Artigo
| Pretreatment strategies for optimizing the lignocellulosic fractionation of pineapple peel residual biomass for energy purposes |
|
Lara Beatriz Pereira de LimaI; Shirlene Kelly Santos CarmoII,*; José Mariano da Silva NetoII,; Stefani da Silva de MeloI; Flávio Luiz Honorato da SilvaIII
I. Departamento de Engenharia Química, Universidade Federal Rural do Semi-Árido, 59625-900 Mossoró - RN, Brasil Received: 12/11/2023 *e-mail: shirlene@ufersa.edu.br Seeking a viable alternative to fossil fuels as the primary energy source, bioethanol has emerged as a promising second-generation fuel derived from lignocellulosic biomass. With Brazil ranking pineapple as the third most produced fruit in temporary crops, this study aims to assess the impact of chemical pretreatment techniques, specifically organosolv and diluted acid, on the residual biomass of Pérola pineapple peel to enhance bioethanol production. Employing an experimental design that incorporates temperature, reaction time, and varying concentrations of organic solvent, the study examines their effects on the susceptibility of the substrate to saccharification for future bioethanol generation. Factors such as alphacellulose, hemicellulose, and lignin were evaluated in triplicate and subjected to statistical analysis using the Tukey test with a confidence level of 95%. Upon application of the pretreatment methods, it was observed that the organosolv solvent approach (utilizing water and ethanol) with sulfuric acid exhibited superior sugar conversion, yielding the highest concentration of xylose at 9.13 g L-1. Additionally, the pretreated biomass contained 1.38 g L-1 of cellobiose, 3.11 g L-1 of arabinose, and 3.76 g L-1 of glucose. This technique demonstrates promising potential for obtaining fermentable sugars crucial for bioethanol production. INTRODUCTION The main source of energy in the world is still fossil fuels, however, their reserves are limited and are running out at an astonishing rate. Renewable energy plays a significant role in the present and future era to overcome the depletion of fossil fuels, this directly contributes to reducing the environmental damage from the emission of greenhouse gases, in addition to controlling the problems associated with pollution. As a viable alternative to replace fossil fuels as a primary energy source, bioethanol has gained strength as a 2nd generation fuel produced from lignocellulosic biomass. It is predominant among various biofuels due to its high-octane number, high heat vaporization, low boiling point, and high fame velocity. Bioethanol has many advantages over fossil fuels. The compression ratio is high, the burn time is shorter and the engine burns thinner, thus resulting in theoretical benefits over petroleum-based fuels.1 Brazil stands out as one of the largest agricultural producers in the world and, as a result, is capable of generating large amounts of agro-industrial waste. These residues, rich in lignocellulosic fibers, appear as an energy alternative from bioethanol, as well as reducing the volume of solid residues inappropriately disposed in the environment. Residual lignocellulosic fibers are polymeric materials of great industrial interest, as they are renewable and biodegradable products. The chemical composition of lignocellulosic biomass generally contains 35-50% cellulose, followed by 20-35% hemicellulose, 10-25% lignin and a small amount of ash and extractives. This chemical composition varies according to the type of biomass.2 These fractions guarantee biomass resistance against microorganism attacks and rigidity. Pineapple (Ananas comosus) is a tropical fruit highly prized for its unique aroma and sweet taste. In addition to being considered a tasty fruit, it is rich in minerals and vitamins that offer several health benefits. Third-placed behind banana and citrus fruits, it shows a growth in the industry from products obtained from its processing and in the processing of its residues.3 Native to Brazil, pineapple is a herbaceous plant, whose cultivation adapts to a wide temperature range, ranging from 5 to approximately 40 ºC. Data from the IBGE (Instituto Brasileiro de Geografia e Estatística)4 indicate that in 2017, 1,502,598 tons of pineapple were produced in Brazil, 594,774 tons in the Northeast region and 363,330 tons in Paraíba. According to Silva,5 a good part of the national pineapple production is destined to the processing of juice, generating an average of 30 to 40% of the total weight of the raw material in solid waste, originating about one million tons of discarded bagasse every year. Pineapple waste has a large amount of fermentable sugars and sucrose. In a study conducted by Botelho et al.,6 aimed at characterizing the dietary fibers found in both the peel and central cylinder of pineapples, it was discovered that these parts of the fruit are significant sources of dietary fiber, particularly cellulose, hemicellulose, and lignin. After saccharification, these structures can be converted into fermentable sugars such as glucose and xylose.7 The high lignocellulosic content of pineapple, composed mainly of cellulose and hemicelluloses, forms strong barriers to saccharification enzymes and microorganisms and prevents easy access to sugars, a phenomenon known as biomass recalcitrance.8 Sustainable bioethanol production requires intensive pretreatment technology for effective recovery of fermentable sugars without decomposition. The choice of pretreatment technology plays a vital role in the cost assessment process of the entire technology, as it contributes about 30 to 35% of the total production cost.9 The use of biomass for energy production requires a pretreatment step, as it aims to increase the enzymatic accessibility of the intracellular sugar in the biomass.10 The literature presents several pretreatment techniques that have already been or continue to be studied, being considered the most important step of the process, since its effects directly influence the yield of the other steps, which makes this the main challenge in obtaining ethanol cellulosic. This fact has intensified the development of research dedicated to seeking more efficient and economical pretreatment methods with little damage to the environment. The organosolv pretreatment is a subcategory of this modality and shows promise, it makes use of organic solvents to deconstruct the lignocellulosic structure and provides an efficient fractionation of the biomass components. The aim of this study was to explore pretreatment processes applied to the biomass of Pérola pineapple peel, aiming to obtain fermentable sugars essential for 2nd generation fuel production. The choice of this material is due to several advantageous reasons: pineapple peel is abundantly discarded after fruit consumption, making it an accessible and sustainable source of raw material for biotechnology. Furthermore, its rich composition in cellulose and hemicellulose facilitates conversion into fermentable sugars, essential in the production of biofuels such as ethanol. In light of the above, pineapple peel presents a series of favorable characteristics, including availability, suitable chemical composition, ease of pretreatment, renewability, and low cost, making it a promising and sustainable option for biofuel production. The fact that Brazil is one of the major producers of materials based on lignocellulosic fibers also demonstrates the great expectation of its sustainable energy potential.
EXPERIMENTAL The research was carried out in the Applied Chemistry and General Chemistry laboratories of the Universidade Federal Rural do Semi-Árido, Campus Pau dos Ferros, Rio Grande do Norte, Brazil. Figure 1 presents the flowchart of the steps performed, from obtaining the biomass (pineapple peel) to the analysis of the sugars resulting from the process of saccharification of the material, through the chemical treatment applied.
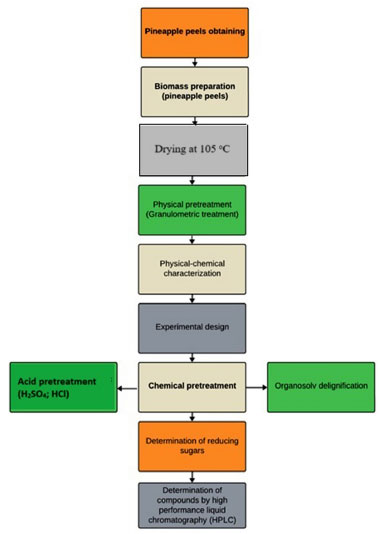 Figure 1. Methodological flowchart
Obtaining and preparing the raw material For research purposes, it was used pineapple peel, Figure 2a, provided by merchants at the local fair in the Pau dos Ferros City, Rio Grande do Norte. The material was initially dried in an oven with air circulation at an average temperature of 105 ºC to remove moisture. The dried residue was then processed in a knife mill (model SL-3) and subsequently sieved at 20 mesh to standardize the granulometry, Figure 2b.
 Figure 2. Pineapple peel waste (a) and dried pineapple peel powder (b)
Characterization of dry and pretreated biomass The dried pineapple peel powder was then characterized in terms of lignin, alphacellulose and hemicellulose, based on the methodology of Morais et al.11 which was based on TAPPI standards. All these analyses were performed in triplicate to ensure greater reliability of the results obtained. Experimental design The pineapple peel biomass was submitted to chemical pretreatment experiments of organosolv delignification and acid pretreatment with sulfuric acid (H2SO4). This was a way used to break the cellular barrier, removing part of the lignin, extractives and hemicellulose, reducing the crystallinity of the structure and increasing the porosity of the material, in order to make the cellulose susceptible to hydrolysis. For the optimization of operational procedures in the process of pretreatment of the lignocellulosic material from the pineapple peel, and to allow the analysis of all possible combinations of the independent variables in order to obtain the best response in relation to the yield in the production of sugars from the saccharification of the material, a complete factorial experimental design and analysis of variance (ANOVA) were performed to determine the statistical significance of the response (p < 0.05). Delignification by the organosolv method was evaluated both in an acid medium using sulfuric acid (H2SO4) and in a basic medium using sodium hydroxide (NaOH). All tests were performed in triplicate, and results were reported as mean and standard deviation. The parameters chosen in this planning were treatment time (min), concentration of reagents (%), and concentration of solvents (%), keeping the temperature fixed at 121 ºC. Tables 1 and 2 show the values defined for the lower (-1) and upper (+1) levels, as well as for the central point (0) and the combinations of factors and levels used in each test, from according to the design matrix.
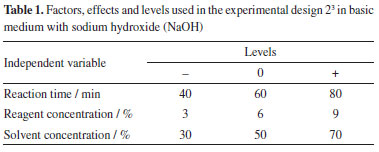
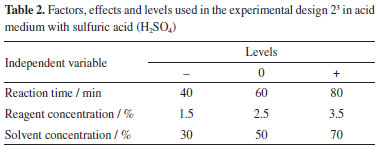
The real and coded levels show the information of the input variables: reaction time, reagent concentration and solvent concentration. Pan et al.12 showed that the process variables that exert a strong influence on the results of the organosolv process stand out: biomass particle size, temperature, reaction time and type of concentration of the organic solvent. Chemical pretreatment Acid pretreatment The biomass was chemically treated with diluted H2SO4 and hydrochloric acid (HCl), for observation of the type of acid that provided the increase in surface area, enabled the decrease in the degree of polymerization and the crystallinity of the cellulose. Based on studies of Silva,13 5.0 g of dried and ground residue were added in a 500 mL Erlenmeyer, 50 mL of 5% diluted acid and a vertical autoclave at 121 ºC was used, with the reaction time of 80 min. Subsequently, the material underwent a filtration process, where the solid fraction was washed with distilled water to remove impurities and obtain a neutral pH, and then taken to an oven (80 ºC) until constant weight. Organosolv delignification In this process, the organosolv solvent (water and ethanol) was used to separate lignin from pineapple peel biomass, in order to enable the hydrolysis of hemicellulose and cellulose and obtain fermentable sugars. Its purpose is to break down the biomass structure by cleaving chemical bonds and reducing cellulose polymerization, thereby enhancing access to cellulose and hemicellulose for conversion into monomeric sugars that can be further processed to produce biofuels. For this procedure, a vertical autoclave was used, evaluating different conditions. In one of them, the alkaline medium with NaOH was used, in the other, the acid medium with H2SO4, with 5 g of dry and ground biomass, setting a temperature of 121 ºC, varying the reaction time (40, 60 and 80 min), base concentration (3, 6 and 9%) acid concentration (1.5, 2.5 and 3.5%) and solvent concentration (30, 60 and 70%). After this step, the material went through the filtration process, and the liquid fraction was quantified for analysis of reducing sugars using the 3,5-dinitrosalicylic acid (DNS) method. Subsequently, the data obtained from the experimental design for pretreating pineapple peel using organosolv in an acidic medium were analyzed utilizing the Statistic 11 software.14 This software is widely recognized for its robustness and versatility in efficiently exploring and interpreting complex data sets. Analysis of reducing sugars (RS) The sugar contents were analyzed in a spectrophotometer (model T80+ UV/Vis, PG instruments Ltd, Porto Alegre, Brazil), at 540 nm and with the aid of a vortex shaker (Warmnest, VX-38, Curitiba, Brazil) to homogenize the samples. The methodology relies on the liquid fraction and employs the 3,5-dinitro salicylic acid (DNS) method, as described by Lorenz Miller15 and adapted by Vasconcelos et al.16 for the reducing sugars analysis. The fermentable sugars, acetic acid, furfural and hydroxymethylfurfural (HMF) quantifications were carried out using high performance liquid chromatography (HPLC) with a Shim-Pack SCR-101H (Shimadzu, Kyoto, Japan) column operating at 65 ºC. Sulfuric acid (5.0 mM) at a flow rate of 0.6 mL min-1 was the mobile phase and a sample with a volume of 20.0 µL was injected into the column. The samples were pre-filtered through 20 µm membranes.
RESULTS AND DISCUSSION Chemical composition of pineapple peel The lignocellulosic characterization of the pineapple peel biomass aimed to know its composition in relation to the cellulose, hemicellulose and lignin contents, which are the most interesting components present in lignocellulosic materials, these contents are presented in Table 3. It is possible to observe a comparison with the content obtained by Banerjee et al.,17 Nordin et al.,18 and Bhatia and Johri.19
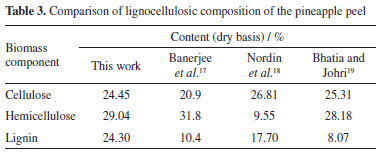
In terms of lignin, the pineapple peel showed values higher than those found by Banerjee et al.17 which was 10.4%, Nordin et al.18 which was 17.10% and as Bhatia and Johri19 which was 8.07%, where these authors used methods different from the one presented in this work. It is also verified that the cellulose and hemicellulose values found for the dried pineapple peel are close to those found by Bhatia and Johri19 when studying the pulverized pineapple peel, the composition value for cellulose was 25.31%, while for hemicellulose it was 28.18%. Hemicellulose is a heteropolysaccharide made up of pentoses (xylose, rhamnose and arabinose), hexoses (glucose, mannose and galactose) and uronic acids (4-O-methyl-glucuronic and galacturonic acids) and provides a more amorphous or non-crystalline nature than the crystalline cellulose polymer with glucose subunits. The amorphous structure of hemicellulose becomes more easily hydrolyzed than cellulose because it has around 100 to 200 sugar units.20 Due to the different ages and periods of biomass harvesting, there are differences in the fiber properties of the plant tissue and in the chemical composition. As the biomass matures, the lignin content increases while the holocellulose composition decreases, especially for amorphous hemicellulose. Evaluation of the experimental design The objective of this study was to fractionate the lignocellulosic biomass of the pineapple peel by the route that presented the best results in this work, that is, to disorganize the organic matrix to allow the hydrolysis step of the carbohydrates. After the organosolv process, the pretreated solid fraction (rich in cellulose - mass recovery) and the liquid fraction were separated. Table 4 presents the values of reducing sugars (RS) obtained for each test in the liquid fraction.
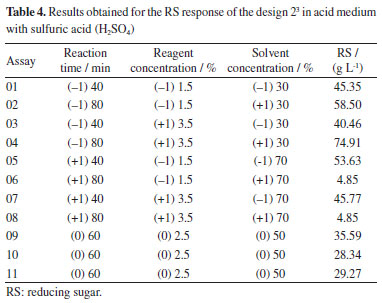
The data from the experimental design carried out with the pretreatment of the pineapple peel using organosolv in acid medium were analyzed using the Statistic 11.0 software14 and with the results, Equation 1 was obtained, which presents the regression model of the experimental data of the 2³ experimental design for the response concentration of RS in the liquid fraction. 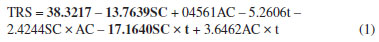 The coefficients presented in the regression in bold are the statistically significant ones at the 95% confidence level and SC, AC, and t represent the solvent concentration, acid concentration, and time, respectively. The determination coefficient (R2) of the regression model was 94.01%. Table 5 presents the ANOVA of the RS concentration-response.
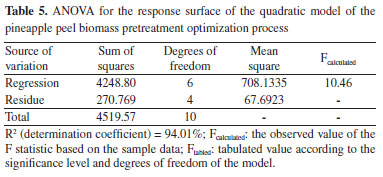
With the data in Table 5, it can be seen that the model is statistically significant, at the 95% confidence level, since the ratio of Fcalculated by Ftabled is equal to 1.70, and is above 1.0. Figure 3 shows the response surface of the influence of time and solvent concentration on the total reducing sugar content. Analyzing Figure 3, it is observed that the increase in time and the decrease in solvent concentration on the reducing sugars content, where operating at levels +1 and -1 for these variables, respectively, yields above 60% are obtained.
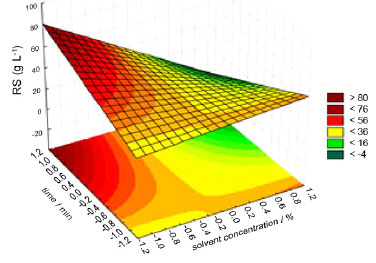 Figure 3. Response surface
In Figure 3, observe that with increasing time and decreasing solvent concentration, keeping the acid concentration fixed at level -1, there is a tendency for the AR concentration to increase. Table 6 presents the biomass recovery in the percentage of the best results obtained, where the liquid fraction obtained was used to determine the percentages of reducing sugars and the solid fraction was used for chemical characterization.
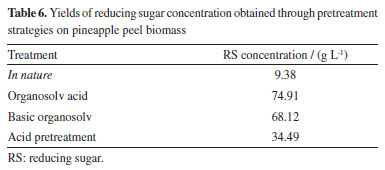
For the residue evaluated in this work, the organosolv pretreatment technique in organic/acid medium, using temperature conditions of 121 ºC, a reaction time of 80 min, the acid concentration of 3.5%, and 30% solvent concentration, resulted in a higher yield of sugars, around 74.91 g L-1. Therefore, there is a significant increase in the amount of sugar compared to its original natural form. A mass yield of 28.17% is obtained under conditions that provide the best sugar yield, where the mass loss is related to the solubilization of part of the lignin, extractives, ash and small fractions of carbohydrates into the liquid phase of the reaction. Thus, from the analysis of the data in Table 6, it appears that the conditions in both the acid and alkaline medium had the greatest mass loss and consequently were the most efficient in the production of reducing sugars. To validate the condition that presented the best yield of sugars, Table 7 shows the compounds, analyzed by high performance liquid chromatography (HPLC), present in the composition of the liquor produced in the treatment condition via acid organosolv technique. Among these, the concentrations of sugars stand out: cellobiose, arabinose, xylose, and glucose.
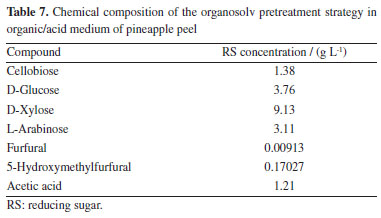
Upon analyzing the experimental data from Table 7, it is noted that 52.5% of the analyzed sugars correspond to xylose and 17.89% to arabinose, indicating the effectiveness of the pretreatment in the deconstruction of lignocellulose and the breaking of chemical bonds, due to the crystallinity and degree of polymerization of the material, where these sugars originate from hemicellulose solubilization. In addition to the mentioned pentoses, lower concentrations of other sugars were identified, with 21.63% being glucose and 7.9% cellobiose. It is expected that with subsequent hydrolysis, the percentage of C6 sugars will increase, as the structure is already more susceptible to degradation, thus elevating the potential of this material for second-generation ethanol production. Michel,21 when studying the acid hydrolysis of soybean husk, obtained 6.14 and 5.15 g L-1 of arabinose and xylose, respectively, and these values are close to those found in this study. Baracho et al.22 found around 7.85 g L-1 of arabinose, when studying the acid hydrolysis process using cactus (Opuntia ficusindica Mill) under conditions of 5% sulfuric acid concentration, temperature of 120 ºC and mass/acid ratio of 1:8. Granada et al.23 highlights in general terms that the chemical composition of pineapple varies greatly depending on the time of year, that is, the harvest tends to occur during the summer, resulting in fruits with high sugar levels and lower levels of acidity. Although the main objective of the acid treatment of lignocellulosic biomass is the removal of hemicellulose to promote cellulose saccharification, this same treatment often involves secondary reactions, which result in byproducts derived from the hydrolysis of acetyl groups present in hemicellulose and the dehydration of monosaccharides, generating acetic acid, furfural and 5-hydroxymethylfurfural as the main products.24 It can be observed in the present work that the concentration of the researched inhibitors, acetic acid (1.21 g L-1), furfural (0.00913 g L-1) and 5-hydroxymethylfurfural (0.17027 g L-1) (Table 7), were distinct and low when compared to the values reported in the literature for hemicellulosic hydrolysates treated with H2SO4. Matos et al.25 after acid treatment of sunflower cake found values of acetic acid (5.27 g L-1), furfural (0.05 g L-1) and 5-hydroxymethylfurfural (0.71 g L-1), while Lima et al.26 found values of 2.1, 3.4 and 0.2 g L-1, respectively. Among the inhibitory agents identified in the pretreatment presented as the best technique in this study, furfural, 5-hydroxymethylfurfural (HMF) and acetic acid stand out as the most harmful to microorganisms used in the fermentation process, both can cause disruption of the cell membrane and interfere with intracellular metabolism.27 Acetic, formic and levulinic acids can also affect the microbial cell membrane and decrease internal pH, increasing turgor pressure and, consequently, causing cell lysis.28
CONCLUSIONS The transition from fossil fuels to renewable energy presents itself as a sustainable alternative to fight against climate change. Renewable energies are the main allies in the decarbonization process. From the studies carried out on the pineapple peel, which presents a great potential for waste generation, mainly in the agroindustry, the results obtained in this research show that the chemical pretreatment of organosolv delignification in acid medium with sulfuric acid allowed a high conversion of polysaccharides into reducing sugars, from their raw natural material composition of 9.38 to 74.91 g L-1. From the results obtained by chromatographic analysis around the fraction of fermentable sugars, the use of lignocellulosic residue from pineapple peel to produce second-generation ethanol is presented as a promising alternative due to its high cellulose content. This fuel brings an unquestionable benefit to the environment, since the biofuel neutralizes the carbon footprint, reduces the emission of greenhouse gases (GHG) in the atmosphere and it is renewable raw natural material, since it is created using resources that can be reused or restocked.
ACKNOWLEDGMENTS The authors would like to thank the Universidade Federal Rural do Semi-Árido (UFERSA) for providing the necessary infrastructure to carry out the research and the Research and Chemical Analysis Group for the efforts of its collaborators in the development of this work.
REFERENCES 1. Verma, D.; Paul, J. S.; Tiwari, S.; Jadhav, S. K.; Waste Biomass Valorization 2022, 13, 4651. [Crossref] 2. Yang, J.; Ching, Y. C.; Chuah, C. H.; Polymers 2019, 11, 751. [Crossref] 3. Ali, M. M.; Hashim, N.; Aziz, S. A.; Lasekan, O.; Food Res. Int. 2020, 137, 109675. [Crossref] 4. Instituto Brasileiro de Geografia e Estatística (IBGE), https://sidra.ibge.gov.br/tabela/5457, accessed in June 2024. 5. Silva, O. O.: Aproveitamento do Bagaço de Abacaxi (Ananas comosus L. Merril) para Produção Biotecnológica de Xilitol; Tese de Doutorado, Universidade Federal de Viçosa, Viçosa, Brasil, 2011. [Link] accessed in June 2024 6. Botelho L.; Conceição A.; Carvalho V. D.; Cienc. Agrotecnol. 2002, 26, 362. [Link] accessed in June 2024 7. Boussarsar, H.; Rogé, B.; Mathlouthi, M.; Bioresour. Technol. 2009, 100, 6537. [Crossref] 8. Sun, Q.; Foston, M.; Meng, X.; Sawada, D.; Pingali, S. V.; O'Neill, H. M.; Li, H.; Wyman, C. E.; Langan, P.; Ragauskas, A. J.; Kumar, R.; Biotechnol. Biofuels 2014, 7, 150. [Crossref] 9. Kumar, M. N.; Ravikumar, R.; Thenmozhi, S.; Kumar, M. R.; Shankar, M. K.; Waste Biomass Valorization 2019, 10, 1693. [Crossref] 10. Boro, M.; Verma, A. K.; Chettri, D.; Yata, V. K.; Verma, A. K.; Environ. Technol. Innovation 2022, 28, 102679. [Crossref] 11. Morais, J. P. S.; Rosa, M. F.; Marconcini, J. M.; Documentos 236: Procedimentos para Análise Lignocelulósica, Embrapa: Campina Grande, 2010. [Link] accessed in June 2024 12. Pan, X.; Gilkes, N.; Kadla, J.; Pye, K.; Saka, S.; Gregg, D.; Ehara, K.; Xie, D.; Lam, D.; Saddler, J.; Biotechnol. Bioeng. 2006, 94, 851. [Crossref] 13. Silva, R. A.: Efeito do Pré-Tratamento Ácido Seguido de Básico na Hidrólise Enzimática do Bagaço de Acerola; Dissertação de Mestrado, Universidade Federal de Campina Grande, Campina Grande, Brasil, 2014. [Link] accessed in June 2024 14. Statistica, version 11; StatSoft Inc., Tulsa, USA, 2012. 15. Lorenz Miller, G.; Anal. Chem. 1959, 31, 426. [Crossref] 16. Vasconcelos, N. M.; Pinto, G. A. S.; de Aragão, F. A. S.; Determinação de Açúcares Redutores pelo Ácido 3,5-Dinitrosalicílico: Histórico do Desenvolvimento do Método e Estabelecimento de um Protocolo para o Laboratório de Bioprocessos, 1ª ed.; Embrapa Agroindústria Tropical: Fortaleza, 2013. [Link] accessed in June 2023 17. Banerjee, S.; Patti, A. F.; Ranganathan, V.; Arora, A.; Food Bioprod. Process. 2019, 117, 38. [Crossref] 18. Nordin, N.; Illias, R. M.; Manas, N. H. A.; Ramli, A. N. M.; Azelee, N. I. W.; Advances in Engineering Research 2020, 200, 10. [Crossref] 19. Bhatia, L.; Johri, S.; Waste Biomass Valorization 2016, 7, 427. [Crossref] 20. Barbosa, F. F.; Tokach, M. D.; DeRouchey, J. M.; Goodband, R. D.; Nelssen, J. L.; Dritz, S. S.; Res. Publ. - Kans., Agric. Exp. Stn. 2008, 158. [Crossref] 21. Michel, A. C. S.: Produção Biotecnológica de Xilitol e Etanol a partir de Hidrolisado de Casca de Soja; Dissertação de Mestrado, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brasil, 2007. [Link] accessed in June 2024 22. Baracho, T. H. A.; Silva, F. L. H.; Torres Neto, A. B.; Congresso de Iniciação Científica da Universidade Federal de Campina Grande; Campina Grande, Brasil, 2009. [Link] accessed in June 2024 23. Granada, G. G.; Zambiazi, C. R.; Mendonça, B. R. C.; B. Ceppa 2004, 22, 405. [Crossref] 24. Jönsson, L. J.; Martín, C.; Bioresour. Technol. 2016, 199, 103. [Crossref] 25. de Matos, J. P.; de Souza, K. R.; dos Santos, A. S.; Pantoja, L. A.; Quim. Nova 2018, 41, 23. [Crossref] 26. Lima, C. S. S.; Conceição, M. M.; Silva, F. L. H.; Lima, E. E.; Conrado, L. S.; Leão, D. A. S.; Appl. Energy 2013, 102, 254. [Crossref] 27. Van der Pol, E. C.; Bakker, R. R.; Baets, P.; Eggink, G.; Appl. Microbiol. Biotechnol. 2014, 98, 9579. [Crossref] 28. Hasunuma, T.; Kondo, A.; Biotechnol. Adv. 2012, 30, 1207. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





