Artigo
| Qualitative analysis of the shallow underground Waters of Benevides, Pará, based on multivariate statistical methods |
|
Neuton T. Vasconcelos JuniorI,*; Paulo P. AraújoII; Kelson C. F. FaialI; Bruno S. CarneiroI
I. Instituto Evandro Chagas, 67030-000 Ananindeua - PA, Brasil Received: 09/14/2023 Groundwater is considered an important source of water aid for human consumption. However, this water can be contaminated by impurities, such contamination spots that might reach the water tables. The aim of this study was to investigate the quality of shallow groundwater in Flores neighborhood, located in the municipality of Benevides, within Belém metropolitan region, State of Pará, Brazil. Recommendations described in Standard Methods for Examination of Water and Wastewater were followed at the time to determine physical-chemical parameters such as potential hydrogen (pH), electrical conductivity (EC), total dissolved solids (TDS), apparent color, turbidity, total hardness (TH), ions and trace elements. Multivariate statistical analysis has shown that most predictive water-quality parameters set for human consumption are associated with natural events involving weathering and leaching processes. Nevertheless, parameters of nitrate, phosphate, EC, TDS and pH are also influenced by anthropic conditions in the surroundings, mainly phosphate and nitrate ions. Therefore, it can be concluded that shallow groundwaters in Flores neighborhood are unsuitable for human consumption, making it necessary for municipal public authorities to take action to solve the problem of water shortage in the borough, thus preventing the population from use those shallow groundwaters, as their consumption can bring health problems. INTRODUCTION Groundwater has been used in Brazil since its colonial period. Despite that, a demand for this resource has begun to rise in the 1970s due to advances in both hydrogeology and well-drilling techniques, most of all to a questionable quality of surface waters. This process has made groundwaters into one of the main sources of public water supply.1-3 For some time, groundwaters were assumingly free from contamination by the action of soil and rock layers, but it is known that natural and anthropic factors can affect its quality.4 The lack of adequate basic sanitation is among the human influences, and it is one of the causes of aquifer contamination, mainly in unconfined aquifers that supply water to shallow wells. Studies conducted by Malek et al.5 and Meschede et al.,6 have associated contamination rates recorded for groundwater used for human consumption with shallow wells and the action of different types of effluents. Studies that have focused on investigating the quality of water intended for human consumption have evidenced its potential to transmit diseases caused by pathogenic microorganisms found in human and animal feces, or by chemical substances at higher concentrations than those set within the potability standards. Therefore, it is necessary to control and monitor water quality, for instance by performing hydrochemical surveys in order to achieve a better understanding of the local geology, seasonality and anthropic activities which impact groundwater quality.7,8 Currently, in Brazil, water must meet the recommendations established by Ordinance GM/MS No. 888,9 issued by the Brazilian Ministry of Health on May 4th, 2021, to suit human consumption in the country. Groundwater must also comply with recommendations set by CONAMA (Conselho Nacional do Meio Ambiente) Resolution No. 396,10 from April 3rd, 2008, which establishes environmental guidelines for groundwater classification, as well as actions for preventing and controlling pollution in these environments. The lack of basic sanitation services is a major issue faced by Benevides, PA. According to Social Indicator Information System of State of Pará (SIIS), that city had 8,049 homes in 2013, being 4,304 (approximately 53.5%) of them served by the general water distribution network, whereas the remaining ones were supplied by other sources such as wells and water springs. Several abstraction wells have been dug indiscriminately. This procedure features an aggravating event, since only 60 households in Benevides counted on regular sewage services and less than 1% of them had their domestic waste discharged in the general sewage network.11 Many researchers use multivariate statistical methods as tool to manage water resources, as these methods help to comprehend and identify water pollution sources.12 Principal component analysis (PCA) and hierarchical cluster analysis (HCA) stand out amid multivariate techniques used in studies about water quality, for they enable selecting the main features participating in each component, as well as defining the physical-chemical water properties to be closely monitored.13 In light of the foregoing, the aim of the current research was to apply multivariate analysis to physical-chemical parameters of shallow groundwaters in Barreiras aquifer, located in Flores neighborhood, municipality of Benevides, Belém Metropolitan Region (BMR), by correlating physical-chemical features of shallow groundwater to the geological nature and to anthropic particularities of the investigated area.
EXPERIMENTAL Research area This study was carried out in the municipality of Benevides, Belém Metropolitan Region (BMR), State of Pará, Brazil. According to estimates, the population of the municipality in 2022 encompassed 63,567 inhabitants. Its urban territory comprises 16 neighborhoods, including Flores neighborhood, as shown in Figure 1.14,15
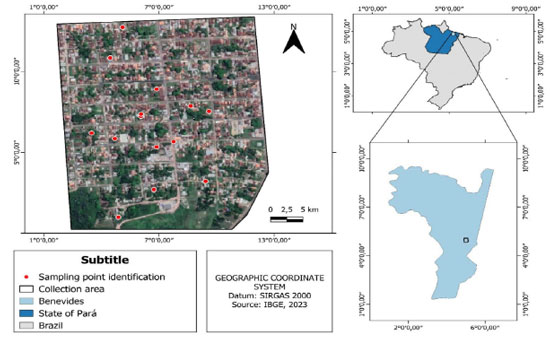 Figure 1. Map of the Flores neighborhood, municipality of Benevides, State of Pará, Brazil (study area) with the location of the wells used in this study
In accordance with a survey conducted by Mineral Resources Research Company (Companhia de Pesquisas de Recursos Minerais, CPRM),16 municipalities belonging to BMR have similar geology, which is defined by lithological units of the Paleozoic age. These units include rocks deriving from marine sedimentation of Pirabas formation, as well as continental and transitional sedimentation rocks from Barreiras and Pós-Barreiras groupings. The assessed aquifer systems belong to two lithostratigraphic units of regional distribution. These aquifers, when free, are highly prone to contamination especially caused by pesticides and fertilizers applied to rural areas, and also by gas stations, septic tanks, domestic and industrial waste in urban zones. It is usually a result of their both lithological composition and proximity to surface. That contamination process can get worse due to high rainfall rates observed in the region.17 Sampling Six samplings were performed in fourteen shallow wells used for domestic purposes in July, September and November 2017 (dry period), likewise in February, April and June 2018 (rainy period), in order to fully cover the hydrological cycle of that location. All sampling points were georeferenced by Garmin eTrex companion Global Positioning System (Vista-hiking®, USA). Informations about latitude and longitude of the sampling spots are shown in Table 1.
Water samples were collected in previously washed 1 L polypropylene bottles with the aid of pre-sterilized stainless pails, and they were subjected to physical-chemical and metal analysis. Physical-chemical analysis was performed through potentiometry technique, with a pre-calibrated multiparameter probe, model HI9828 (Hanna®, Italy). It was done to determine water pH, electrical conductivity (EC) and total dissolved solids (TDS) as recommended by Standard Methods for Examination of Water and Wastewater (SMEWW) 500-H+ B, 2510 B and 2510 A methods, respectively. The samples were placed in vials, refrigerated and sent to the Toxicology Laboratory, Environment Department at Evandro Chagas Institute to acquire additional physical-chemical parameters and also main ionic and metal forms present into them. The adopted analytical methods have followed procedures and recommendations described in the Standard Methods for Examination of Water and Wastewater.18,19 Sample analysis Physical-chemical parameters as apparent color and turbidity were analyzed according to recommendations of the respective methods SMEWW 2120 C and 2130 B. Furthermore, total suspended solids (TSS) and total hardness (TH) parameters complied with recommendations of Hach® 8006 and USEPA 9056 A methods, respectively. The analyses were undertaken by applying spectrophotometry analytical technique, using a DR-3900 spectrophotometer (Hach®, USA) with the following wavelengths: 455 (apparent color), 635 (turbidity), 810 (total suspended solids) and 522 nm (total hardness). Cations such as calcium (Ca2+), magnesium (Mg2+), sodium (Na+) and potassium (K+) and anions such as chloride (Cl-), sulfate (SO42-), phosphate (P-PO43-) and nitrate (N-NO3-) were measured as recommended by SMEWW 4110 B method. All parameters were subjected to ion chromatography through an ICS 2100 DUAL system (DIONEXTM, USA). Chromatographic method operating conditions were controlled in Chromeleon software 6.80 SR6a.20 Conditions adopted in that chromatographic method are shown in Table 2.
Bicarbonate (HNO3-) ion, in its turn, was determined through a volumetric method suggested by ABNT NBR 13736 method.21 Titration with standardized sulfuric acid (H2SO4) (Sigma-Aldrich® Merck, USA) solution at 0.1 M concentration was used to determine the final neutralization reaction by using methyl orange (1% v/v) (Hach®, USA) as indicator. A 50 mL sample aliquot was taken to establish total metals such as aluminum (Al), iron (Fe), barium (Ba), manganese (Mn) and zinc (Zn). The aliquot was acidified with 500 µL of nitric acid (HNO3) at 65% (Emsure® Merck, USA), at high purity degree, in order to generate a 1% v/v solution. Trace elements were analyzed by a simultaneous custom-designed detector inductively coupled plasma atomic emission spectrometer (CCD ICP-AES, Vista-MPX®, Australia) model at axial configuration, equipped with automatic sampling system, as recommended by SMEWW 3120 B method. The ICP-AES operating conditions were controlled by ICPExpert Vista software 4.1.0.22 Established instrumental criteria for metal determination are shown in Table 3.
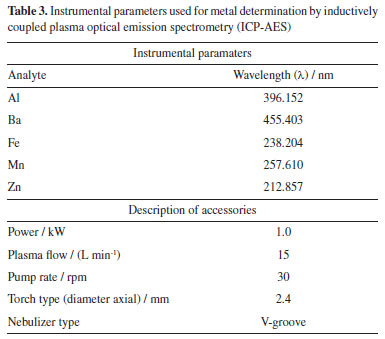
Analytical method validation Validation parameters of the analytical methods used for this research are linearity, accuracy (recovery), limit of detection (LOD) and limit of quantification (LOQ). All reagents for ion and metal analysis were analytical grade; solutions were prepared with high-purity deionized water (resistivity of 18.2 MΩ cm) obtained from a Milli-Q system (Millipore®, USA). The linearity of the ion chromatography method was set according to analytical response of calibration curves at a concentration range from 10 to 500 mg L-1; multielement standards (DionexTM, Thermo Fisher ScientificTM, USA) were used for that, as well as micropipettes and previously calibrated 100 mL volumetric flasks. Starting from 1000 mg L-1 solutions, aliquots of 1, 5, 10, 25 and 50 mL were taken to prepare calibration curves at concentrations of 10, 50, 100, 250 and 500 mg L-1, respectively. After adding the required volumes for standard solutions, their final volumes were gauged with reagent water and each solution was homogenized. Regarding the analytical technique to quantify metals, its linearity was assessed by interpreting calibration curves generated from standard solutions of Al, Ba, Fe, Mn and Zn (Inorganics Venture®, USA); aliquots were prepared with dilution method, covering the dynamic range from 0.1 to 3.2 mg L-1. Starting from 1000 mg L-1 solutions, aliquots of 10, 20, 40, 160 and 320 µL were taken to prepare calibration curves at concentrations of 0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 mg L-1, respectively. After adding the required volumes for standard solutions, the samples were acidified with 1 mL of nitric acid (HNO3) at 65% (Emsure® Merck, USA), their final volumes were gauged with reagent water and each solution was homogenized. Fortified blanks at concentration of 1 mg L-1 were used to check accuracy (recovery) of the ion chromatography method. To do this, 10 mL of a 10 mg L-1 solution containing the analytes of interest were transferred to a 100 mL volumetric flask and the solution was gauged to its final volume. The percentage recovery was calculated according to Equation 1: 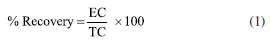 where: EC is the experimentally determined concentration of the fortified blank and TC is the initial concentration of the analytes added to the fortified blank. For quantification of Al, Ba, Fe, Mn and Zn, standard reference material (SRM) 1640a trace elements in water (NIST, USA) was applied. Recovery was calculated according to Equation 2: 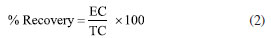 where: EC is the experimentally determined concentration and TC is the certified concentration in the standard reference material. For quantifying ions and metals, the limit of detection (LOD) and limit of quantification (LOQ) of the analytical method were determined by reading ten analytical blanks. To do this, analytical blanks were prepared in accordance with INMETRO normative document DOQ-CGCRE-008.23 The validation parameters were calculated with the following formulas: 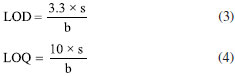 where: s is the standard deviation of the blank response and b is the slope of the analytical curve. Statistical data processing Basic descriptive statistics was applied to results in this current study. Data entries were tabulated using Microsoft Office Excel® software24 and results were inputted in Minitab 18 Statistical® software25 for multivariate statistical analysis.
RESULTS AND DISCUSSION Descriptive statistical analysis Table 4 shows values registered by the recovery test for ions and metals to assess the accuracy of the employed analytical methods. These are average values for six samples collected between July 2017 and June 2018. For the method to be considered accurate and specific, the calculated recovery rates should correspond to 80-110% of the real value for the standard reference material. This was based on criteria adopted by AOAC, Appendix F: Guidelines for Standard Method Performance Requirements.26
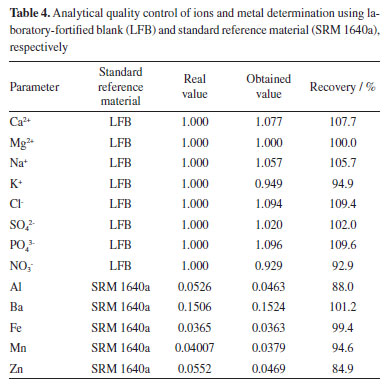
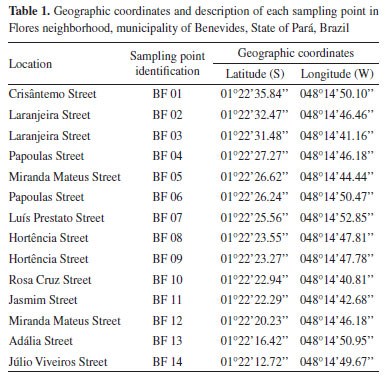
It was not possible to run recovery tests for the following parameters: pH, electrical conductivity (EC), total dissolved solids (TSD), color, total suspended solids (TSS), turbidity, total hardness (TH) and bicarbonate ion, as these parameters do not have standard solutions or standard reference materials. Physical-chemical quality of shallow groundwater Table 5 shows mean overall values and their standard deviations, and also minimum and maximum, LOD and LOQ values for the investigated parameters. Table 6 presents maximum acceptable values (MAV) established in GM/MS Ordinance No. 888,9 which was issued by Brazilian Ministry of Health through CONAMA Resolution No. 396,10 in partnership with the research of Gandhi et al.,27 who cite a concentration of 0.1 mg L-1 of phosphate as MAV, based on the guidelines of the World Health Organization (WHO) for drinking water quality.
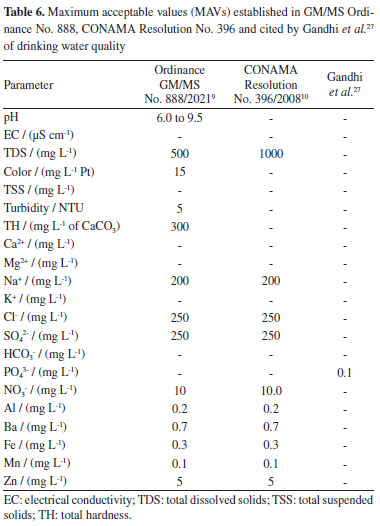
Based on the ordinances adopted for the current research, parameters such as Ca2+, Mg2+, K+, HCO3-, TSS and EC do not have MAV. However, Santos Filho et al.,28 have listed EC values higher than 100 µS cm-1 as contamination indicators. This is an important observation, since 70% of samples analyzed in their research registered EC values higher than 100 µS cm-1, which is indicative of their susceptibility to contamination. The main contamination agents comprised septic systems near to wells, dump activities and shallow wells. Values reported for TH, TDS, Na+, Cl-, SO42-, Ba, Mn and Zn were in compliance with MAV set by GM/MS Ordinance No. 888 as well as by CONAMA Resolution No. 396, except for the first two parameters of them which are only referenced by the Ministry of Health regulation. With respect to pH, turbidity and apparent color parameters, respectively 92, 3.5 and 13% of the samples were non-compliant with values set by GM/MS Ordinance No. 888. Consumption of low pH water can lead to increased incidence of autoimmune diseases such as diabetes and encephalomyelitis. In counterpart, excessive turbidity and apparent color in water for human consumption are aesthetically unpleasant and can make consumers feel disgusted.29,30 Al, Fe and N-NO3- concentrations were not in compliance with GM/MS Ordinance No. 888 nor with CONAMA Resolution No. 396. Excessive Al concentrations are often associated with degenerative lesions observed in Alzheimer's patients, whereas excessive Fe concentrations may intensify human immunodeficiency and hepatitis C virus replication.31,32 Two sampling spots presented N-NO3- ion concentrations higher than MAV. However, NO3- concentrations higher than 3 mg L-1 indicate groundwater contamination scenarios. Thus, all fourteen wells herein selected as sampling spots have shown signs of contamination.33 According to Ward et al.,34 studies performed by International Agency for Research on Cancer have concluded that nitrate and nitrite ions are likely carcinogenic when ingested under conditions capable of inducing endogenous nitrosation. The same authors have shown that once these anions enter the human body, their contents in the blood increase by up to twenty times. The ordinances used for comparison in this current research do not present MAV of P-PO43- for drinking water; therefore, the value stipulated by Gandhi et al.27 was taken as reference. Accordingly, 54.8% of analyzed samples have pointed P-PO43- concentrations above the recommendations set out by the aforementioned organization. Excessive phosphate concentrations trigger toxicity symptoms and cause its retention in body, as well as greater occurrence of vascular calcification in patients suffering from chronic kidney diseases.35,36 Spearman correlation matrix To assess the normality of data distribution, Shapiro-Wilk test was applied at a 5% significance level. This trial revealed that only 3 of the 21 variables in the database had a normal distribution, as the p-value was below the 5% significance level, which rules out the null hypothesis and indicates that majority of the data do not follow a normal distribution, as shown in Figures 1S to 3S (Supplementary Material). To identify outliers, data were subjected to graphical analysis using boxplots, as illustrated in Figures 4S to 6S, in the Supplementary Material. All occurrences were individually examined to check whether they needed to be excluded in order to avoid distorting results, but no data was excluded, since outliers can happen naturally into environmental measurements. Table 1S (Supplementary Material) presents the Spearman correlation coefficients. This statistical tool was used to characterize correlations between physical-chemical and chemical parameters of the examined groundwaters; about 44% of all calculated correlation coefficients were significant (p ≤ 0.05). Spearman correlation coefficient was employed to check the dependence or independence of variables regarding each other. This method was helpful to comprehend physical-chemical and chemical processes which occur in the investigated underground systems. Nonetheless, its choice was also based on the ease that it allows in monitoring water quality when several parameters are included in the analytical process. Moderate and positive correlation between pH and P-PO43- has suggested that groundwater acidity is not only resulted from the influence of natural factors such as rain or weathering processes, but likewise from unsatisfactory sanitary conditions over the region. The presence of P-PO43- ion in groundwater is often attributed to septic systems located too closely to capture wells, as described in the survey conducted by Mechtensimer and Toor.37 Moderate correlations between EC and Cl- (r = 0.52; p < 0.01) and between TDS and Cl- parameters (r = 0.53; p < 0.01) indicate that chloride is the major anion found in the Flores neighborhood underground system. Considering that Barreiras sediments are of a sandy-clay nature,17 it is likely this hydrochemical class prevails due to the material of the sediment deposit where the shallow groundwater is inserted, given that Cl- is present in originating minerals of these sediments.38 According to Bertolo et al.,39 environments with mineralogical settings made up of sandy clay materials, such as the Barreiras system, tend to have a chloride nature. There was strong and positive correlation between EC and TDS parameters (r = 0.99; p < 0.01). That outcome was expected to happen because EC multiplication by a factor ranging from 0.55 to 0.75 provides good TDS estimate for water samples.40 Moderate correlations were also observed between TSS and turbidity (r = 0.50; p < 0.01). Turbidity parameter had low values for most of the samples. This finding suggests that soil-water interaction presented mainly low clay and silt rates. For this reason, turbidity behavior was similar to TSS variability, demonstrating the ability of suspended materials to obstruct light transmittance in water samples and, consequently, affecting turbidity values.41 Ca2+ and Mg2+ ions have shown a particularly good correlation to each other (r = 0.84; p < 0.01), corroborating the close relationship that is widespread in scientific literature, since they have similar chemical features. The ion behavior has also indicated leached environment, which reflected on low mean concentrations.42 K+ ion has shown moderate correlation to Ca2+ (r = 0.60; p < 0.01) and strong correlation to Mg2+ (r = 0.72; p < 0.01) ions. That outcome implies an effect from lithological factors, because the typical lithostratigraphic unit in the investigated area has a sand and clay interlayer system. Clay minerals can easily adsorb K+ ions and may include other alkaline elements as Ca2+ and Mg2+ in their composition.43-45 Barium behavior seems to be associated with a leaching process, which derives from sedimentary Barreiras group formations, for trace amounts of this metal can be found in sedimentary rocks.46 This parameter (barium) has shown moderate correlation to NO3- (r = 0.53; p < 0.01) and Mn (r = 0.56; p < 0.01). N-NO3- ion behavior points to contamination scenarios, especially to the ones linked to sanitary aspects, as seen at the studied location. However, Ba contamination sources are related to steel and agricultural activities (not observed around the investigated region), whereas Mn sources are often natural and may be connected to anthropogenic causes such as industrial effluents and leachate from sewage systems and landfills. Although evidence has indicated that Mn behavior in shallow groundwater was related to rainfall events which have enabled its leaching, as reported in other surveys conducted on BMR.42,47,48 The leaching process might explain the moderate Mn correlation to Ca2+ and Mg2+ (r = 0.61; p < 0.01 and r = 0.57; p < 0.01, respectively) and both Ba and Mn low concentrations. This also has the potential to associate the correlation of Ba with NO3- ion to mineral dissolution processes. Hierarchical cluster analysis (HCA) For a better interpretation of the multivariate data, a standardized data matrix was constructed corresponding to 84 groundwater samples and 19 raw values variables. Those data were submitted to a hierarchical analysis of clusters (HCA) in order to complement the statistical information generated via Spearman rank correlation coefficient. A matrix comprising original data was built on self-scaling method to facilitate multivariate analysis application. Self-scaled data associated with each variable should have mean equal to zero and standard deviation of 1, so all variables would have the same importance, i.e., the same weight to enable data normalization (procedure capable of eliminating the influence of differing measurement units). As said before, HCA performed in this research was based on normalized data about 84 shallow groundwater samples and 19 raw values variables. Cl- and NO3- parameters were not included in this treatment for having smaller sample size. Ward hierarchical agglomerative method was used to minimize original information losses during clustering process. Normalized Euclidean distances served to obtain a dendrogram to verify degrees of similarity among distinct variables or clusters. The vertical lines on the dendrogram shown in Figure 2 represent the analyzed variables. The horizontal lines represent the similarity measures calculated via Euclidean distances that have been considered to form similarity clusters between variables, taking normalized data as a basis.
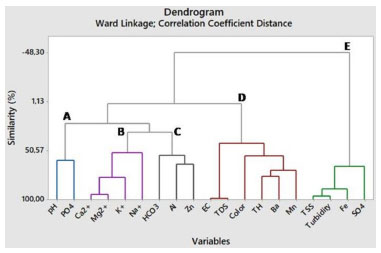 Figure 2. Dendrogram obtained for the variables based on Euclidean distances and the Ward linkage method
It was possible observing a formation of five clusters separated by maximum dissimilarity. These clusters were identified as A, B, C, D and E. Cluster A was formed by pH and P-PO43- parameters and it was represented on the left side of the dendrogram. Mean pH has shown the acidic nature of the shallow groundwater in Flores neighborhood. According to Bahia et al.,49 low pH values for groundwater available in BMR hydrogeological systems are attributed to tropical climate and significant rainfall levels over the region. However, Easa and Abou-Rayan50 have stated that groundwater acidity may be a sign of well contamination by septic tanks and domestic sewage. Cluster B, which comprised four variables, has shown 95.22% similarity between Ca2+ and Mg2+ ions, which confirmed a strong correlation and has indicated similar behavior between both. K+ ion, in its turn, has demonstrated 77.95% similarity to the parameters mentioned above. Finally, these ions have shown 52.58% similarity to Na+. All of them can be associated to the acidic nature of the groundwater, given the slight 22.85% similarity to the cluster formed by pH. Like Ca2+ and Mg2+, the presence of K+ and Na+ ions is suggestive of leaching processes.42,51 Al and Zn parameters included in group C have shown 65.02% similarity, consequently, the pair of metals has evidenced 55.10% similarity to HCO33- ion. There are scientific reports pointing out that the soil in the area is an oxisol type, with increasing clay content and like the soil found in the municipality of Benevides.52 Hence, the similarity between Zn and Al can be imputed to the soil type of the location, for Zn is a common element present in all soil types. On the other hand, aluminum oxides are strongly associated with oxisols. In addition to leaching processes resulting from the strong rainfall regime upon the region, these factors intensify groundwater acidity.53-56 Presence of HCO33- anion in the same group as Al and Zn (elements ruled by acidic nature of that groundwater) may be the consequence of dissolved CO2 converting into bicarbonate ions in acid environments. Moreover, this anion has discrete similarity to ions who are ruled by acidic nature of groundwater, such as Ca2+ and Mg2+, as well as presents 22.85% similarity to pH. According to Souza Filho et al.,57 rainfall events (which reflect low pH values in the investigated area) lead to an increase of HCO33-. Group D is on the right side of the dendrogram and is represented by six parameters. The 99.13% similarity to EC and TDS confirms the strong dependence between these parameters. A similar association was observed by Carvalho et al.,58 who ascribed that dependence to both geochemical and anthropic factors such as effluent discharges. The same group has also revealed substantial similarity to Ba and TH. This behavior indicates that Ba concentrations greatly contributed to the TH value registered for shallow groundwater in Flores neighborhood. This is likely because Ba has analogous physical and chemical properties to those of Ca2+ and Mg2+ and because it has been found in aquifers containing sandy formations. In addition, Ba is easily absorbed by clay minerals which constitute the Barreiras formation.45,55,59-61 This pair of parameters (Ba and TH) also has exhibited 71.16% similarity to Mn. The sandy-clay material of the Barreiras formation has pointed this association between Ba and Mn in groundwaters of BMR. Finally, the apparent color parameter presented 54.97% similarity to the Mn-Ba-TH trio. However, color values were assigned to manganese oxides at colloidal state. Watercolor was related to humic acids, fulvic acids and tannins which derive mostly from plant decomposition processes. Several wells selected as sampling spots have presented some plant species inside, and this may have influenced the apparent color values reported, although this behavior might also be associated with leaching processes.42,62 The fifth and last group E has demonstrated 96.33% similarity between TSS and turbidity parameters, which emphasized a strong dependence between both and has also shown 89.31% similarity to Fe. This behavior may be correlated with the geological material found in the Barreiras formation. This behavior has suggested that ferruginous compounds can increase turbidity in natural water due to Fe2+ ion oxidation into Fe3+ (less soluble form of iron).63 Freddo Filho,64 who has investigated the quality of shallow groundwater in Barreiras aquifer, associates the occurrence of SO42- in water samples with dissolved pyrite minerals. The variation in SO42- concentrations could be related to redox properties of the aquifer system, since its highest concentrations were detected when that system exhibited a reducing characteristic. This process has caused sulfur oxidation and SO42- was its main oxidized species.65 As the local literature states,64,66 wells collecting water from Barreiras aquifer often present dissolved Fe contents higher than 0.3 mg L-1, which corresponds to the maximum value established by current regulations. However, only 3.6% of samples analyzed in this research had registered concentrations above this limit; this finding indicates a less soluble form of iron is mostly found in shallow groundwater.
CONCLUSIONS Findings of the investigated physical-chemical criteria have revealed that 7 of the 21 tested parameters have exceed MAV established in current standards for drinking water, with emphasis on pH, P-PO43-, N-NO3- and Al. Multivariate statistical analysis has helped explaining the association between parameters, as well as differentiating how natural and anthropic actions can affect the quality of an investigated groundwater. The acidic nature of analyzed shallow groundwaters was like that of groundwaters in Belém Metropolitan Region, disclosing natural influences. Nevertheless, multivariate statistical treatment has indicated that pH behavior was slightly analogous to that of P-PO43- ions. Water acidity in the region has also been impacted by anthropic causes, including septic systems nearby collection wells. Thus, it is possible to conclude that dissolved Fe concentrations exceeding the maximum permitted by current regulations have not performed this way due to reducing characteristics observed for the investigated area. The least soluble form of this metal was the most abundant species in shallow groundwaters, a fact noted in its intrinsic association with other parameters such as turbidity and TSS, as demonstrated by HCA and Spearman correlation coefficient. This research has indicated that the limiting factor for shallow groundwater consumption around the region concerned lies especially in septic systems which are the main sources of P-PO43- and N-NO3- ions. Furthermore, poor construction of collection wells and unhealthy conditions have pointed that finding a solution to this issue means going beyond conventional water treatments.
SUPPLEMENTARY MATERIAL Complementary material for this work is available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
REFERENCES 1. Rebouças, A. C.; Braga, B.; Tundisi, J. C.; Águas Doces no Brasil: Capital Ecológico, Uso e Conservação, 3a ed.; Escrituras editora: São Paulo, 2006. 2. Estrada, M. M. P.; Cadernos do Programa de Pós-Graduação em Direito/UFRGS 2005, 3, 209. [Crossref] 3. Cappi, N.; Ayach, L. R.; dos Santos. T. M. B.; Guimarães, S. T. L.; Geografia Ensino & Pesquisa 2012, 16, 77. [Crossref] 4. Elhag, A. B.; Journal of Geology & Geophysics 2016, 6, 1. [Crossref] 5. Malek, A.; Kahoul, M.; Bouguerra, H.; J. Water Land Dev. 2019, 41, 91. [Crossref] 6. Meschede, M. S. C.; Figueiredo, B. R.; Alves, R. I. S.; Segura-Muñoz, S. I.; Revista Ambiente e Água 2018, 13, 1. [Crossref] 7. Subramani, T.; Elango, L.; Damodarasamy, S. R.; Environ. Geol. (Heidelberg, Ger.) 2005, 47, 1099. [Crossref] 8. Ramkumar, T.; Venkarramanan, S.; Anithamary, I.; Ibrahim, S. M.; Arabian J. Geosci. 2012, 6, 101. [Crossref] 9. Ministério da Saúde; Portaria GM/MS No. 888, de 4 de maio de 2021, Altera o Anexo XX da Portaria de Consolidação GM/MS No. 5, de 28 de setembro de 2017, para Dispor sobre os Procedimentos de Controle e de Vigilância da Qualidade da Água para Consumo Humano e seu Padrão de Potabilidade; Diário Oficial da União (DOU), Brasília, No. 85, de 07/05/2021, p. 127. [Link] accessed in May 2024 10. Conselho Nacional do Meio Ambiente (CONAMA); Resolução No. 396, de 3 de abril de 2008, Dispõe sobre a Classificação e Diretrizes Ambientais para o Enquadramento das Águas Subterrâneas e dá Outras Providências; Diário Oficial da União (DOU), Brasília, No. 66, de 07/04/2008, p. 64-68. [Link] accessed in May 2024 11. Ministério Público do Estado do Pará, https://www2.mppa.mp.br/sistemas/gcsubsites/index.php?action=MenuOrgao.show&id=2098&oOrgao=53, accessed in May 2024. 12. Noshadi, M.; Ghafourian, A.; Environ. Monit. Assess. 2016, 188, 13. [Crossref] 13. Nosrati, K.; Van Den Eeckhaut, M.; Environ. Earth Sci. 2012, 65, 344. [Crossref] 14. Souza, L. A.: Produção do Espaço em Ocupações no Município de Benevides, PA: Interfaces entre Rural e Urbano; Dissertação de Mestrado, Universidade Federal do Pará, Belém, Brasil, 2011. [Link] accessed in May 2024 15. Instituto Brasileiro de Geografia e Estatística (IBGE), https://cidades.ibge.gov.br/brasil/pa/benevides/panorama, accessed in May 2024. 16. Companhia de Pesquisa de Recursos Minerais (CPRM); Projeto Estudos Hidrogeológicos da Região Metropolitana de Belém e Adjacências, CPRM: Belém, 2002. 17. Araújo, P. P.: Variações Sazonais dos Componentes Nitrogenados, em Aqüífero Livre na Zona Urbana de Santa Isabel do Pará, Nordeste do Estado do Pará; Dissertação de Mestrado, Universidade Federal do Pará, Belém, Brasil, 2001. [Link] accessed in May 2024 18. APHA; Standard Methods for the Examination of Water and Wastewater; 23rd ed.; Baird, R. B.; Eaton, A. D.; Rice, E. W., eds.; Amer Public Health Assn: Washington, 2017, part 2000. 19. APHA; Standard Methods for the Examination of Water and Wastewater; 23rd ed.; Baird, R. B.; Eaton, A. D.; Rice, E. W., eds.; Amer Public Health Assn: Washington, 2017, part 3000. 20. Chromeleon®, version 6.80 SR6a; Thermo Scientific Inc., Waltham, MA, USA, 2009. 21. ABNT NBR 13736: Água - Determinação de Alcalinidade - Métodos Potenciométrico e Titulométrico, ABNT: Rio de Janeiro, 1996. [Link] accessed in May 2024 22. ICPExpert Vista ®, version 4.1.0; Varian Inc., Mulgrave, Victoria, AU, 2005. 23. DOQ-CGCRE-008: Orientação sobre Validação de Métodos Analíticos, INMETRO: Rio de Janeiro, 2020. [Link] accessed in May 2024 24. Microsoft Office Excel®, version 2010; Microsoft Inc., Redmond, WA, USA, 2010. 25. Minitab 18 Statistical®, version 18; Minitab Inc., State College, PA, USA, 2017. 26. Official Methods of Analysis (AOAC): Guidelines for Standard Method Performance Requirements (Appendix F), Maryland, 2016. [Link] accessed in May 2024 27. Gandhi, R. K.; Sharma, N.; Raina, A. K.; Environ. Conserv. J. 2018, 19, 17. [Crossref] 28. Santos Filho, M. G.; Hirata, R.; Luiz, M. B.; Conicelli, B.; Rev. Inst. Geogr. Geol., Sao Paulo 2017, 38, 47. [Crossref] 29. Amfo-Otu, R.; Agyenim, J. B.; Nimbabumah, G. B.; Environmental Research, Engineering and Management 2014, 67, 24. [Crossref] 30. Jayaraman, S.; Jayaraman, A.; J. Diabetes Res. 2018, 2018, 6. [Crossref] 31. Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B. B.; Beeregowda, K. N.; Interdiscip. Toxicol. 2014, 7, 72. [Crossref] 32. Weinberg, E. D.; Oxid. Med. Cell. Longevity 2009, 2, 109. [Crossref] 33. Biguelini, C. P.; Gumy, M. P.; Revista Faz Ciência 2012, 14, 175. [Crossref] 34. Ward, M. H.; Jones, R. R.; Brender, J. D.; De Kok, T. M.; Weyer, P. J.; Nolan, B. T.; Villanueva, C. M.; Van Breda, S. G.; Int. J. Environ. Res. Public Health 2018, 15, 31. [Crossref] 35. Fukagawa, M.; Hamada, Y.; Nakanishi, S.; Tanaka, M.; J. Bone Miner. Metab. 2006, 24, 438. [Crossref] 36. Razzaque, M. S.; Clin. Sci. 2011, 120, 97. [Crossref] 37. Mechtensimer, S.; Toor, S.; PloS One 2017, 12, 14. [Crossref] 38. Ramage, L.: Hidrogeoquímica do Sistema Aquífero Granular Cenozóico do Município de Porto Alegre, RS; Dissertação de Mestrado, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brasil, 2005. [Link] accessed in May 2024 39. Bertolo, R.; Hirata, R.; Fernandes, A.; Rev. Bras. Geocienc. 2007, 37, 515. [Crossref] 40. Paul, M. K.; Sen, S.; Curr. World Environ. 2012, 7, 258. [Crossref] 41. Hannouche, A.; Chebbo, G.; Ruban, G.; Tassin, B.; Lemaire, B. J.; Joannis, C.; Water Sci. Technol. 2011, 64, 2452. [Crossref] 42. Bahia, V.; Fenzl, N.; Morales, G. P.; Resumos do 15o Congresso Brasileiro de Águas Subterrâneas; Natal, Brasil, 2008. [Link] accessed in May 2024 43. Iwasaki H.; Iwasaki F.; Suzuki, C. K.; Oliveira, V. A. R.; Hummel, D. C. A.; Shinohara, A. H.; Abstracts of 40th Annual Symposium on Frequency Control; Philadelphia, USA, 1986. [Link] accessed in May 2024 44. Iwasaki, H.; Iwasaki, F.; Oliveira, V. A. R.; Hummel, D. C. A.; Pasquali, M. A.; Guzzo, P. L.; Watanabe, N.; Suzuki, C. K.; Jpn. J. Appl. Phys. 1991, 30, 1495. [Crossref] 45. Cabral, N. M. T.; Lima, L. M.; Bol. Mus. Para. Emilio Goeldi: Geol. 2006, 1, 166. [Crossref] 46. Garlipp, A. B.: Variação Espacial e Sazonal de Elementos Maiores e Traços no Estuário do Rio Curimataú (RN), através de Dados Geoquímicos e de Sensoriamento Remoto; Tese de Doutorado, Universidade Federal do Rio Grande do Norte, Natal, Brasil, 2006. [Link] accessed in May 2024 47. Reimann, C.; de Caritat, P.; Chemical Elements in the Environment: Factsheets for the Geochemist and Environmental Scientist, 1st ed.; Springer Verlag: Berlim, 1998. 48. British Columbia Ground Water Association (BCGWA); Water Stewardship Information Series: Iron & Manganese in Groundwater, BCGWA: British Columbia, 2007. [Link] accessed in May 2024 49. Bahia, V. E.; Fenzl, N.; Leal, L. R. B.; Morales, G. P.; Luiz, J. G.; Águas Subterrâneas 2011, 25, 56. [Crossref] 50. Easa, A.; Abou-Rayan, A.; Abstracts of 14th International Water Technology Conference; Cairo, Egypt, 2010. [Link] accessed in May 2024 51. Bahia, V. E.; Fenzl, N.; Morales, G. P.; Geochim. Bras. 2006, 20, 311. [Crossref] 52. Secco, C.; Secco, N.; Batista, F.; Porto, A.; Delgado, G.; Camarini, G.; Resumos do11o Encontro Latino Americano de Iniciação Cientifica & Encontro Latino Americano de Pós-Graduação; São José dos Campos, Brasil, 2007. [Link] accessed in May 2024 53. Resende, M.; Curi, N.; Rezende, S. B.; Corrêa, G. F.; Pedologia: Base para Distinção de Ambientes, 1a ed.; NEPUT: Viçosa, 1998. 54. Mcphail, D. C.; Summerhayes, E.; Welchl, S.; Brugger, J. In Advances in Regolith: Proceedings of the CRC LEME Regional Regolith Symposia; Roach, I. C., ed.; CRC LEME: Tablelands, 2003, ch. 69. 55. Kabata-Pendias, A.; Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, 2011. 56. Mertens, J.; Smolders, E. In Heavy Metais in Soils: Trace Metais and Metalloids in Soils and their Bioavailability; Alloway, B. J., ed.; Springer: Dordrecht, 2012, ch. 17. 57. Souza Filho, O. A.; Veríssimo, L. S.; Silva, C. M. S. V.; Santiago, M. M. F.; Resumos do 13o Congresso Brasileiro de Águas Subterrâneas; Cuiabá, Brasil, 2004. [Link] accessed in May 2024 58. Carvalho, F. I. M.; Lemos, V. P.; Dantas Filho, H. A.; Dantas, K. G. F.; Rev. Virtual Quim. 2015, 7, 2241. [Crossref] 59. Bodek, I.; Lyman, W. J.; Reehi, W. F.; Rosenblatt, D. H.; Environmental Inorganic Chemistry: Properties, Processes, Estimation Methods, 1st ed.; Pergamon Press: New York, 1988. 60. https://www.pca.state.mn.us/sites/default/files/wq-am1-10.pdf, accessed in May 2024. 61. Matta, M. A. S.: Fundamentos Hidrogeológicos para Gestão Integrada dos Recursos Hídricos da Região de Belém/Ananindeua - Pará, Brasil; Tese de Doutorado, Universidade Federal do Pará, Belém, Brasil, 2002. [Link] accessed in May 2024 62. da Silva, A. B.; de Brito, J. M.; Silva, R. A.; Braz, A. S.; da Silva Filho, E. D.; Águas Subterrâneas 2017, 31, 109. [Crossref] 63. Rocha, P. S. G.: Análise da Influência da Turbidez em Resultados de Amostra de Água Subterrânea; Trabalho de Conclusão de Curso, Escola Superior da CETESB, São Paulo, Brasil, 2019 [Link] accessed in May 2024 64. Freddo Filho, V. J.: Qualidade das Águas Subterrâneas Rasas do Aquífero Barreiras: Estudo de Caso em Benevides - PA; Dissertação de Mestrado, Universidade Federal do Pará, Brasil, 2018. [Link] accessed in May 2024 65. Nelson, D.; Groundwater Foundation Annual Meeting; Springfield, Oregon, 2002. [Link] accessed in May 2024 66. Picanço, F. E. L.; Lopes, E. C. S.; Souza, E. L. Resumos do 12º Congresso Brasileiro de Águas Subterrâneas; Florianopólis, Brasil, 2002. [Link] accessed in May 2024 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access