed based on the IC50, considering the concentration capable of scavenging 50% of free radicals calculated from the percentage of inhibition (Equation 1) against the EO concentration using the Origin®8.0 software.20 Antimicrobial activity The P. ovatum DSFHs activity was evaluated against the gram-positive and gram-negative bacteria Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 8739), respectively, and the yeast Candida albicans (ATCC 90028), using the disk diffusion method.21 The initial analysis of the activity was performed with a mixture of DSFHs from the vegetation physiognomic regions of the Cerrado (CSS, CR, C and MG). Briefly, suspensions of each microorganism evaluated, containing about 108 colony-forming units per mL, were spread on plates containing solid brain-heart infusion agar medium (Neogen Culture Media, USA). Filter paper discs of 4.2 mm in diameter were soaked with the mixture of DSFHs, negative controls with buffered saline solution at pH 7.4, or positive controls with the antibiotics penicillin and streptomycin (10,000 IU mL-1 and 10 mg mL-1, respectively) in the case of bacteria, or the antifungal amphotericin B (1.0 mg mL-1) in the case of yeast. Three discs of the same group were placed in each inoculated plate. The plates were incubated for 24 h at 37 ºC and then, the inhibition zones were measured. In the case of C. albicans, for which the DSFH was proven to be active, a second experiment was carried out with the DSFHs extracted individually from the regions of CSS, CR, C and MG, following the same protocol. The experiments were carried out in triplicate on two separate occasions, totaling 6 discs per group. Statistical analysis The evaluations of DSFHs yield and antioxidant activity were performed in triplicate; biological assays were performed in sextuplicate; results were compared by multivariate ANOVA and Tukey's post-hoc test using GraphPad Prism 8.0,22 with a 95% confidence level.
RESULTS AND DISCUSSION The yield of DSFHs leaves of P. ovatum from the CSS region differed significantly from the other physiognomies investigated (average yield of 0.17 ± 0.06%) (Figure 1). The oil essential from P. ovatum leaves collected in the Cerrado region at the Universidade de Rio Verde (UniRV), in Rio Verde, Goiás, Brazil (17º 47'53"S and 50º 55'41" W) in July 2015 yielded 0.3%,6 which is higher than the percentage found in this study developed with the same species (physiognomie CSS). The other physiognomies showed higher performance than previously reported.6 The variations of yield in DSFH may be related to edaphic factors such as geomorphological formations and soil fertility, which vary according to the vegetation physiognomy.23
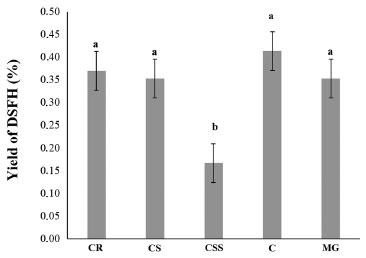 Figure 1. Average yield of Protium ovatum leaf dichloromethane soluble fraction of hydrolate (DSFH) from physiognomic types of the Cerrado of Goiás, Brazil. CR: Campo Rupestre; CS: Campo Sujo; CSS: Cerrado stricto sensu; C: Cerradão; MG: Mata de Galeria. Bars followed by the same letters are not significantly different by Tukey's test at 5% probability
Figure 2 shows the chromatographic profile of the DSFHs from the leaves of P. ovatum collected from different physiognomies of the Brazilian Cerrado. Table 2 shows their chemical composition. Forty-five compounds were identified, including oxygenated hemiterpenes (0.44%), hydrocarbon monoterpenes (2.00-17.50%), hydrocarbon sesquiterpenes (80.50-95.80%) and oxygenated sesquiterpenes (1.15-11.57%). Souza et al.14 showed that the essential oil extracted from the leaves of P. ovatum consisted predominantly of sesquiterpenes, which accounted 81.0% of their total chemical composition, however, only 20 compounds were identified.
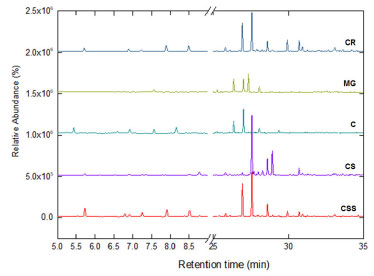 Figure 2. GC-MS chromatogram of chemical constituents of Protium ovatum leaf dichloromethane soluble fraction of hydrolate (DSFH) from physiognomic types of the Cerrado of Goiás, Brazil. CR: Campo Rupestre; CS: Campo Sujo; CSS: Cerrado stricto sensu; C: Cerradão; MG: Mata de Galeria
Significant chemical variation was found between DSFHs from P. ovatum leaves collected from the different sites. The compounds α-copaene (4.01-12.03%), (E)-caryophyllene (9.70-19.36%), α-humulene (0.39-2.60%), germacrene D (0.88-21.04%) and δ-cadinene (6.36-8.89%) were frequently found in the samples from the different locations. These compounds can also be found in essential oils of other species of the genus Protium in different geographic regions. Estevam et al.6 identified spathulenol (17.6%), caryophyllene oxide (16.4%), β-caryophyllene (14.0%) and myrcene (8.4%) in the essential oil of P. ovatum from leaves collected in the Cerrado region at the Universidade do Rio Verde (UniRV), in Rio Verde, Goiás, Brazil. The EOs of species in this genus cultivated in the Amazon and sold in a popular market consisted mainly of p-cymene (with concentrations ranging from 21.9 to 51.9%), α-pinene (22.7%), β-phellandrene, α-phellandrene, β-pinene, trans-dihydro-α-terpineol, α-terpineol and α-terpinene.24 Zoghbi et al.25 investigated the chemical composition of EOs from different species of Protium collected from forests in the Amazon and identified the following major compounds: α-pinene (78.6%) in P. decandrum; α-pinene (31.7%), p-cymene (31.2%), and α-phellandrene (24.1%) in P. pilosum; sabinene (56.3%) in P. spruceanum. The resins extracted from P. strumosum and P. altsonii showed a different pattern of essential oil composition. Limonene (75.5%) was the major compound of P. strumosum resin, however, p-cymene (31.5%) and trans-dihydro-α-terpineol (25.8%) are the major components found in the resin of P. altsonii. It is possible to observe that the chemical composition of DSFHs may vary widely depending on both genetic and environmental features such as temperature, soil nutrient level, sun exposure and relative humidity. This variability is very common when the constituents, such as monoterpenes and sesquiterpenes, are volatile.6,24,25 In the principal component analysis (PCA) biplots, PC1 and PC2 account for 66.7% of the variance between the samples collected in different regions (Figure 3). This multivariate statistical analysis indicates the variables (loadings) correlated to the samples (scores) clustering similar samples. The essential oils from the CSS (Cerrado stricto sensu), MG (Mata de Galeria) and C (Cerradão) regions were grouped in the negative part of PC1, showing high similarity. These essential oils also correlated with germacrene D and B, limonene, α-pinen and trans-isolimonene, whereas the DSFH from the C region correlated with germacrene B, myrcene and α-acoradiene. The DSFH from CS (Campo Sujo) were richer in selin-11-en-4α-ol, (E)-γ-bisabolene, β-bisabolene, cubebol, β-cubebene. The compounds viridiflorol, α-cadinene, 4,5-di-epi-aristolochene, (3Z)-hexenol, cyclopentanol, cis-muurol-5-en-4-α-ol and γ-cadinene were found only in the CR (Campo Rupestre) region. Metabolites that were found in only one of the samples of DSFHs can be used as biomarkers for the regions assessed. Other authors have used principal component analysis (PCA) to identify possible relationships in essential oils. Carvajal et al.26 used PCA to identify possible relationships between volatile components and the origin zone or sampling month of essential oils of Protium colombianum. Alolga et al.27 used unsupervised PCA and supervised PLS-DA to observe the differences between the essential oils from Xylopia aethiopica fruits, in terms of metabolite levels, collected in Ghana and Nigeria.
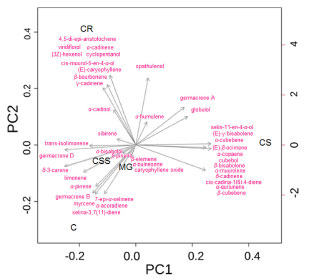 Figure 3. Biplot of principal component analysis (PCA) of Protium ovatum leaf dichloromethane soluble fraction of hydrolate (DSFH) from physiognomic types of the Cerrado of Goiás, Brazil. CR: Campo Rupestre; CS: Campo Sujo; CSS: Cerrado stricto sensu; C: Cerradão; MG: Mata de Galeria. The scores characterize the different physiognomic regions of the vegetation studied. The loadings represent the chemical compounds of the DSFH analyzed by GC-MS
Permutation tests (p < 0.01) were used to validate the partial least-square (discriminant analysis) (PLS-DA), with both R2 and Q2 ~ 0.9. The variable importance projection (VIP) was used to list the compounds and to correlate them with the area of collection in the Heatmap. The significant VIPs were organized in the Heatmap, showing the distribution of compounds in each essential oil (Figure 4). At the top of the map, the samples were organized by similarity using the Euclidean distance and Ward's method as the clustering algorithm in the hierarchical cluster analysis (HCA). The resulting dendrogram shows the formation of three groups of similarity between the chemical constituents. The DSFHs from the regions MG, C and CSS are closer to each other, as in the PCAs, while the DSFHs from CS and CR were more distant. The significant metabolites selected by PLS-DA can be used as biomarkers to classify EOs. This work suggests that the quality and quantity of chemical compounds of P. ovatum DSFHs vary with the different regions of the Brazilian Cerrado. Previous studies have shown that variations in environmental, geological and physiological factors can explain such differences.9,28,29
Figure 4. Heatmap of the samples (dendrogram of the hierarchical cluster analysis, HCA) at the top using the Euclidean distance and Ward clustering algorithm, representing the chemical similarities of Protium ovatum leaf dichloromethane soluble fraction of hydrolate (DSFH) from physiognomic types of the Cerrado of Goiás, Brazil. CR: Campo Rupestre; CS: Campo Sujo; CSS: Cerrado stricto sensu; C: Cerradão; MG: Mata de Galeria. The VIPs compounds (from PLS-DA) were selected. The plot shows the variation of compounds in different regions: the red color represents more intense in the sample and the blue color, when less intense or absent
The DSFHs of P. ovatum presented antioxidant potential with IC50 values ranging from 0.58 to 0.98 mg mL-1 (data not shown). The best result was obtained for the DSFH of CR with IC50 = 0.58 mg mL-1. The antioxidant activity of the DSFH of P. ovatum may be associated with a high content of sesquiterpenes (Table 2). Similar results were presented for the essential oil from the resin of P. Heptaphyllum.30 Antioxidant compounds can delay or inhibit the oxidation of an oxidizable substrate, producing toxic compounds such as ketones, aldehydes, hydrocarbons and alcohols.31 Mancini et al.32 state that the antioxidant activity of essential oils composed of phenolic monoterpenes and sesquiterpenes is due to their ability to donate hydrogen. The mechanism of autoxidation of non-phenolic terpenes, especially the unsaturated, is a reaction of the abstraction of hydrogen atoms by peroxyl radicals (ROO·) or by the addition of ROO· to an unsaturated carbon.31 However, the antioxidant activity of volatile oils is commonly attributed to the synergy between all constituents, not one or two separately.33 This study shows that the DSFH of P. ovatum contains a significant group of unsaturated monoterpenes and sesquiterpenes with high antioxidant potential when acting in synergy. Table 3 presents the inhibition zone diameters produced by the DSFHs against gram-positive (S. aureus) and gram-negative (E. coli) bacteria, and yeast (C. albicans). The mixed sample of DSFHs obtained from the different vegetation physiognomies showed no significant activity against S. aureus and E. coli; thus, they were not analyzed separately. The mixture inhibited the growth of the yeast C. albicans; as also, each of the DSFH separately showed activity corresponding to one third of the inhibition by the positive control (amphotericin B). The post hoc Tukey test showed that there was no significant difference between the DSFHs from the leaves of P. ovatum collected from the different physiognomies. These results corroborate the results presented by da Silva et al.,24 in which the essential oils of Protium spp. samples of popular Amazonian markets did not show antimicrobial activity against S. aureus and E. coli. These authors suggest that the antimicrobial activity of some essential oils is related to high concentrations of phenolic compounds, such as phenylpropanoids (eugenol, methyl eugenol and 4-ethyl-guaiacol) and phenolic monoterpenes (thymol and carvacrol), which were not detected in the essential oils used in this study.
Dichloromethane soluble fraction of hydrolate (DSFH) from the four Cerrado physiognomies showed fungicide activity against C. albicans, with an inhibition zone of 6.0 to 7.0 mm. The best result was presented for the mixture of oils OEH (7.5 mm) (Table 3). These results corroborate the data presented by Carvajal et al.,26 where essential oils of P. colombianum showed cytotoxicity against Fusarium isolates. The antifungal and antimicrobial properties of EOs from aromatic plants have, in some cases, been explained in terms of their main constituents, but in other cases, it was found that the activity of the EO cannot be easily correlated with any individual component, but by a mixture of compounds, which reflect the higher antimicrobial activity of the whole EO than the major constituents do when tested separately.33 The lipophilic character of the EOs is directly related to the antimicrobial action.34 According to Oliveira et al.,35 essential oils modify the permeability of the fungal cell membrane, allowing solutes into the cell and affecting its osmotic balance. This process can lead to decreased cytoplasm content and loss of important ions and proteins, ultimately causing cell damage and death.36 Essential oils of trees from the genus Boswellia (Burseraceae) were analyzed by gas chromatography coupled with mass spectrometry. The components identified and their range in the oils include α-pinene (2.0-64.7%), α-thujene (0.3-52.4%), β-pinene (0.3-13.1%), myrcene (1.1-22.4%), sabinene (0.5-7.0%), limonene (1.3-20.4%), p-cymene (2.7-16.9%) and β-caryophyllene (0.1-10.5%). The antimicrobial activity (minimum inhibition concentration assay) of the oils was investigated against five reference test organisms and the activity ranged from 4-16 mg mL-1 (S. aureus); 1.5-8.3 mg mL-1 (Bacillus cereus); 4.0-12.0 mg mL-1 (E. coli); 2.0-12.8 mg mL-1 (Proteus vulgaris) and 5.3-12.0 mg mL-1 (C. albicans).37 These results corroborate those presented for C.albicans; however, the essential oils were not tested separately for S. aureus so that the comparison could be made. Therefore, this study shows promising results for the antioxidant and antifungal potential of the DSFH of P. ovatum.
CONCLUSIONS The results show that the different physiognomic regions affect the yield, chemical composition and oxidant activity of DSFHs extracted from leaves of P. ovatum. A significant variation was found in the chemical composition between the regions. Thus, genetic and environmental factors need to be considered to ensure the quality of the DSFH chemical composition. Additionally, DSFHs from different vegetation physiognomies of the Brazilian Cerrado exhibited antioxidant and fungicidal activity against C. albicans.
ACKNOWLEDGMENTS The authors thank FAPEG, FAPEMIG, FAPESP, CNPq, CAPES, and IF GOIANO - Campus Rio Verde for the financial support.
REFERENCES 1. Gottsberger, G.; Gottsberger, I. S.; Acta Botanica Brasilica 2018, 32, 434. [Crossref] 2. Oliveira, L. G. S.; Ribeiro, D. A.; Saraiva, M. E.; Macêdo, D. G.; Macedo, J. G. F.; Pinheiro, P. G.; Costa, J. G. M.; Souza, M. M.; Menezes, I. R. A.; Ind. Crops Prod. 2017, 97, 455. [Crossref] 3. Hyldgaard, M.; Mygind, T.; Meyer, R. L.; Frontiers in Microbiology 2012, 3, 1. [Crossref] 4. Alencar Filho, J. M. T.; Araújo, L. C.; Oliveira, A. P.; Guimarães, A. L.; Pacheco, A. G. M.; Silva, F. S.; Cavalcanti, L. S.; Lucchese, A. M.; Almeida, J. R. S.; Araújo, E. C. C.; Rev. Bras. Farmacogn. 2017, 27, 440. [Crossref] 5. Ud-Daula, A. F. M. S.; Demirci, F.; Salim, K. A.; Demirci, B.; Lim, L. B. L.; Baser, K. H.; Ahmad, N.; Ind. Crops Prod. 2016, 84, 189. [Crossref] 6. Estevam, E. B. B.; Deus, I. P. B.; Silva, V. P.; Silva, E. A. J.; Alves, C. C. F.; Alves, J. M.; Cazal, C. M.; Magalhães, L. G.; Pagotti, M. C.; Esperandim, V. R.; Souza A. F.; Miranda, M. L. D.; An. Acad. Bras. Cienc. 2017, 89, 3005. [Crossref] 7. Cutro, A. C.; Castelli, M. V.; López, S. N.; Rosales, M. A.; Hollmann, A.; Rodriguez, A. S.; Nat. Prod. Res. 2021, 35, 2931. [Crossref] 8. Olmedo, R. H.; Asensio, C. M.; Grosso, N. R.; Ind. Crops Prod. 2015, 69, 21. [Crossref] 9. Tohidi, B.; Rahimmalek, M.; Arzani, A.; Food Chem. 2017, 220, 153. [Crossref] 10. El-Jalel, L. F. A.; Elkady, W. M.; Gonaid, M. H.; El-Gareeb, K. A.; Future Journal of Pharmaceutical Sciences 2018, 4, 156. [Crossref] 11. Rosalem, P. F.; Picão, T. B.; Rodrigues-Lisoni, F. C.; Martins, A. R.; Rev. Bras. Farmacogn. 2017, 27, 673. [Crossref] 12. Castelo, A. V. M.; Del Menezzi, C. H. S.; Resck, I. S.; Cerne 2010, 16, 573. [Crossref] 13. Flora do Brasil, https://floradobrasil2015.jbrj.gov.br/FB81476, accessed in May 2024 14. Souza, W. C.; Oliveira Filho, J. G.; Alves, C. C. F.; Forim, M. R.; Cazal, C. M.; Aust.J. Crop Sci. 2021, 15, 570. [Crossref] 15. Zhang, D. Y.; Yao, H. X.; Duan, M. H.; Wei, F. Y.; Wu, G. H.; Li, L.; Ind. Crops Prod. 2015, 77, 772. [Crossref] 16. Adams, R. P.; Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp: Illinois, 2007, ch. 3. 17. Xia, J.; Mandal, R.; Sinelnikov, I. V.; Broadhurst, D.; Wishart, D. S.; Nucleic Acids Res. 2012, 40, 127. [Crossref] 18. Nguyen, E.; Jones, O.; Kim, Y. H. B.; Martin-Gonzalez, F. S.; Liceaga, A. M.; Fish. Sci. 2017, 83, 317. [Crossref] 19. Yehye, W. A.; Rahman, N. A.; Ariffin, A.; Abd Hamid, S. B.; Alhadi, A. A.; Kadir, F. A.; Yaeghoobi, M.; Eur. J. Med. Chem. 2015, 101, 295. [Crossref] 20. OriginLab, version 8.0; OriginLab Corp., Northampton, MA, USA, 2007. 21. National Committee for Clinical Laboratory Standards; Approved Standard M2-A8. Performance Standards for Antimicrobial Disk Susceptibility Tests, vol. 23, 8th ed.; National Committee for Clinical Laboratory Standards: Wayne, 2003. 22. GraphPad Prism, version 8.0.0; GraphPad Software, San Diego, CA, USA, 2018. 23. Harisadan, M.; Braz. J. Plant Physiol. 2008, 20, 183. [Crossref] 24. da Silva, E. R.; Oliveira, D. R.; Leitão, S. G.; Assis, I. M.; Veiga-Junior, V. F.; Lourenço, M. C.; Alviano, D. S.; Alviano, C. S.; Bizzo, H. R.; J. Essent. Oil Res. 2013, 25, 171. [Crossref] 25. Zoghbi, M. G. B.; Andrade, E. H. A.; Lima, M. P.; Silva, T. M. D.; Daly, D. C.; J. Essent.Oil Bear. Plants 2005, 8, 312. [Crossref] 26. Carvajal, D.; Alvarez, R.; Osorio, E.; J. Pest Sci. 2016, 89, 241. [Crossref] 27. Alolga, R. N.; León, M. A. S. C.; Osei-Adjei, G.; Onoja, V.; J. Pharm. Pharmacol. 2019, 71, 1544. [Crossref] 28. Czelusniak, K. E.; Brocco, A.; Pereira, D. F.; Freitas, G. B. L.; Rev. Bras. Plant. Med. 2012, 14, 400. [Crossref] 29. Tian, J.; Zeng, X.; Zhang, S.; Wang, Y.; Zhang, P.; Lü, A.; Peng, X.; Ind. Crops Prod. 2014, 59, 69. [Crossref] 30. Bandeira, P. N.; Fonseca, A. M.; Costa, S. M. O.; Lins, M. U. D. S.; Pessoa, O. D. L.; Monte, F. J. Q.; Nogueira, N. A. P.; Lemos, T. L. G.; Nat. Prod. Commun. 2006, 1, 117. [Crossref] 31. Amorati, R.; Foti, M. C.; Valgimigli, L.; J. Agric. Food Chem. 2013, 61, 10835. [Crossref] 32. Mancini, E.; Senatore, F.; Del Monte, D.; de Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Feo, V.; Molecules 2015, 20, 12016. [Crossref] 33. Burt, S.; Int. J. Food Microbiol. 2004, 94, 223. [Crossref] 34. Vieira, P. R. N.; Morais, S. M.; Bezerra, F. H. Q.; Ferreira, P. A. T.; Oliveira, I. R.; Silva, M. G. V.; Ind. Crops Prod. 2014, 55, 267. [Crossref] 35. Oliveira, L. B. S.; Batista, A. H. M.; Fernandes, F. C.; Sales, G. W. P.; Nogueira, N. A. P.; Rev. Bras. Plant. Med. 2016, 18, 511. [Crossref] 36. Mendes, J. L.; Araújo, T. F.; Carvalho, M. G.; Catunda Júnior, F. E. A.; Costa, R. A.; Sci. World J. 2019, 2019, 1. [Crossref] 37. Van Vuuren, S. F.; Kamatou, G. P. P.; Viljoen, A. M.; S. Afr. J. Bot. 2010, 76, 686. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access