Artigo
| Screening of Schinus terebinthifolia Raddi fruit by access of its functional properties, antioxidant and antimicrobial capacity and metal content |
|
Maria C. da S. SauthierI, II,*; Walter N. L. dos SantosI, III; Jamile da C. CaldasI; Isaac M. de J. SilvaI; José J. C. L. de Souza JúniorI; I. Departamento de Ciências Exatas e da Terra I, Universidade do Estado da Bahia (UNEB), Rua Silveira Martins, 2555, Cabula, 41195-001 Salvador - BA, Brasil Received: 11/06/2023 *e-mail: celestesauthier@gmail.com Fruits of Schinus terebinthifolia Raddi were investigated. A fast and efficient method for the simultaneous determination of 11 polyphenolic substances by high performance liquid chromatography-diode array detector (HPLC-DAD) was applied. Limits of quantification ranged between 0.78 and 3.14 mg L−1. High concentrations of kaempferol were found. An exploratory evaluation was performed using principal component analysis (PCA) for fruit origin differentiation. Total phenolic content (TPC) ranged from 274 to 517 mg GAE 100 g−1; total flavonoid content (TFC) ranged from 101 to 283 mg QE 100 g−1. In vitro analyses showed that antioxidant capacity with 2, 2-diphenyl-1-picrylhydrazyl (DPPH) ranged from 1.7 to 7 µM DPPH 100 g-1. The bacteriostatic and bactericidal effect were also evaluated against: Bacillus subtilis, B. cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella choleraesuis, Candida albicans and C. glabrata, using broth microdilution assay. It can be inferred that S. terebinthifolia Raddi fruit has the potential to act as an ally in the search for new alternatives in the action against pathogens, and it can be used as a potential natural antioxidant and antimicrobial agent. Metals (Cu, Fe, Mg, Mn and Zn) were also determined by flame atomic absorption spectrometry (FAAS), with emphasis on high concentrations of Mg, Zn and Fe. INTRODUCTION Aroeira fruit, also known as Brazilian pink pepper, originates from the aroeira tree, belonging to the Anacardiaceae family, and scientifically named Schinus terebinthifolia Raddi. Apart from being renowned for its versatility in cooking, it is rich in substances beneficial to human health.1, 2 The Anacardiaceae family comprises large branches, encompassing more than 70 genera and 600 known species due to their fruitfulness and high-quality wood.3 The traditional uses of various plants, along with their ethnomedical applications, are well-established and recognized by the ancestral culture of South America.4 In folk medicine, decoction, teas, infusions or tinctures made from the flowers, stems, fruits and leaves of the aroeira tree have been used to treat cancer, ulcer, breathing problems, wounds, rheumatism, gout, diarrhea, skin illnesses, rheumatoid arthritis, urinary disorders. In addition to these antioxidative properties, studies5, 6 have already proved the action of substances isolated from S. terebinthifolia as febrifuge, analgesic, antiseptic, anti-inflammatory and antimicrobial agents, among others. Aroeira pepper is native to South and Central Americas and it has also been found in subtropical and tropical regions of the United States and Africa. It is widespread across Brazil, but the primary reference for pink pepper cultivation is the Espírito Santo State, which exported an average of 700 to 800 tons of the spice in 2022. The main consumer markets for our pink pepper are Europe (internationally) and São Paulo State (nationally). In terms of the ready-to-eat product, a kilo reaches R$ 220, approximately 40 dollars. Pink pepper finds extensive use in the preparation of various products, extending beyond gourmet cooking. Crop waste is used in the distillation and production of essential oils, cosmetics, perfumery, fertilizers, disinfectants, honey, cachaça, beer, etc.7 Phenolic compounds are believed to contribute to allelopathic effects,8 and are known for their high antioxidant capacity. Their consumption has been associated with a reduced risk of developing diseases such as cancer, diabetes and cardiovascular diseases.9 Fruits and vegetables have been extensively studied for their phenolic composition, antioxidant activity and other biological properties.10, 11 Research12 indicates that society is increasingly seeking new food sources beneficial to human health. Functional foods are gaining popularity in Brazilian meals, not only for basic nutrition, but also for physiologically active compounds. Several health benefits associated with pink pepper result from the presence of bioactive compounds and antioxidants in its matrix, making it commercially promising.13 This fruit contains several polyphenolic compounds, including phenolic acids, flavonoids, flavones, anthocyanins, which exhibit antioxidant, anticancer and antifungal properties.14-16 Various analytical methods can be used to determine bioactive phenolic compounds. However, due to the complexity of the matrix and the potential analyte forms in the sample, separation techniques with high power resolution are required. Over the last two decades, high-performance liquid chromatography (HPLC) has dominated the separation and characterization of phenolic compounds in fruits, plant extracts and their derivatives.17, 18 However, total phenolic compounds and antioxidant capacity have been primarily determined using spectrophotometry.19, 20 An exploratory evaluation involving five samples (in triplicate) was conducted using principal component analysis (PCA), considering mean phenolic concentrations (mg 100 g−1) by high performance liquid chromatography-diode array detector (HPLC-DAD) for sample differentiation and the results of spectrophotometry. Moreover, several plant phenols are known for their antimicrobial properties; thus, these substances might change the composition of the colonic flora.21 This research aims to apply a fast and efficient analytical method for the simultaneous determination of 11 polyphenolic compounds by HPLC-DAD in pink pepper samples. Additionally, official methods adapted for total phenolic content (TPC), total flavonoid content (TFC) and antioxidant capacity (using 2, 2-diphenyl-1-picrylhydrazyl or DPPH) were used. Antimicrobial activity was assessed against Bacillus subtilis, B. cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella choleraesuis, Candida albicans and C. glabrata using broth microdilution assay. Furthermore, metals (Cu, Fe, Mg, Mn, and Zn) were determined using flame atomic absorption spectrometry (FAAS) after sample acid digestion. Despite its importance in popular medicine, few scientific studies have examined the biological activity and phytochemical composition of Schinus terebinthifolia Raddi fruits consumed in Salvador, Bahia, Brazil.
EXPERIMENTAL Reagents and standards In the experiments of this study, several reagents and analytical standards with high purity (> 95%) were used: acetic acid, phosphomolybdic acid, hydrochloric acid, phosphoric acid, sodium tungstate and DPPH, all obtained from Merck (Darmstadt, Germany). Aluminum chloride and sodium carbonate were purchased from Vetec Química (Rio de janeiro, Brazil). The analytical standards included: caffeic acid, (+)-catechin, chlorogenic acid, ellagic acid, ferulic acid, gallic acid, kaempferol, p-coumaric acid, quercetin, rutin, syringic acid and vanillic acid, all purchased from Sigma-Aldrich (St. Louis, USA). Ultrapure water was obtained (18.2 MΩ cm) from a Milli-Q water purification system (Millipore, Bedford, USA). Chloramphenicol and ciclopirox olamine (Loprox, São Paulo, Brazil) were used as positive controls for bacteria and fungi, respectively. All solutions were prepared using ultrapure water obtained from a Milli-Q water purification system (Millipore, USA). For analysis by FAAS, 1000 mg L-1 stock solutions (Merck, Darmstadt, Germany) of each element were used to prepare the multi element standard solutions. Hydrogen peroxide (30% v/v) and concentrated nitric acid, both sourced from Merck (Darmstadt, Germany), were used for sample digestion. Equipment For the determination of bioactive compounds, the following equipment was used: a UV-Vis spectrophotometer (SP-22, Biospectro, Brazil) and a lyophilizer (K202, Liotop, Brazil). The extracts were concentrated using a rotary evaporator (IKA RV 10, Brazil), and the Falcon tubes were stirred on a shaker table (0225M, Quimis, Brazil). Additionally, a high-performance liquid chromatography system (HPLC, LC-20AD Prominence, Shimadzu, Japan) was used. This HPLC system was equipped with four high-pressure pumps (LC-20AD, Shimadzu, Japan), diode array detectors (DAD, SPD-20A, Shimadzu, Japan), serially interfaced (CBM-20A, Shimadzu, Japan) with an RP 18 "LiChrospher" column (5 μm, 4.6 × 250 mm, Agilent, Brazil), and controlled by the LC-System software. For the evaluation of the antimicrobial activity, a unidirectional vertical flow (FUV 12, Grupo Veco, Brazil) and an incubator stove (Quimis, Brazil) were used. Plant materials In this study, 0.5 kg of Schinus terebinthifolia Raddi fruit from various sampling locations were obtained in several commercial points in Salvador, Bahia, Brazil, and its surrounding areas, including natural product markets (I: Itapuã pepper) and four organic plantations: Governador Mangabeira, Nazaré, Cabula and Arembepe (M: Mangabeira pepper; C: Cabula pepper; A: Arembepe pepper; N: Nazaré pepper). The plant material was collected from a specimen located in the city of Salvador-BA. Botanical identification was conducted by Eric Oliveira Carvalho, and the exsiccata prepared from the studied specimen was compared with that previously deposited at the Institute (Herbario Radambrasil, HRB), under voucher number 62157. The sample of each collection point was dried in a lyophilizer (Liotop, K202) at −42 ºC and 0.025 mbar for 72 h. The resulting material was homogenized using a domestic food processor, sieved through a Nylon® sieve (100 mesh) and stored in opaque plastic containers, in desiccators. All analyses were performed in triplicate for fruits collected in each sampling point and the results were expressed as mean and standard deviation.22 Preparation of extracts The optimal extraction conditions, evaluated by the determination of total phenolics, which followed the methodology described by Singleton and Rossi,23 were: 0.5 g of the sample with 30 mL of methanol acidified with 10 μL of concentrated HCl, and stirring on a shaker table, at 10 G-force and 25 ºC for 30 min. The extracts were filtered and concentrated on a rotary evaporator (IKA RV 10, China) at 60 ºC and 40 mbar. The resulting material, solubilized in 1.5 mL of methanol, was stored at −20 ºC and filtered through a polytetrafluoroethylene (PTFE) syringe filter (0.45 μm), prior to HPLC and spectroscopic determinations.24 Determination of total polyphenol content (TPC) The determination of total polyphenol content (TPC) followed the methodology described by Singleton and Rossi,23 with some modifications. In summary, 10 μL of the extracts were used, mixed with 500 μL of Folin-Denis reagent (1 M), 400 μL of 7.5% Na2CO3 and deionized H2O to make 10 mL. Incubation was then performed for 30 min at room temperature and absorbance was determined at 760 nm with a UV-Vis spectrophotometer. Gallic acid was used as a standard to construct a calibration curve (0-10 ppm) and TPC values were expressed as gallic acid equivalent per 100 g dry weight (GAE 100 g-1 DW).25 Determination of total flavonoid content (TFC) The total flavonoid content (TFC) was determined using the aluminum chloride method, adapted from Sauthier et al.26 Thus, 100 μL of the methanolic extract of each sample were used in triplicate, transferred to 5 mL volumetric flasks; 3.0 mL of 2.0% dehydrated aluminum chloride (m/v in methanol) were added, as well as methanol, until reaching 5.0 mL. After 30 min, absorbance was measured at 415 nm, using a UV-Vis spectrophotometer. Quercetin was used as a standard for the construction of a calibration curve (0.5-20 mgL-1) and the results were expressed as quercetin equivalent per 100 g dry weight (QE 100 g−1 DW). Determination of total antioxidant capacity by the capture of the free radical DPPH The DPPH method is based on the scavenging of the DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical by antioxidant compounds.27 A 60-μM solution of DPPH was prepared by dissolving 2.4 mg of DPPH in methyl alcohol and adjusting the volume to 100 mL in a volumetric flask with the same solvent. Subsequently, a 50 μL aliquot of the extract was transferred into test tubes containing 3.9 mL of the DPPH (60 μM) radical solution and mixed thoroughly. The kinetic test was conducted with a control solution (3.9 mL of DPPH (60 μM) methanolic solution with 50 μL of methanol) and a solution for determination (3.9 mL of DPPH (60 μM) methanolic solution with 50 μL of extract). Absorbance was measured at 515 nm, and the reaction was monitored chronometrically until absorbance stabilization, typically within 2 min. An analytical curve (10-60 μM DPPH) was constructed at 515 nm. All procedures for this assay were conducted in the absence of light. The results were expressed as the equivalent antioxidant capacity in μM DPPH per 100 g dry weight (µM DPPH 100 g-1 DW). Antimicrobial activity Antimicrobial tests were conducted using broth microdilution according to Santos et al.28 The antimicrobial activity of the extracts was assessed as minimum inhibitory concentration (MIC) by using the successive microdilution assay in 96-well plates. The strains were obtained from the André Tosello Foundation. Tests were carried out in the Bioassay laboratory of the Special Building of the Department of Exact and Earth Sciences I of the State University of Bahia (UNEB). Nutrient broth (Acumedia, USA) and malt extract (Acumedia, USA) were used as culture media for bacterial and fungal growth, respectively. Chloramphenicol (0.19-25 μg mL-1), gentamicin (0.039-5 μg mL-1) and ciclopirox olamine (0.39-50 μg mL-1) were used as positive controls. Stock solutions were prepared by dissolving extracts with dimethyl sulfoxide (DMSO) in water (20% v/v). For this reason, DMSO was used as a negative control. After serial dilution and addition of 100 μL of the microorganism inoculum in all wells (0.5 McFarland), the tested concentration range of the samples was of 7500 to 58.59 μg mL-1. The 96-well plates were incubated at 36 ºC (24 h) and 26 ºC (72 h) for bacterial and fungal growth, respectively. The MIC was determined through the emergence of turbidity in the wells. Antimicrobial activity was assessed against Bacillus subtilis (ATCC 6633), B. cereus (CCT 0096), Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 94863), Pseudomonas aeruginosa (CCT 0090), Salmonella choleraesuis (ATCC 14028), Candida albicans (ATCC 18804) and C. glabrata (CCT 0728). All analyses were performed in triplicate. The MIC was determined through the absence of turbidity in the wells and extracts were considered active when they inhibited microbial growth at concentrations below or equal to 468.75 μg mL-1. From those wells that showed the absence of turbidity, 10 μL of the content were inoculated in solid nutrient broth or malt extract to evaluate whether the observed activity was microbiostatic or microbiocide.28 All samples were tested in triplicate. Analysis by HPLC-DAD Separation was efficiently conducted at 40 ºC, with a continuous flow of 1.0 mL min−1 and an injection volume of 20 μL, following the previously developed and validated methodology.29 All samples and standards were analyzed in triplicate and precision was evaluated by the performance of intra-day and inter-day assays, by six replicated injections of the standard solutions. Limits of detection (LOD) and quantification (LOQ), detected as the injection concentration, with peak heights 3- and 10-fold the signal-to-noise ratio (s/n), were acquired.25, 26 The LOQ ranged between 0.78 and 3.14 mg L−1 and the individual recovery values obtained for the spiked samples ranged from 80 to 120%. The analytical solvents constituting the binary mixture used as the mobile phase were: (A) ultrapure water containing 1.0% acetic acid (v/v), and (B) methanol. The selected elution gradient complied with the following program: 0-10 min, 100% A; 10-20 min, 70% A; 20-30 min, 10% A; 30-37 min, 70% A and 37-40 min, 100% A. The compounds were tested at the wavelengths (nm) of the highest absorption, which provided better resolution between the analytes: 260 for vanillic acid and ellagic acid; 272 for syringic acid; 280 for gallic acid, ferulic acid, trans-cinnamic acid and (+)-catechin; 310 for p-coumaric acid; 330 for chlorogenic acid and caffeic acid; and 360 for rutin, quercetin and kaempferol.30 Analysis by FAAS For metal determination by FAAS, after acid digestion,31 sample masses of 300 mg were weighed directly into a digester tube using an analytical balance. Subsequently, 7.0 mL of 65% (m/m) HNO3 and 1.0 mL of 30% (m/v) H2O2 were added to the digester tube. Digestion was then carried out under the following conditions: temperature: 180 ºC; power: 1000 W; pressure: 100 bar; time: 30 min. After the procedure, the digested sample was transferred to a 50-mL Falcon tube and the solution was made up to 25 mL with ultrapure water (purifier brand). The solutions were stored under refrigeration for later procedures. To determine metal composition by FAAS, five elements were determined, namely: Fe, Cu, Zn, Mn, Mg. An air-acetylene flame was used for all analytes, with a gas flow rate of 1.5 L min-1 for acetylene and 5.0 L min-1 for air. The sample solution introduction rate was 5.0 mL min-1. The reading mode used for absorbance measurements was signal integration and the reading time was 3 s in triplicate. Metal determinations were carried out at the following wavelengths (nm): Fe (248.30); Cu (324.70); Zn (213.90); Mn (279.50) and Mg (202.60). Statistical analysis All extraction assays were carried out in triplicate and each extract was analyzed. The results were expressed as means and standard deviation (SD). Means were compared using analysis of variance (ANOVA). Means of each compound followed by at least one letter in the column do not differ at 5% probability by the Tukey test. Statistical differences were considered to be significant. The relationship between the assays was assessed using Pearson's correlation test.
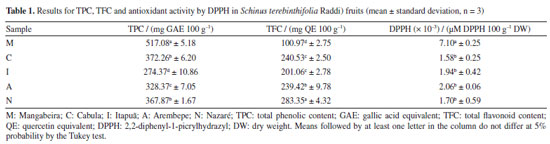
RESULTS AND DISCUSSION Spectrophotometric determinations (TFC, TPC, DPPH) The results obtained in the determination of total phenolics (TPC), total flavonoids (TFC) and antioxidant activity by DPPH in S. terebinthifolia Raddi fruits are presented in Table 1. The results for TPC and TFC ranged from 274.37 to 517.08 mg GAE 100 g-1 and 100.97 to 283.35 mg QE 100 g-1, respectively. The most significant TPC values were observed, in Mangabeira (M) and Cabula (C) samples, both from organic cultivation. For TFC, the lowest values were found in Mangabeira (M) (100.97 mg GAE 100 g-1) and Itapuã (I) fruits (201.06 mg GAE 100 g-1).
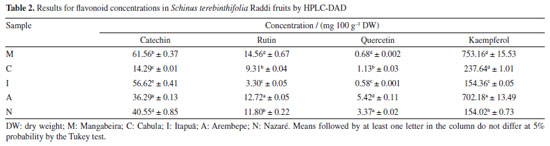
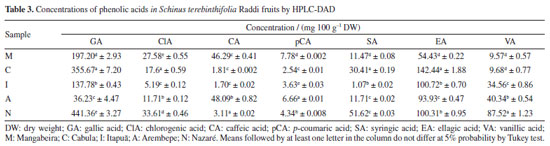
Total antioxidant activity (DPPH) ranged from 0.0016 to 0.0071 µM DPPH 100 g-1. There were no significant differences between the analyzed samples for antioxidant activity, except in the Mangabeira sample (M). Following the application of the Tukey similarity test, it was observed that, at a significance level of 5%, samples: C and N (TPC), C and I (TFC) and C, I, A and N (DPPH) did not present significant differences. Applying Pearson's correlation test, the observed values were: r (correlation coefficient) = 0.87 (TPC × DPPH); r = -0.66 (TPC × TFC) and r = -0.92 (DPPH × TFC), demonstrating a positive correlation between total phenolic content and antioxidant activity by DPPH, and a very strong negative correlation between TPC and FTC, as well as TFC and DPPH. Values greater than 0.561 for this test indicate a significant correlation between the variables, according to critical values table of Pearson's r coefficient. In research25 about phenolic compounds in freeze-dried peels from three tropical fruits grown in Yucatan, Mexico: purple star apple (Chrysophyllum cainito L.), yellow cashew and red cashew (Anacardium occidentale), the TPC values for yellow cashew and red cashew were about 633 and 1317 mg GAE 100 g-1, respectively. The best result found for pink pepper (517 mg GAE 100 g-1) was close to yellow cashew and lower than that of red cashew. The TFC values for yellow cashew and red cashew were about 628 and 833 mg QE 100 g-1, respectively. The best result found for pink pepper (283 mg QE 100 g−1) was lower. In other paper,32 it was determined approximately 1366 mg GAE 100 g-1 (TPC) and 33 mg QE 100 g−1 (TFC) for the methanol and water solution (80:20 v/v) extract of S. terebinthifolius obtained by ultrasound-assisted extraction (30 min) and centrifugation (5 min). In the research of Ennigrou et al.,33 it was determined approximately 270 mg GAE 100 g-1 extract for the methanolic extract of S. terebinthifolia Raddi obtained by ultrasound-assisted extraction and maceration for 30 min and 24 h, respectively. In another study,34 the phenolic compounds of S. terebinthifolia Raddi fruit extracts prepared by different methods (Soxhlet, ultrasound-assisted extraction and supercritical fluid extraction) and solvents (hexane, ethanol, and ethyl acetate) were evaluated, and found values ranging from 2.9 ± 0.4 (supercritical fluid extraction/150 bar/60 ºC) to 60 ± 1.0 mg GAE 100 g-1 extract (soxhlet/ethanol). Extracts of the exocarp and the internal part of S.terebinthifolia fruits were analyzed.35 The authors used caffeic acid as a standard and detected around 50 and 14 mg caffeic acid equivalent per g of extract, respectively. In Melo and de Araújo36 study carried out with mango (Mangifera indica L.), a fruit belonging to the Anacardiaceae family, determined, through the Folin-Ciocalteu method, its total phenolic content. The concentrations found varied between 126.08 and 279.54 mg 100 g-1, which are lower than those found in this study. Thus, based on the total phenolic content, S. terebinthifolia fruit is a good source of bioactive substances compared to mango, which is a tropical fruit of great economic importance. HPLC determinations The results obtained for the determination of flavonoid and phenolic acid concentrations in S. terebinthifolia Raddi fruit by HPLC are shown in Tables 2 and 3, respectively. From the analysis of the content of phenolic and flavonoid compounds, the highest concentrations (mg 100 g−1) found for phenolic acids were: gallic acid (36.23 to 441.36), ellagic acid (54.43 to 142.44) and vanillic acid (9.57 to 87.52). Among the flavonoids, they are: catechin (14.29 to 61.56) and kaempferol (154.36 to 753.16). The concentration of phenolic bioactive compounds in fruits depends on the degree of maturity, variety, climate, soil composition, geographic location and storage conditions, besides other factors.2
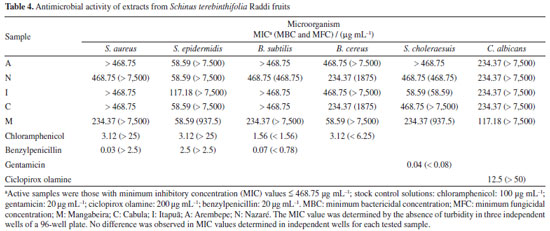
In the Moo-Huchin et al.25 study, six phenolic compounds were identified by HPLC UV-Vis in the peel from the tropical fruits studied: ferulic, caffeic, sinapic, gallic, ellagic acids and myricetin. The results in S. terebinthifolia Raddi fruit for caffeic, sinapic, gallic and ellagic acids had higher concentrations. Analyzing the phenolic profile generally determined in S. terebinthifolia Raddi, it is noticed that gallic acid and catechin are found in this species.37 As kaempferol is a polyphenol found in fruits and vegetables, its determination was expected. However, due to its high concentration, as shown in Table 2, in S. terebinthifolia Raddi fruit, it is perceived that this fruit contains this important strong antioxidant substance. In de Oliveira et al.32 research, potential antioxidant components such biflavonoids (tetrahydroamentoflavone, agathisflavone and hinokiflavone) were determined by ultra-high performance liquid chromatography/electrospray ionization mass spectrometry in S. terebinthifolia Raddi fruit extracts. In another study,38 gallic acid, catechin, chlorogenic acid, epicatechin, quercetin and rutin were also determined in S. terebinthifolia Raddi bark extracts. Antimicrobial activity The obtained values of minimum inhibitory concentrations (MICs) are within the range recommended by the Clinical and Laboratory Standards Institute (CLSI, 2020)39 for antimicrobial compounds (range of 512 to 0.125 μg mL-1). Pink pepper extract samples are made up of a mixture of compounds; therefore, different concentrations were considered for the antimicrobial evaluation (in the range of 7500 to 58.59 μg mL-1). The MIC values of extracts from pink pepper samples that were equal to or less than 468.75 μg mL-1 are shown in Table 4.
Regarding antimicrobial activity analysis, all Gram-positive bacteria were more sensitive to the extracts from S. terebinthifolius fruits than Gram-negative strains, especially S. epidermidis and B. cereus. The extracts obtained from Mangabeira (M), Cabula (C), Arembepe (A) and Nazaré (N) showed a bacteriostatic effect against S. epidermidis, with a MIC = 58.59 μg mL-1. S. epidermidis is present in the microbiota of the human skin. However, it is an opportunistic bacteria, frequently associated with hospital infections in immunocompromised patients, due to its ability to develop in medical material, such as catheters; therefore, this antibacterial effect may contribute to the investigation of new agents against S. epidermidis. The M extract showed bacteriostatic effect for B. cereus, with a MIC of 58.59 μg mL-1. Fruit extracts of this species should be useful for the control of infections caused by Bacillus sp., as observed for the antibacterial activity presented by the methanolic extracts of the fruits against B. cereus and by the effect already reported in the literature40 against B. subtilis of its extracts in acetone (MIC = 4 μg mL-1). Among all the extracts tested, the I extract showed a higher bacteriostatic and bactericidal effect against the Gram-negative bacteria S. typhimurium (S. choleraesuis), with MIC and minimum bactericidal concentration (MBC) values of 58.59 μg mL-1. Regarding fungal effect, all extracts were selective for C. albicans and presented only microbiostatic effect with MIC values in the range of 234.37-117.18 μg mL-1. None of the samples tested showed an effect against the Gram-negative bacteria E. coli or P. aeruginosa, and against the fungi C. glabrata. However, the antifungal effect was evaluated in an extract obtained from leaves, an organ different from that investigated in this study. The M extract showed higher bacteriostatic effect, with a MIC value of 117.2 μg mL-1. In general, the M extract presented higher antimicrobial activity against all the microorganisms tested, except S.typhimurium, which had the highest activity observed for the I extract. Studies41 show that extracts obtained in different solvents (water, hexane and acetone) from S. terebinthifolius fruits demonstrated antimicrobial effects against S. aureus, B. subtilis, E. coli, P. aeruginosa, Candida sp., among others, by agar diffusion and broth microdilution. These previous studies, together with the results obtained, reinforce the antimicrobial potential of S. terebinthifolius fruits. However, they cannot be compared, due to the difference in extraction solvents and geographic distribution of the specimens. The difference in the composition of secondary metabolites in specimens of a plant influences its biological properties. Admittedly, biotic and abiotic factors, such as habitat, exposure to solar radiation, type of soil, climatic differences, among others, interfere with the biosynthesis of secondary metabolites in specimens.42, 43 Therefore, the specific conditions of each location in the municipality of Salvador, where S. terebinthifolius fruits were obtained, can alter the chemical composition of the extracts and, consequently, their antimicrobial activity. Chemometric analysis The results of the chemical analyses were correlated by multivariate analysis using PCA. One-way analysis of variance (ANOVA) and multivariate analysis was carried out by the Statistica software, version 7.0.44 Data were then autoscaled. An exploratory evaluation involving five samples (in triplicate) was performed using PCA, comprising 5 variables: chlorogenic acid (ClA); ellagic acid (EA); gallic acid (GA); catechin (CAT) and kaempferol (KAE) mean phenolic concentrations (mg 100 g−1) by HPLC-DAD (Tables 2 and 3). PCA analysis was applied, after auto-scaled. Components (PC1 × PC2) describe 81.18% of total data variance and provide discriminatory information related to the samples. The major component (PC1) accounts for 52.27% of the total variance. The bioactive compounds: chlorogenic, ellagic and gallic acids are the dominant variables on this PC (better scores 0.3), thereby causing greater variability among these samples (PC1 = -0.33ClA - 0.33EA - 0.36GA + 0.14CAT + 0.14KAE). The second major component (PC2) accounts for 28.91% of the total variance and the phenolics, catequin and kaempferol (better scores 0.4), are the dominant variables on this PC (PC2 = -0.34ClA - 0.23EA - 0.16GA + 0.40CAT + 0.57KAE). Figure 1a shows the score plots (PC1 × PC2) and Figure 1b the loading plots of the PCA at different S. terebinthifolia Raddi fruit samples in relation to phenolic concentrations (mg 100 g-1) by HPLC.
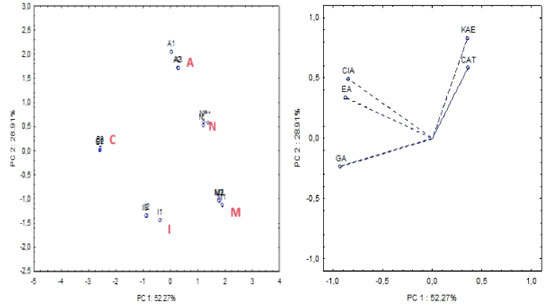 Figure 1. (a) Score and (b) loading plots: PC1 vs. PC2 of Schinus terebinthifolia Raddi fruit samples in relation to phenolic concentrations (mg 100 g-1 DW) by HPLC-DAD. M: Mangabeira; C: Cabula; I: Itapuã; A: Arembepe; N: Nazaré; GA: gallic acid; ellagic acid; ClA: chlorogenic acid; KAE: kaempferol; CAT: catechin; DW (dry weight)
The obtained data underwent PCA, focusing on evaluating the most effective substances in the samples, namely, catechin (CAT) and kaempferol (KAE) (flavonoids), along with chlorogenic (ClA), ellagic (EA) and gallic (GA) acids. By examining the score clustering displayed in Figure 1a, which corresponds to specific groups of samples, and the positioning of variables (Figure 1b) strongly correlated with the respective principal components (PC1 × PC2), an attempt was made to identify how the phenolic compounds markers contribute to the classification of different groups via PCA. Specifically, ellagic acid was identified with a marker for samples from the Cabula region (C), located in the upper left quadrant, while kaempferol served as a marker for samples from Arembepe (A), situated in the upper right quadrant (Figure 1). In addition, PCA was based on spectrophotometric analysis involving 5 samples (in triplicate) of pink pepper, incorporating 3 variables: TPC, TFC and antioxidant activity assessed by DPPH (refer to Table 1). The components (PC1 × PC2) accounted for 98.32% of total data variance, providing discriminative information regarding the samples. PC1 represented 65.64% of total variance, while PC2 represented 32.68% of the variance (Figure 2).
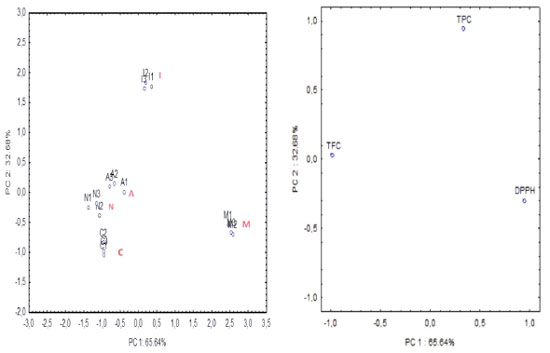 Figure 2. (a) Score and (b) loading plots: Schinus terebinthifolia Raddi fruit samples in relation to phenolic concentrations (TPC, TFC) and antioxidant activity (DPPH) by spectrophotometric analysis. M: Mangabeira; C: Cabula; I: Itapuã; A: Arembepe; N: Nazaré; TPC: total phenolics concentration in (mg 100 GAE g-1); TFC: total flavonoids concentration in (mg QE 100 g-1); DPPH: antioxidant activity by 2, 2-diphenyl-1-picrylhydrazyl in (µM DPPH 100 g-1 of dry sample), (mean ± standard deviation, n = 3)
The dominant variables are DPPH in PC1 and TPC in PC2 (Figure 2). Comparing the scores (Figure 2a) with the loading (Figure 2b) plot, it becomes evident that there is a strong correlation between TFC and samples A, N and C, as indicated by their placement in the left quadrant (Figure 2), which is further supported by the data presented in Table 1. Similarly, sample I exhibits a higher correlation with TPC, as evidenced by its position in the upper quadrant (Figures 2a and 2b). Moreover, DPPH shows correlation for sample M (Mangabeira) (as shown in Figure 2, right quadrant). Furthermore, soil composition and environmental conditions to which the fruit is exposed are crucial factors that can influence the concentrations of these bioactive compounds in the studied matrix. The Mangabeira sample (M) originated from an organic plantation situated approximately 100 km away from Salvador, where the majority of the other samples were obtained. This geographical distinction may account for the isolated discrimination of this sample in the two PCA plots. Results for FAAS determination The predominant element in all S. terebinthifolia Raddi fruit samples was Mg and their concentration exceeds that of several fruits, such as banana and papaya.45 Magnesium is required for nucleic acids in protein synthesis and human reproduction. It can also be considered a good source of Zn and an excellent source of Fe. No other studies on the mineral composition of pink pepper were found to establish a more adequate comparison. The results for metal determination in S. terebinthifolia Raddi fruit using FAAS are presented in Table 5.
CONCLUSIONS Through the use of chromatographic, spectrophotometric, spectrometric and serial dilution techniques in this study, it was demonstrated that S. terebinthifolia Raddi fruit is a significant source of bioactive phenolics. It was possible to identify and quantify 11 analytes in less than 20 min by high-performance liquid chromatography. These analytes include phenolic acids such as caffeic, chlorogenic, p-coumaric, ellagic, gallic, syringic and vanillic acids, along with flavonoids as catechin, quercetin, rutin and kaempferol. It is noteworthy that the high concentration of kaempferol is particularly valuable, given its abundance in plants and its known properties, including anti-diabetic and anti-inflammatory action. Multivariate analysis with PCA suggests the presence of potential markers that can aid in sample identification and differentiation, based on their geographical origin. Considering the properties of the identified analytes, the methanolic extract of S. terebinthifolia Raddi fruits exhibited remarkable activity against the tested microorganisms, demonstrating bacteriostatic and bactericidal effects against microorganisms such as S. epidermidis. This suggests that pink pepper may serve as a valuable ally in the search for novel alternatives against pathogens. Moreover, the study also identified some important elements crucial for the proper functioning of organisms, with a particular emphasis on the high concentrations of magnesium and iron found in the samples.
ACKNOWLEDGMENTS The authors are grateful to Brazilian agencies that supported the study: Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Programa de Apoio a Núcleos de Excelência (PRONEX).
REFERENCES 1. Morais, T. R.; Coutinho, A. P. R.; Camilo, F. F.; Martins, T. S.; Sartorelli, P.; Massaoka, M. H.; Figueiredo, C. R.; Lago, J. H. G.; J. Braz. Chem. Soc. 2017, 28, 492. [Crossref] 2. Ferreira, M. D.; de Souza Neta, L. C.; Brandão, G. C.; dos Santos, W. N. L.; Biotechnol. Appl. Biochem. 2023, 70, 1001. [Crossref] 3. Cardoso, M. P.; Lima, L. S.; David, J. P.; Moreira, B. O.; Santos, E. O.; David, J. M.; Alves, C. Q.; J. Braz. Chem. Soc. 2015, 26, 1527. [Crossref] 4. Pinilla, L. C. A.; López, Á. M.; Gálvez, A. F.; Corpas, E. J.; Rosero-Moreano, M.; Stashenko, E. E.; J. Braz. Chem. Soc. 2021, 32, 552. [Crossref] 5. Barreira, C. F. T.; de Oliveira, V. S.; Chávez, D. W. H.; Gamallo, O. D.; Castro, R. N.; Damasceno Júnior, P. C.; Sawaya, A. C. H. F.; Ferreira, M. S.; Sampaio, G. R.; Torres, E. A. F. S.; Saldanha, T.; Food Chem. 2023, 403, 134347. [Crossref] 6. Tlili, N.; Yahia, Y.; Feriani, A.; Labidi, A.: Ghazouani, L.; Nasri, N.; Saadaoui, E.; Khaldi, A.; Ind. Crops Prod. 2018, 122, 559. [Crossref] 7. A Gazeta, Produtores do ES Apostam na Pimenta-Rosa, Valiosa para Culinária e Beleza, https://www.agazeta.com.br/es/agro/produtores-do-es-apostam-na-pimenta-rosa-valiosa-para-culinaria-e-beleza-0923, accessed in July 2024. 8. Jasim, I. R.; Yaqub, H. M.; Ibrahim, F. K.; Al-KitabJ. Pure Sci. 2023, 7, 89. [Crossref] 9. Santos, B. O.; Tanigaki, M.; Silva, M. R.; Ramos, A. L. C. C.; Labanca, R. A.; Augusti, R.; Melo, J. O. F.; Takahashi, J. A.; de Araújo, R. L. B.; J. Braz. Chem. Soc. 2022, 33, 1058. [Crossref] 10. Barizão, É. O.; Boeing, J. S.; Rotta, E. M.; Volpato, H.; Nakamura, C. V.; Maldaner, L.; Visentainer, J. V.; J. Braz. Chem. Soc. 2021, 32, 2206. [Crossref] 11. de Magalhães, B. E. A.; Santana, D. A.; Silva, I. M. J.; Minho, L. A. C.; Gomes, M. A.; Almeida, J. R. G. S.; dos Santos, W. N. L.; Microchem. J. 2020, 155, 104683. [Crossref] 12. de Magalhães, B. E. A.; dos Santos, W. N. L.; An. Acad. Bras. Cienc. 2020, 92, e20190646. [Crossref] 13. da Silva, B. G.; do Prado J. M.; Fileti, A. M. F.; Foglio, M. A.; Vieira e Rosa, P. T.; Chem. Eng. J. Adv. 2023, 15, 100514. [Crossref] 14. Lima, N. N. C.; Faustino, D. C.; de Almeida, B. S.; de Magalhães, B. E. L.; Santos, L. F. P.; Santana, D. A.; Pinto, L. C.; Eur. Food Res. Technol. 2023, 249, 1627. [Crossref] 15. Rodrigues, C. A.; Nicácio, A. E.; Jardim, I. C. S. F.; Visentainer, J. V.; Maldaner, L.; J. Braz. Chem. Soc. 2019, 30, 1229. [Crossref] 16. Pereira, D. P.; da Silva, A. I. B.; Nunes, L. E.; de Sá-Filho, G. F.; Ribeiro, L. H. F.; Revista Saúde e Meio Ambiente 2021, 13, 25. [Link] accessed in July 2024 17. da Silva, V. C.; de Magalhães, B. E. A.; Magalhães, T. B. S.; Guimarães, E. T.; Guedes, A. S.; Mota, M. D.; dos Santos, W. N. L.; Cerqueira, B. A. V.; Santos Júnior, A. F.; Rev. Colomb. Cienc. Quim.-Farm. 2023, 51, 1341. [Crossref] 18. Bernal, F. A.; Orduz-Díaz, L. L.; Coy-Barrera, E.; Quim. Nova 2016, 39, 160. [Crossref] 19. Azevedo, R. S. A.; Teixeira, B. S.; Sauthier, M. C. S.; Santana, M. V. A.; dos Santos, W. N. L.; Santana, D. A.; Food Chem. 2019, 273, 39. [Crossref] 20. dos Santos, A. M. P.; Silva, E. F. R.; dos Santos, W. N. L.; da Silva, E. G. P.; dos Santos, L. O.; Santos, B. R. S.; Sauthier, M. C. S.; dos Santos, W. P. C.; Microchem. J. 2018, 138, 98. [Crossref] 21. da Silva, J. H. S.; Simas, N. K.; Alviano, C. S.; Alviano, D. S.; Ventura, J. A.; de Lima, E. J.; Seabra, S. H.; Kuster, R. M.; Nat. Prod. Res. 2018, 32, 1365. [Crossref] 22. dos Santos, W. N. L.; Sauthier, M. C. S.; Cavalcante, D. D.; Benevides, C. M. J.; Dias, F. S.; Santos, D. C. M. B.; An. Acad. Bras. Cienc. 2016, 88, 1243. [Crossref] 23. Singleton, V. L.; Rossi, J. A.; Am. J. Enol. Vitic. 1965, 16, 144. [Crossref] 24. Burneo, N. F.; Mora-Medina, M.; Figueroa, J. G.; Quim. Nova 2022, 45, 621. [Crossref] 25. Moo-Huchin, V. M.; Moo-Huchin, M. I.; Estrada-León, R. J.; Cuevas-Glory, L.; Estrada-Mota, I. A.; Ortiz-Vázquez, E.; Betancur-Ancona, D.; Sauri-Duch, E.; Food Chem. 2015, 166, 17. [Crossref] 26. Sauthier, M. C. S.; da Silva, E. G. P.; Santos, B. R. S.; Silva, E. F. R.; Caldas, J. C.; Minho, L. A. C.; dos Santos, A. M. P.; dos Santos, W. N. L.; Food Chem. 2019, 273, 115. [Crossref] 27. Brand-Williams, W.; Cuvelier, M. E.; Berset, C.; LWT - Food Sci. Technol. 1995, 28, 25. [Crossref] 28. Santos, R. L.; Miguêz, L. S.; Castro, J. O.; Silva-Jardim, I.; Bastos, T. M.; de Sousa, K. A. F.; Soares, M. B. P.; de Souza, A. J.; Santana, A. N.; de Jesus, A. S.; Pereira, M. G.; de Souza Neta, L. C.; Nat. Prod. Res. 2023, 37, 2951. [Crossref] 29. dos Santos, W. N. L.; Sauthier, M. C. S.; dos Santos, A. M. P.; Santana, D. A.; Azevedo, R. S. A.; Caldas, J. C.; Microchem. J. 2017, 133, 583. [Crossref] 30. Sá, R. R.; Matos, R. A.; Silva, V. C.; Caldas, J. C.; Sauthier, M. C. S.; dos Santos, W. N. L.; Magalhães, H. I. F.; Santos Júnior, A. F.; Microchem. J. 2017, 135, 10. [Crossref] 31. Santos Júnior, A. F.; Sá, R. R.; Silva, L. O. B.; Magalhães, H. I. F.; Tarantino, T. B.; Korn, M. G. A.; J. Braz. Chem. Soc. 2017, 28, 2163. [Crossref] 32. de Oliveira, V. S.; Augusta, I. M.; Braz, M. V. C.; Riger, C. J.; Prudêncio, E. R.; Sawaya, A. C. H. F.; Sampaio, G. R.; Torres, E. A. F. S.; Saldanha, T.; Food Chem. 2020, 315, 126274. [Crossref] 33. Ennigrou, A.; Casabianca, H.; Laarif, A.; Hanchi, B.; Hosni, K.; S. Afr. J. Bot. 2017, 108, 407. [Crossref] 34. Andrade, K. S.; Poncelet, D.; Ferreira, S. R. S.; J. Food Eng. 2017, 204, 38. [Crossref] 35. Feuereisen, M. M.; Barraza, M. G.; Zimmermann, B. F.; Schieber, A.; Schulze-Kaysers, N.; Food Chem. 2017, 214, 564. [Crossref] 36. Melo, E. A.; de Araújo, C. R.; Semina: Cienc. Agrar. 2011, 32, 1451. [Crossref] 37. Zhuang, Y.; Chen, L.; Sun, L.; Cao, J.; J. Funct. Foods 2012, 4, 331. [Crossref] 38. Migues, V. H.; David, J. M.; David, J. P.; Anal. Methods 2020, 12, 1478. [Crossref] 39. Clinical and Laboratory Standards Institute (CLSI); Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, USA, 2020. [Link] accessed in July 2024 40. Hussein, H. S.; Salem, M. Z. M.; Soliman, A. M.; Sci. Hortic. 2017, 216, 111. [Crossref] 41. Salem, M. Z. M.; El-Hefny, M.; Ali, H. M.; Elansary, H. O.; Nasser, R. A.; El-Settawy, A. A. A.; El Shanhorey, N.; Ashmawy, N. A.; Salem, A. Z. M.; Microb. Pathog. 2018, 120, 119. [Crossref] 42. Ribeiro, D. A.; Camilo, C. J.; Nonato, C. F. A.; Rodrigues, F. F. G.; Menezes, I. R. A.; Ribeiro-Filho, J.; Xiao, J.; Souza, M. M. A.; da Costa, J. G. M.; Food Chem. 2020, 315, 126277. [Crossref] 43. Formato, F.; Scharenberg, F.; Pacifico, S.; Zidorn, C.; Phytochemistry 2022, 203, 113385. [Crossref] 44. Statistica®, version 7.0; StatSoft Inc., Tulsa, USA, 2007. 45. Lima, D. G.; Padovani, R. M.; Rodriguez-Amaya, D. B.; Farfán, J. A.; Nonato, C. T.; de Lima, M. T.; Salay, E.; Colugnati, F. A. B.; Galeazzi, M. A. M.; Tabela Brasileira de Composição de Alimentos - TACO, 4th ed.; NEPA-UNICAMP: Campinas, 2011. [Link] accessed in July 2024
Editor handled this article: Jorge M. David |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access