Artigo
| Passive-sampler employed for antifouling booster biocides analyses in seawater |
|
Marta S. D. FreitasI; Rodrigo M. BatistaII; Andressa R. C. CostaI,*; Ozelito P. de Amarante JuniorI,II,; Teresa C. R. S. FrancoIII; Gilberto FillmannII; Natilene M. BritoI
I. Departamento Acadêmico de Química, Instituto Federal de Educação, Ciência e Tecnologia do Maranhão, 65035-005 São Luís - MA, Brasil Received: 01/16/2024 *e-mail: andressacastro.c@gmail.com The antifouling booster biocides are frequently studied for toxic effects on the aquatic ecosystems. The present investigation proposes passive silicone rubber samplers as a collection method for biocides, once these methods can concentrate substances in aqueous matrices at very low levels. Through the passive sampler-water partition coefficient (Ksw) and the analyte chemical nature, we can optimize their extraction from the membrane to apply in the sample medium. We used the co-solvent method to determine the Ksw of three third-generation antifouling biocides, chlorothalonil, dichlofluanid, and dichlorooctylisothiazolinone (DCOIT), with log Ksw = 2.24, 4.01, and 2.38, respectively. Improving extraction also led to a recovery range higher than 70%, determinations were carried out by gas chromatography with an electron capture detector. Biocides concentration in seawater samples from Itaqui port (São Marcos Bay, northern Brazil) ranged from 0.058 to 0.72 µg L-1 for chlorothalonil, 0.001 to 0.008 µg L-1 for dichlofluanid, and 0.018 to 0.64 µg L-1 for DCOIT. INTRODUCTION Aquatic ecosystems are susceptible for toxic substances due to irregular chemical disposal directly into the water, contaminant transport in river channels, and wet/dry atmospheric deposition, among other input sources of toxic substances.1 Dichlofluanid, chlorothalonil, and DCOIT (4,5-dichloro-2-n-octyl-4-isothiazolin-3-one) are among the most popular third-generation antifouling booster biocides, being frequently studied for toxic effects on the ecosystem,2 such as algal growth inhibition,3 carcinogenic and mutagenic effects on marine invertebrates.4 These biocides usually have a relatively short half-life in the environment, for example the DCOIT biocide has a half-time that can range between 1-13 days in seawater due to the diverse environmental conditions that affect its degradation.5 However, they may degrade into even toxic products, chlorothanonil can be highlighted, once degradation products are more toxic than the parent compound.6,7 Chlorothalonil and dichlofluanid are also used as pesticides (fungicides), and applied to several crops worldwide.8 DCOIT, also known as Sea-Nine®, was developed for antifouling purposes, and it is highly toxic.9 Solid-phase extraction (SPE), liquid-liquid extraction (LLE), and solid-phase microextraction (SPME) are the main methods to prepare environmental matrices for analysis.6,7,10-14 These are very expensive and time-consuming techniques, requiring high-cost materials.15 The low level of these contaminants in aqueous matrices usually hampers their detection in small volumes of water, as they can fall below the limits of detection and quantification of traditional chromatographic methods. Only a few studies report chlorothalonil, dichlofluanid, and DCOIT in seawater using conventional extraction techniques, such as SPE, LLE, and SPME.16 Studies using those extraction methods and chromatographic techniques often do not detect biocides.7,17-19 Thus, silicone passive samplers are an alternative to traditional procedures as they may be reusable, are mechanically resistant, and they also can feature temporal integration and non-discrete sampling.20,21 Unlike in traditional extractions, passive sampler does not require a large water volume because it is immersed in the aquatic environment, eliminating costs with transportation, storage, collect, and treatment of large volumes of water.22-24 The present investigation proposes passive silicone rubber samplers as a collection method for third generation biocides, once these methods can guarantee data of compounds bioavailability integrated to time.25 This occurs because silicone rubbers have the characteristic of accumulating pollutants in a similar way to what happens with living tissues.20 Regarded to time, unlike active sampling, passive samplers are exposed for a period, accumulating substances present in natural waters, such as living organisms. These three compounds chosen in this study are components of antifouling paints currently used to protect submerged surfaces. It should be noted that these substances (DCOIT, chlorothalonil, dichlofluanid) have sampler-water partition coefficient (Ksw) < 3.0, different from what occurs with the compounds previously studied for the material of the sampler.26 This is the present study main contribution, since it expands this silicone rubbers use in the passive sampling of organic pollutants in natural waters.
EXPERIMENTAL Chemical reagents and materials All experiments used silicone rubber (0.5 mm thick) purchased from AlteSil (Bude, UK). Alkaline detergent (Dinâmica®, Indaiatuba, Brazil) was used and running water to wash the glassware, followed by immersion for 24 h in a 10% alkaline detergent solution and a 10% nitric acid (Alphatec, Carlsbad, USA) bath. Glassware was placed in a drying oven at 36 ºC for 3 h. Chlorothalonil, dichlofluanid, and DCOIT analytical standards (98%) were obtained from Sigma-Aldrich (St. Louis, USA). Acetonitrile (ACN), methanol (MeOH), toluene, hexane (Hex), and acetone (AcO) chromatographic grade from Merck (Darmstadt, Germany) and ethyl acetate from JT Baker (Phillipsburg, USA). PCB-30 (2,4,6-trichlorobiphenyl), used as internal standard, and PCB-112 (2,3,3',5,6-pentachloro-1,1'-biphenyl), used as surrogate standard, were purchased from Merck and Dr. Ehrestorfer (Augsburg, Germany) (> 99%), respectively. Individual stock solutions were prepared in toluene and working solutions were prepared by dilution in hexane. Sampling location and deployments Itaqui Port (02o35'12" S and 044o23'30" W) was chosen due to the high volume of large vessel traffic and the mangrove ecosystem. This port is located in São Marcos Bay, São Luís (MA), capital of Maranhão State, northern Brazil, in the Legal Amazon zone (Figure 1). The sampling site (Pier 108) was selected considering vessel traffic, ease of access, the safety of samplers, and enough depth to ensure full structure coverage, despite the wide tidal range, protecting the samplers from atmospheric exposure.
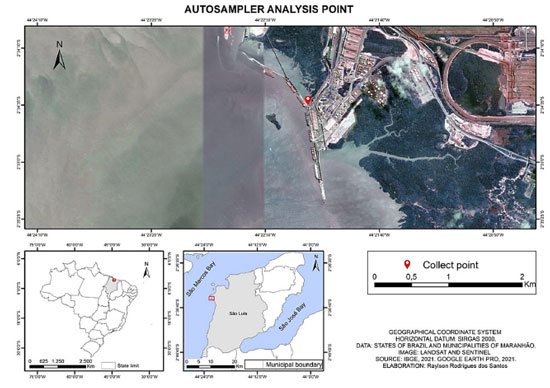 Figure 1. Sampling point localization: Pier 108 of Itaqui Port, São Marcos Bay, northern Brazil, in the Legal Amazon zone
Twenty-four silicone rubber samplers were fixed to two stainless-steel frames and deployed for six weeks according to agreed standard operating procedures.27 Structural fabrication procedures and field blanks were carried out as described in Vrana et al.28 Immediately after retrieval, sheet surfaces were cleaned from fouling by wiping them using a pre-cleaned (in methanol and dried) nylon scouring pad while immersed in water collected at the sampling site. Samplers were stored in pre-cleaned glass flasks, identified, and kept frozen until extraction and analysis. The silicone rubber samplers were immersed in Pier 108 of Itaqui Port to verify the presence of chlorothalonil, dichlofluanid, and DCOIT for two 6-week periods, this sampler remained for 6 weeks to guarantee the partition balance between the analytes and the membrane. The first sampling period went from August 08th to September 28th, 2018, and the second from September 28th to November 14th, 2018. All rubbers were immersed under similar conditions of temperature, luminosity, pH, and depth. The rubber batches were divided into three groups of 6 rubbers, ensuring that extractions and analyses were performed in triplicate for each sampling period. Analytical methods Passive sampler preparation Translucent silicone polymer sheets with 0.5 mm thickness (Altesil, Bude, UK) were cut into 5.5 × 9.0 cm sheets and Soxhlet-extracted with ethyl acetate for 100 h to remove short-chain branched polymers that may interfere with the analyses. The silicone rubbers' size increase has been observed due to ethyl acetate adsorption. The sheets were then immersed in methanol for 8 h, this process was performed twice.29 This allowed the removal of ethyl acetate from the sheet. Before their usage, sheets were stored in glass bottle closed with aluminum foil. At each sampling campaign, 18 sheets were transported to the area to be sampled, of which 12 were exposed to water during the sampling period and 6 returned to the laboratory as blank samples. After its usage, each sheet was washed with methanol again and stored. Extraction procedure Two extraction procedures using acetonitrile:methanol (1:1) were tested: (i) mechanical agitation at 40 rpm for 1 h, followed by another 1 h in an ultrasound bath, and (ii) only one ultrasound bath for 1 h. After extraction, the solvent was evaporated to 1 mL in a Syncore Analyst® (London, England) drying system. The extract obtained was concentrated up to 0.4 mL under nitrogen flow, and ethyl acetate was added to complete 1 mL, because the analyses were carried out with GC system. The clean-up step was performed in an open column filled with 3 g of silica (activated at 160 ºC for 4 h) and 1 cm top layer of sodium sulfate (Na2SO4) to remove moisture. The extract was eluted three times with a 5 mL acetone-hexane (1:2), then evaporated to 1 mL under nitrogen flow and fortified with 40 ng of PCB-30 (internal standard). Recovery standard (PCB-112) was added to the rubbers before every extraction to monitor the process efficiency on real samples. The recovery of the analytes was calculated according to Equation 1, where C1 is the concentration of the analyte in the fortified sample, C2 is the concentration of the analyte in the unfortified sample, and C3 is the concentration of the analyte added to the fortified sample. 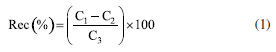 Analysis by gas chromatography The analytes were analyzed in a PerkinElmer® Clarus® 500 (Waltham, USA) gas chromatograph equipped with an electron capture detector (CG-ECD) and a Phenomenex® Zebron™ ZB-5MS capillary column (30 m × 0.25 mm × 0.25 µm) in an isocratic elution mode at 280 ºC. Helium was used as carrier gas (1.5 mL min-1) and nitrogen was employed as make up gas. The run-time and injection volume were 30 min and 2 µL, respectively. The injector and detector were kept at 280 ºC. Analytical curves were obtained by injecting solutions between 0.50 and 50.0 ng mL-1, for each analyte, analyzed in triplicate, under the chromatographic conditions described.19 Validation parameters To evaluate the precision and accuracy, recovery studies were carried out. Each extraction procedure was investigated with 6 sheets fortified with biocides and the recovery standard (PCB-112), at three different level concentrations. Increased sheets volume indicates successful absorption of the solution. Hexane was used as a solvent in fortification solutions due to analyte absorption improvement attributable to its hydrophobicity.30 Each sampler was individually placed inside a 100 mL flask containing 60 mL of ACN:MeOH (1:1, v:v) and both extractions methods were performed as previously mentioned. Recovery tests were carried out in triplicate by fortifying rubbers with studied analytes at three different concentrations, between 5.0 and 50.0 ng mL-1. For repeatability purposes, all experimental replicates were made by same analyst operating the same equipment. To assess whether there are significant differences between the values obtained and the fortification concentrations, two-tailed Student's t-test was performed with 95% confidence. The null hypothesis (H0) is that there is no significant difference between the determined concentrations and the fortification concentrations. Limit of detection (LOD) calculation employed residual standard deviation (sy/x) approach, obtained in place of blank standard deviation (sB), which is a more accurate estimate of value blank sign (yB) than a simple blank measurement. Limit of quantification (LOQ) was determined by estimating from the analytical curve, which provides better results at the trace level. The LOQ is the smallest amount of the analyte in the sample that can be quantitatively determined with acceptable precision and accuracy.31 Passive sampler-water partition coefficients (Ksw) The sampler-water partition coefficients were determined according to other works.23,32 Each 1 g rubber silicone was fortified with the biocides and transferred to 100 mL flasks. Solvent system used was changed over time, starting with 100% methanol and adding water up to the limit of 50%, as shown in Table 1. During the experiments, these flasks were kept under constant agitation.
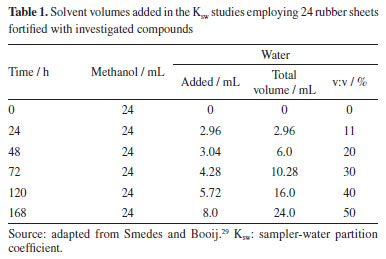
Extraction was conducted right after the fortification process to determine the initial concentration (initial Cs). This co-solvent experiment is carried out to prevent molecules migration to glass of the flasks due to the investigated compounds hydrophobicity, interfering in the results of interaction between silicone rubber and water. After each 24-h interval, three sheets were removed from this experiment for extraction and chromatographic analysis and water was added to the solvent in remaining flasks, as described in Table 1. A graph was built relating the concentrations determined in the sampler (Cs) after this exposure period versus the co-solvent percentage. From the obtained equation, by extrapolation, analytes concentration in silicone rubber for 100% water can be calculated (final Cs). Analyte concentrations in water (Cw) was considered as the difference between final and initial concentration in silicone rubber. The Equation 2 describes sampler partition coefficient calculation:  Calculation of freely dissolved concentrations Biocides aqueous concentrations (Cw) from real samples were calculated using the first-order uptake model to equilibrium according to Smedes and Booij.29 After silicone rubber exposition to the environment, each sheet was subjected to developed extraction process and biocides concentrations found in silicone rubbers (Cs) and calculated partition coefficients were used in the equation to estimate water concentrations (Cw).
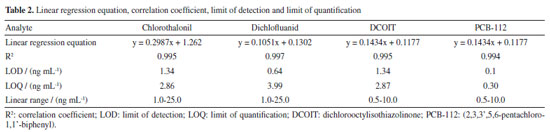
RESULTS AND DISCUSSION Analytical curves data provided correlation coefficients (R) with values above 0.99, showing a very strong correlation between the analyte concentration and the analytic signal.33,34 Linear range was determined as described by Ribani et al.,35 and values for chlorothalonil and dichlofluanid were found between 1.0 and 25 ng mL-1 and between 0.5 and 10 ng mL-1 for DCOIT and PCB-112. Limit of quantification (LOQ) and limit of detection (LOD) are shown in Table 2.
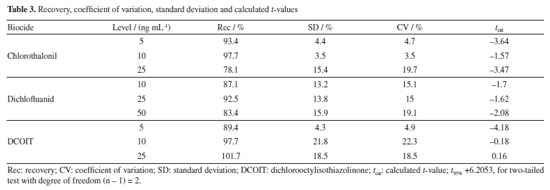
Accuracy and precision Recovery assay was used to assess accuracy and precision, both obtained during fortification tests of silicone rubber samplers. Accuracy was considered as the recoveries ratio and precision was estimated as the coefficients of variation (CV) for each studied compound. Average of recoveries for employed surrogate standard PCB-112 were 116.86%, with CV of 5.95%. Recovery values for PCB-112 were within the acceptable range for methods for pesticide residue analysis.34 Table 3 shows the recovery values obtained for the three booster biocides fortified in the silicone rubber samplers. All three biocides presented recovery percentages within the range indicated in the scientific literature for all three concentration levels investigated, thus the method was considered to have adequate accuracy. Also, the method precision for residue analyses of microcontaminants was considered acceptable since CVs were below 20%.36,31 A two-tailed Student's t-test (95% confidence) compared recovery mean values with fortification concentration values, resulting in t-values lower than the tabulated t-value, indicating no significant differences between the actual value and those found in the recovery study.34
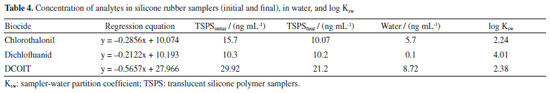
Once the method proved accurate and precise, it was applied to determine sampler-water partition coefficients and then passive samplers were used in the waters of Itaqui Port. Partition coefficients (Ksw) Extraction only by ultrasound bath showed best recoveries results for all the studied compounds. Wille et al.37 had already stated that combining solvent mixture and sonication was the best option to extract pesticides and drugs with log Kow from -0.39 to 4.51, which matches the investigated biocides. Just as Kow is used to measure a compound lipophilicity and relates to a substance bioavailability, bioaccumulation, biomagnification, and toxicity, Ksw can assess the accumulation of target compounds on silicone rubber materials.38 Analyses of the extracts from the fortified translucent silicone polymer samplers (TSPS) in contact with the solvent allowed us to plot the analyte concentration in the rubber vs. methanol concentration in the solvent mixture. Straight-line equation was employed to determine the analyte concentration in 0.5, 1, 5, and 10% methanol solutions and studied compounds Ksw, as showed on Table 4. All three biocides showed affinity to the silicone sampler, as it is showed by their log Ksw values. These results suggest that the material studied has the potential to be used as passive samplers. Dichlofluanid highest affinity can be explained by its hydrophobicity, once its value of log Kow is 3.70.7,27
Booster biocides in the environment After silicone rubber samplers were immersed in Pier 108 of Itaqui for 7-week periods, rubber batches were submitted to cleaning, extraction and analysis procedures as previously described. After chromatographic analysis and determination of Cs (concentration absorbed by the sampler during the exposure period), the Ksw of each analyte was used to calculate their concentration in the water (Cw). In a study carried out in the Czech Republic,39 researching polyaromatic hydrocarbons, polychlorinated biphenyls and organochlorine pesticides, condition of equilibrium between the concentration in water and the concentration in the samplers was obtained in 28 days for compounds with Kow close to those presented by the analytes investigated in this present work. Due to this, it was possible to consider that 7 weeks was enough to reach the equilibrium between Cw and Cs for the investigated analytes. Discrepant results were discarded and analyte concentrations in water are shown in Table 5.
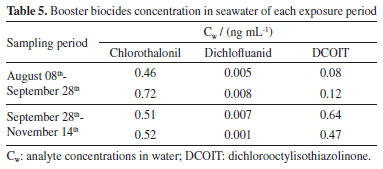
Average concentrations were 0.59 ng mL-1 with 0.18 ng mL-1 of SD for chlorothalonil in the first period of sampling and 0.52 ng mL-1 with 0.01 ng mL-1 of SD in the second period. For dichlofluanid, an average of 0.007 ng mL-1 (SD of 0.002 ng mL-1) in the first period and an average of 0.004 ng mL-1 (SD of 0.004 ng mL-1) in the second period were found. DCOIT showed an average of 0.10 ng mL-1 (SD of 0.03 ng mL-1) in the first period and an average of 0.56 ng mL-1 (SD of 0.12 ng mL-1) in the second period. As the first record in this estuary, those data are worrying once it is in the Legal Amazon zone, covered mainly by mangrove vegetation, providing large fishing activity and shellfish harvesting for commerce and feeding the locals. Comparing our data with other studies, only dichlofluanid presented lower values in São Luís than in other locations. Chlorothalonil was found in Greece at concentrations ranging from 0.031 to 0.063 ng mL-1.18 Other studies8,12 detected about 0.030 ng mL-1 of this fungicide on the coast of South Korea and more than 0.010 ng mL-1 in Spain. DCOIT was found in Japanese waters varying from 0.0001 to 0.011 ng mL-1.40 In Greece, the concentration reached 0.049 ng mL-1 in recreational areas, while in Denmark and Spain, DCOIT concentration ranged from 0.005 to 0.283 ng mL-1.12,19,41 Dichlofluanid was also found in Greece at concentrations between 0.024 and 0.284 ng mL-1.19 Other studies10,12 showed concentrations below 0.030 ng mL-1 on the Spanish coast and below 0.001 ng mL-1 in Italy. As dichlofluanid degrades rapidly (t1/2 = 1.2 h), we suggest quantifying its major metabolite DMSA (N,N-dimethyl-N'-phenylsulfamide), which is more stable to photodegradation and hydrolysis processes.7,19 Campos et al.42 argue that DCOIT, dichlofluanid and chlorothalonil are among the biocides with the greatest risk to coastal ecosystems, highlighting that chlorothalonil is up to 400-fold more toxic for non-target organisms than to target ones. These authors sustain that DCOIT degradation ranges between less than a day and 13 days, and it was found concentrations ranging from 0.03 to 3.7 ng mL-1 in Mediterranean, Atlantic and Pacific waters samples. They indicate that chlorothalonil present half-live around 1.8 days, but it has been detected in Atlantic water samples in France (0.01 ng mL-1) and UK (1.38 ng mL-1). Dichlofluanid has the fastest degradation with half-life about 3 h, however it has been found in coast water from different countries with higher concentrations, in Portugal (0.02 ng mL-1) and Spain (3.37 ng mL-1). Those studies were carried out with active sampling methods that obtain instantaneous information, which usually reflects the moment the sample was collected but may not represent the reality of the environment. Nevertheless, the present study was performed applying passive samplers that provide an average of the exposition period, about six weeks in this case. Dispersion of concentration data in each sampling period showed variations that can be attributed to biofouling and water flow on the surface of the sampler. The biofouling process forms layers on the rubber during exposure, which may hinder absorption once it let a less surface of rubber exposed. Those data fluctuation may be related to the high tide variation (up to 7 m) that can explain a longer residence time in this bay water. Paz-Villarraga et al.2 stand that chlorothalonil has not been registered to use in nautical paints which can explain why these low levels were determinate in the present investigation. Dichlofluanid can be employed as pesticide in crops protection and dock at this bay from rivers water. Overall, this information can be used to investigate the potential impacts of high vessel traffic, most of all large draft ones, in Itaqui Port and create a monitoring program to better understand the dynamics of these contaminants.
CONCLUSIONS Silicone rubbers were tested as passive samplers to determine the presence and quantify third-generation antifouling biocides, commonly used as reinforcing agents in underwater paints. This sampling technique allows us to detect biocide levels usually described as below the limit of quantification since the sampler is exposed to the environment for a period to accumulate the contaminants. Combining these samplers with the chosen extraction method ensured recovery percentages suitable for applying the technique to real samples. The chromatographic conditions provided an efficient separation and quantification of the analytes, despite their very low levels in the water. This paper identified these biocides in Itaqui Port (São Luís, Maranhão, Brazil) waters for the first time. The estuary location is an area of environmental concern as it exhibits high biodiversity, mangrove vegetation and provides a nursery for various marine species. Besides the environmental risk, these compounds can also represent a health and economic threat since the area provides fish and shellfish for household feeding and commerce. Chlorothalonil showed the highest levels in this bay water, followed by DCOIT, while dichlofluanid presented lower levels. Beside chlorothalonil is not registered to be used in nautical paints, international vessels traffic can bring this compound to the studied area. Dichlofluanid fast degradation was not sufficient to guarantee its absence in this environment. It can be related to recent and continuous dock. However, degradation does not mean environmental safety, as their metabolites can also be toxic. Passive sampling captures these biocides as they are continuously released in the water and thus is more suitable to monitor the environment than active sampling. We suggest including degradation products in the upcoming investigations and promoting new campaigns to better understand the dynamics of contaminants and environmental fate.
ACKNOWLEDGMENTS We would like to thank Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA) for the research grant and Empresa Maranhense de Administração Portuária (EMAP) - Itaqui Port's environmental technicians. G. F. was a research fellow of CNPq (PQ 314202/2018-8), while R. M. B. was a PhD fellow of CAPES (Finance Code 001).
REFERENCES 1. Amarante Junior, O. P.; Marcotti-Murua, M.; Stephens, F. S.; International Journal of Hydrology 2020, 4, 191. [Crossref] 2. Paz-Villarraga, C. A.; Castro, I. B.; Fillmann, G.; Environ. Sci. Pollut. Res. 2022, 29, 30090. [Crossref] 3. Onduka, T.; Ojima, D.; Ito, M.; Ito, K.; Mochida, K.; Fujii, K.; Fish. Sci. 2013, 79, 999. [Crossref] 4. Castro, I. B.; Westphal, E.; Fillmann, G.; Quim. Nova 2011, 34, 1021. [Crossref] 5. Subbaiyan, R.; Ganesan, A.; Dhanuskodi, S.; Appl. Biochem. Biotechnol. 2024, 196, 1752. [Crossref] 6. Thomas, K. V.; Brooks, S.; Biofouling 2010, 26, 73. [Crossref] 7. Hamwijk, C.; Schouten, A.; Foekema, E. M.; Ravensberg, J. C.; Collombon, M. T.; Schmidt, K.; Kugler, M.; Chemosphere 2005, 60, 1316. [Crossref] 8. Lee, S.; Lee, Y. W.; Mar. Pollut. Bull. 2016, 113, 253. [Crossref] 9. Jacobson, A. H.; Willingham, G. L.; Sci. Total Environ. 2000, 258, 103. [Crossref] 10. Ansanelli, G.; Manzo, S.; Parrella, L.; Massanisso, P.; Chiavarini, S.; Di Landa, G.; Ubaldi, C.; Cannarsa, S.; Cremisini, C.; Regional Studies in Marine Science 2017, 16, 254. [Crossref] 11. Castro, I. B.; Westphal, E.; Fillmann, G.; Quim. Nova 2011, 34, 1021. [Link] accessed in June 2024 12. Giráldez, I.; Chaguaceda, E.; Bujalance, M.; Morales, E.; J. Chromatogr. A 2013, 1271, 17. [Crossref] 13. Konstantinou, I. K.; Albanis, T. A.; Environ. Int. 2004, 30, 235. [Crossref] 14. Kot, A.; Namieśnik, J.; TrAC, Trends Anal. Chem. 2000, 19, 446. [Crossref] 15. Sanson, A. L.; Baeta, B. E. L.; Rodrigues, K. L. T.; Afonso, R. J. C. F.; Quim. Nova 2014, 37, 150. [Crossref] 16. Silva‐Barni, M. F.; Smedes, F.; Fillmann, G.; Miglioranza, K. S. B.; Environ. Toxicol. Chem. 2019, 38, 340. [Crossref] 17. Batista-Andrade, J. A.; Caldas, S. S.; Arias, J. L. O.; Castro, I. B.; Fillmann, G.; Primel, E. G.; Mar. Pollut. Bull. 2016, 112, 415. [Crossref] 18. Sakkas, V. A.; Konstantinou, I. K.; Albanis, T. A.; J. Chromatogr. A 2001, 930, 135. [Crossref] 19. Sakkas, V. A.; Lambropoulou, D. A.; Albanis, T. A.; Chemosphere 2002, 48, 939. [Crossref] 20. Martin, A.; Margoum, C.; Randon, J.; Coquery, M.; Talanta 2016, 160, 306. [Crossref] 21. Smedes, F.; Bakker, D.; Weert, J.; The Use of Passive Sampling in WFD Monitoring, Deltares: Netherlands, 2010. [Link] accessed in June 2024 22. Lohmann, R.; Booij, K.; Smedes, F.; Vrana, B.; Environ. Sci. Pollut. Res. 2012, 19, 1885. [Crossref] 23. Smedes, F. In Comprehensive Analytical Chemistry; Greenwood, R.; Mills, G.; Vrana, B., eds.; Elsevier: Amsterdam, 2007, ch. 19. [Crossref] 24. Vrana, B.; Allan, I. J.; Greenwood, R.; Mills, G. A.; Dominiak, E.; Svensson, K.; Knutsson, J.; Morrison, G.; TrAC, Trends Anal. Chem. 2005, 24, 845. [Crossref] 25. Aminot, Y.; Belles, A.; Alary, C.; Readman, J. W.; Mar. Pollut. Bull. 2017, 119, 92. [Crossref] 26. Rusina, T. P.; Smedes, F.; Koblizkova, M.; Klanova, J.; Environ. Sci. Technol. 2010, 44, 362. [Crossref] 27. Lohmann, R.; Vrana, B.; Muir, D.; Smedes, F.; Sobotka, J.; Zeng, E. Y.; Bao, L. J.; Allan, I. J.; Astrahan, P.; Barra, R. O.; Bidleman, T.; Dykyi, E.; Estoppey, N.; Fillmann, G.; Greenwood, N.; Helm, P. A.; Jantunen, L.; Kaserzon, S.; Macías, J. V.; Maruya, K. A.; Molina, F.; Newman, B.; Prats, R. M.; Tsapakis, M.; Tysklind, M.; van Drooge, B. L.; Veal, C. J.; Wong, C. S.; Environ. Sci. Technol. 2023, 57, 9342. [Crossref] 28. Vrana, B.; Smedes, F.; Hilscherová, K. In In Situ Bioavailability and Toxicity of Organic Chemicals in Aquatic Systems; Brinkmann, M.; Seiler, T. B., eds.; Humana: New York, 2022, p. 29. [Crossref] 29. Smedes, F.; Booij, K.; ICES Tech. Mar. Environ. Sci. 2012, 52, 20. [Link] accessed in June 2024 30. Seethapathy, S.; Górecki, T.; Anal. Chim. Acta 2012, 750, 48. [Crossref] 31. Instituto Nacional de Metrologia, Normalização e Qualidade Industrial (INMETRO); DOQ-CGCRE-008, Orientações sobre Validação de Métodos Analíticos, INMETRO: Rio de Janeiro, 2020. [Link] accessed in June 2024 32. Yates, K.; Davies, I.; Webster, L.; Pollard, P.; Lawton, L.; Moffat, C.; J. Environ. Monit. 2007, 9, 1116. [Crossref] 33. Agência Nacional De Vigilância Sanitária (ANVISA); Resolução No. 166, de 24 de julho de 2017, Dispõe sobre a Validação de Métodos Analíticos e Dá Outras Providências; Diário Oficial da União (DOU), Brasília, No. 141, de 25/07/2017. [Link] accessed in June 2024 34. Brito, N. M.; Amarante Junior, O. P.; Polese, L.; Ribeiro, M. L.; Pesticidas: Revista de Ecotoxicologia e Meio Ambiente 2003, 13, 129. [Crossref] 35. Ribani, M.; Bottoli, C. B. C.; Collins, C. H.; Jardim, I. C. S. F.; Costa Melo, L. F.; Quim. Nova 2004, 27, 771. [Crossref] 36. Guilhen, S. N.; Pires, M. A. F.; Dantas, E. S. K.; Xavier, F. V.; Quim. Nova 2010, 33, 1285. [Crossref] 37. Wille, K.; Kiebooms, J. A. L.; Claessens, M.; Rappé, K.; Vanden Bussche, J.; Noppe, H.; Van Praet, N.; De Wulf, E.; Van Caeter, P.; Janssen, C. R.; De Brabander, H. F.; Vanhaecke, L.; Anal. Bioanal. Chem. 2011, 400, 1459. [Crossref] 38. O'Hara, S.: Silicone Rubber Passive Samplers for Water Quality Monitoring of Persistent Organic Pollutants in the Marine Environment; Masters Dissertation, Technological University Dublin, Dublin, Ireland, 2009. [Crossref] 39. Prokeš, R.; Vrana, B.; Klánová, J.; Environ. Pollut. 2012, 166, 157. [Crossref] 40. Mochida, K.; Hano, T.; Onduka, T.; Ichihashi, H.; Amano, H.; Ito, M.; Ito, K.; Tanaka, H.; Fujii, K.; Environ. Pollut. 2015, 204, 233. [Crossref] 41. Steen, R. J. C. A.; Ariese, F.; Van Hattum, B.; Jacobsen, J.; Jacobson, A.; Chemosphere 2004, 57, 513. [Crossref] 42. de Campos, B. G.; Figueiredo, J.; Perina, F.; Abessa, D. M. S.; Loureiro, S.; Martins, R.; Crit. Rev. Environ. Sci. Technol. 2022, 52, 3179. [Crossref]
Associate Editor handled this article: Cassiana C. Montagner |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access