Artigo
| Occurrence of polybrominated diphenyl ethers (PBDEs) in surface sediments of an urban artificial lake in Brazil |
|
Fernando F. SodréI,* I. Instituto de Química, Universidade de Brasília, 70910-900 Brasília - DF, Brasil Received: 04/01/2024 *e-mail: ffsodre@unb.br Polybrominated diphenyl ethers (PBDEs) are persistent flame retardants widely used in everyday materials, with the potential to leach out and contaminate indoors and receiving waters. Despite their well-documented ecological risks, there has been limited reporting on PBDE levels in sediments from Brazilian freshwaters, motivating the monitoring of sediments from Paranoá Lake, in the Brazilian capital. After sampling, extraction, and analysis of congeners BDE-28, -47, -66, -85, -99, -100, -138, -153, and -154 by gas chromatography with electron capture detection, Σ9PBDE levels ranged from 3.9 ± 0.2 to 19 ± 1 ng g-1 dw, with higher concentrations attributed to effluent discharge from wastewater treatment plants (WWTPs), followed by the influence of storm drainage water contamination. The distribution of PBDE congeners suggested a predominant influence of the commercial pentaBDE formulation, likely DE-71. However, in points influenced by WWTPs, the prevalence of BDE-66 suggests biotic reductive debromination of high-brominated PBDE, possibly BDE-209, indicating the possible influence of the decabrominated formulation. The distribution of PBDEs in the environment, estimated using the EQC (equilibrium criterion) model, level I, indicated low concentrations in the water column, but also noteworthy levels in biota and sediment precursor materials. Finally, preliminary ecological risk assessment indicated the need for future further surveys. INTRODUCTION Polybrominated diphenyl ethers (PBDEs) are synthetic chemicals extensively employed as flame retardants in various consumer products since the 1970s.1,2 These compounds play a crucial role in modern society by enhancing fire safety in everyday items, critical infrastructures, and transportation vehicles, among others. Their addition to materials like plastics, textiles, electronics, and building materials helps reduce fire risk and slow down the spread of flames, thus mitigating potential damage and loss of life associated with fires. Despite their beneficial fire-retardant properties, PBDEs raise significant concerns due to their ability to persist in the environment for extended periods and accumulate in environmentally relevant particles and living organisms, particularly in fatty tissues.3 This bioaccumulation leads to significant concentrations of PBDEs in organisms at higher trophic levels, posing risks to ecosystems, wildlife predators and humans consuming contaminated food.4 Recognizing their potential persistence, bioaccumulation and risks, several PBDE congeners have been banned or restricted in many countries. Three major commercial formulations of PBDEs, penta, octa, and decaBDE, have been extensively used worldwide.5,6 While penta and octaBDE were listed as persistent organic pollutants (POPs) by the Stockholm Convention in 2009, decaBDE was only added in 2017.2 Despite regulations, PBDEs continue to persist in the environment due to their historical use and ongoing release from existing products and waste. Environmental levels of PBDE congeners are expected to persist for decades, even after production ceases, due to their large inventory.1 Their primary sources of contamination include volatilization, effusion, and improper disposal during production, recycling, and dismantling processes.5 Particularly, e-waste recycling, including incineration of PBDE-infused products, still remains an important route of PBDE release.7 Considerable attention has been paid to understanding the fate and transport of PBDEs in various environments.5,8 Although research on their global occurrence remains limited to certain areas and countries,5 it is well-known that PBDEs often bind to atmospheric particles, accumulating in water, sediment, soil, and organisms, with a potential long-range transport through atmospheric deposition and bioaccumulation.9 In urban areas, migration pathways of PBDEs include their accumulation to dust, physical transfer through product abrasion, and direct contact with dust on product surfaces.5,10 These pathways contribute to indoor PBDE sources, where material abrasion and direct material-dust partitioning play significant roles, influencing PBDE fate indoors.11,12 Additionally, local release of PBDEs from road, urban surfaces, and vehicle leaching is expected due to their use in construction materials and automobile parts.13 As they are not chemically bound to the materials, they contaminate indoor and outdoor dust, being transported via surface runoff or municipal sewer systems to wastewater treatment plants (WWTPs) or, depending on regional sanitation conditions, directly into receiving waters.14-16 In WWTPs, several studies showed that PBDEs are present in both effluents and sludge, although the effluent levels tends to be much lower,17,18 mainly due to the high partitioning of PBDEs to solids.19 However, there are also reports20,21 that between 52 and 80% of PBDEs could persist in WWTP effluents, serving as a significant source to receiving waters depending on the nature of the final effluent and the treatment processes. When raw sewage undergoes proper treatment, effluents from WWTPs are likely a significant source of PBDE contamination, particularly in receiving water bodies surrounding these facilities.22 Due to the hydrophobic nature of PBDE congeners, significant attention has been devoted to their contamination of living organisms and various types of sediments, given their important role as reservoirs and sinks for a diversity of POPs. While the vast majority of studies carried out in Brazil explore the presence and possible effects of PBDEs on aquatic biota, mainly in marine and estuarine environments,23-27 there is a virtual absence of studies involving sediment contamination, notably in freshwater ecosystems.28 Ferrari et al.29 reported levels of up to 5.4 ± 0.2 ng g-1 in sediments from the direct recharge area of the Guarani Aquifer in Ribeirão Preto, Brazil. In a study tangentially related to the topic, Cristale et al.30 investigated the occurrence of PBDE, new brominated, and organophosphorus flame retardants in soil, dust, leachate, and well water samples from a landfill in Araraquara, Brazil, and observed significant amounts of waste containing flame retardants in the landfill, as well as high concentrations in soils and dusts in nearby areas where the wastes are handled and stored. Due to the scarcity of available data on the presence of this significant class of persistent pollutants in freshwater sediments across Brazil, this study seeks to investigate the sources and concentrations of PBDE congeners, specifically in sediments from Paranoá Lake, a vital artificial reservoir located in the capital of the nation.
EXPERIMENTAL Study area and sampling points Located within the Brazilian Federal District (FD), Paranoá Lake is an artificial reservoir covering an area of 38 km2, with an average water volume of around 500 million m3 and depth ranging from 13 to 40 m. During the construction of the capital of Brazil, Brasília, in the 1950s, the Paranoá River was dammed, resulting in the flooding of areas below 1000 m above sea level and the creation of Paranoá Lake. This was done to ameliorate the harsh climate of the region, provide hydroelectric power, and offer recreational opportunities. Originally designed for 500,000 inhabitants, the population of Brasília now exceeds 3.0 million, putting significant pressure on local water sources.31 Presently, the hydroelectric potential of Paranoá Lake remains largely untapped, primarily serving for recreation and as the main receiver of domestic wastewater. Along its shores are embassies, sports clubs, restaurants, residential areas, and two wastewater treatment plants located in the north and south wings of Brasília’s Pilot Plan, which resembles the shape of an airplane. In addition to receiving wastewater from these treatment plants, Paranoá Lake also receives substantial amounts of rainwater runoff.31 Since October 2017, it has also served as a source of drinking water. Figure 1 illustrates the study area, the location of the sampling points selected for this study, and the WWTPs Brasília-North (BN) and Brasília-South (BS). In this lake water flows from west to east.
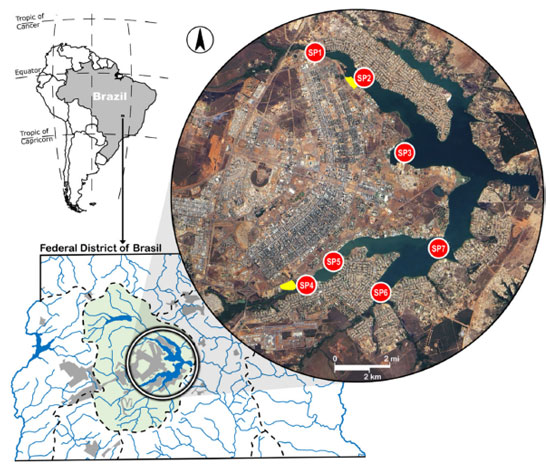 Figure 1. Map showing the location of the Federal District of Brazil, Paranoá watershed (in light green) and the location of the sampling points in the Paranoá Lake. Brasília-North wastewater treatment plant (BN-WWTP) and Brasília-South wastewater treatment plant (BS-WWTP) are highlighted in yellow
Sampling point 1 (SP1) is situated in one of the four lake branches, immediately after the inflow of water from Bananal Creek, which drains a relatively pristine area encompassing the National Park of Brasília.32 Still within the Bananal Branch, sampling point SP2 is located 2.3 km from SP1, towards the center of the lake. SP2 is located nearby the BN-WWTP, which is responsible for treating wastewater from approximately 145,000 inhabitants.33 Located in the Brasília Yacht Club Bay, SP3 serves as a critical sampling site, receiving not only loads from the urban storm drainage system but also contributions from irregular domestic sewage connections.34 In the Riacho Fundo Branch of the lake, to the south, the SP4 sampling point is located in the proximity of the BS-WWTP, serving about 525,000 people in the FD.33 Riacho Fundo Creek is considered the most polluted tributary, receiving effluents from other WWTPs and draining an area primally occupied by urban activities.35 In the vicinities of the Nippo-Brazilian Club is located the SP5 sampling point, 2.1 km from SP4. This area is also influenced by stormwater discharges, but from the southern part of Brasília. Sampling point SP6 is located downstream of the inflow of Gama Creek, which drains a relatively undisturbed area characterized by limited agricultural and residential development. Downstream of the confluence of the Gama and Riacho Fundo branches, sampling point SP7 is located beneath the Juscelino Kubitschek Bridge, renowned as one of the prominent landmarks of Brasilia. Chemicals and reagents Pesticide-grade acetone, iso-octane, and n-pentane were purchased from Tedia (Fairfield, USA). Anhydrous sodium sulfate (99%), obtained from Vetec (Rio de Janeiro, Brazil), underwent pre-treatment at 150 °C for 2 h prior to usage. Silica gel 60 (0.05 to 0.2 mm), also from Vetec, served as the clean-up sorbent in its acidic, neutral, and basic forms. Detailed information regarding the preparation of silica gel can be found elsewhere.36 A mixed analytical standard solution (10 μg mL-1) containing the congeners BDE-28, -47, -66, -85, -99, -100, -138, -153, and -154 (AccuStandard, New Haven, USA) was used to prepare working solutions at concentrations of 0.01, 0.1, 1.0, 5.0, 10.0, and 20.0 ng mL-1. The chemical structures of the investigated PBDEs are depicted in Figure 2.
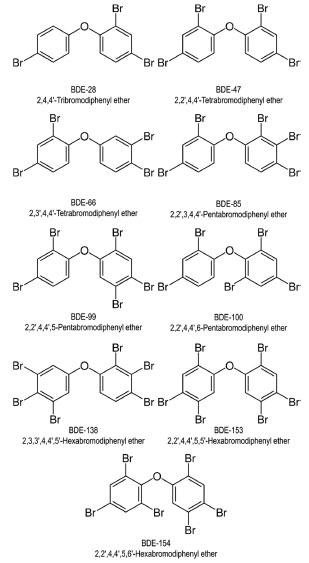 Figure 2. Structure, Ballsmitter-Zell (BZ) numbers, and International Union of Pure and Applied Chemistry (IUPAC) names of the nine PBDE congeners examined in Paranoá Lake sediments
Sampling and sample preparation A boat, kindly provided by the Environmental Military Police Battalion (BPMA/PMDF), was used to access the sampling points. Samples were then collected from the upper sediment layer using a van Veen grab sampler. After retrieval, the dredge was opened onto a plastic tray to allow for the removal of branches, glass, plastics, and other larger impurities. Using a spatula, the sediment was transferred to pre-labeled collection bags and stored in a cooler box for transport to the laboratory, where it was preserved at 4 °C until drying under ambient temperature and airflow in a laboratory hood. The analytical methods employed in this study were previously developed and validated and are succinctly described elsewhere.36 Briefly, 3.000 g of dried sediment samples were transferred to previously cleaned glass tubes containing 10 mL of an acetone:n‑pentane (1:1 v/v) mixture. The suspensions underwent sonication for 5 min and were subsequently centrifuged for 5 min at 1,000 × g. The resulting suspension was centrifuged for additional 5 min at 1000 × g, and the supernatant was then transferred into a 250 mL flat-bottomed flask. This procedure was repeated five times and a composite was produced. Then, iso-octane was added as a solvent keeper (1.0 mL), and the extract was rotary-evaporated to approximately 2 mL. Interferences were suppressed by eluting the extract with 50 mL of n-pentane through a 30-cm column packed with acid, basic, and neutral silica gel (3, 2, and 3 g, respectively), as well as with anhydrous sodium sulfate. After re-concentration to 1.0 mL of the keeper, the extracts were treated with metallic copper strips for 24 h in the dark at 4 °C to mitigate interferences from sulfur compounds during the quantification analyses. Quantification of PBDE congeners Polybrominated diphenyl ether (PBDE) concentrations were assessed by gas chromatography with an electron capture detector (Shimadzu GC 2010 Plus, Kyoto, Japan). A volume of 2.0 µL of the extract was injected in splitless mode (split ratio of 1:20), using a purge time of 1 min and a purge flow of 3 mL min-1, all maintained at a constant temperature of 270 °C. Separation was achieved using a SLB-5MS capillary column (30 m × 0.25 mm × 0.25 μm film thickness, Supelco, Bellefonte, USA) with helium as the carrier gas. The oven temperature program was initiated at 100 °C (held for 2 min), followed by a ramp to 160 °C at a rate of 15 °C min-1, then to 280 °C at 4 °C min-1 (held for 10 min), and finally to 300 °C at 10 °C min-1 (held for 10 min), resulting in a total run time of 58 min. Quantification of all target PBDE congeners was performed using external calibration curves in triplicate, with retention time used as a confirmation parameter. Limits of detection (LOD) and quantification (LOQ), chromatographic retention times and analytical recovery for the PBDE congeners are available in Table 1S presented in the Supplementary Material. PBDE distribution and risk assessment The investigation of PBDEs distribution in the studied environment, according to their fugacity, was carried out using the equilibrium criterion model (EQC model) at its level I.37 This level involves assessing the equilibrium distribution or steady state in a closed environment and suggests the phases or environmental compartments to which a chemical will partition. To evaluate the distribution of PBDEs in the investigated area, a model input was generated, comprising chemical and environmental properties, along with emissions data. The chemical properties included molar mass, water solubility, vapor pressure, melting point, and octanol/water partition coefficient. As for environmental properties, a standard EQC environment model was adopted, incorporating predetermined volumes and densities for air, aerosol, water, suspended solids, fish, soil, and sediment. Additionally, the model accounted for the lipid fraction of fish and the organic carbon of suspended solids, soil, and sediment. All data employed in the EQC model, level I, are shown in Tables 2S and 3S of the Supplementary Material. Hazard quotients (HQs) were calculated to evaluate the ecological risk posed by the detected PBDEs in the sediment environment, using Equation 1. In this equation, MEC represents the measured environmental concentration (ng L-1) of each congener. The predicted no‑effect concentration (PNEC) is the concentration below which acute or chronic adverse effects in the environment are not expected to occur and, in the present study, was obtained using the ECOSAR software38 for each congener based on LD50 values for Daphnia magna. In this case, owing to the limited availability of toxicity data for PBDEs in sediment, the values obtained with ECOSAR consider the water/sediment partitioning of PBDEs and toxicity data for the water column.  Given that the partitioning between water and sediment depends on the organic carbon fraction within the matrix, MEC values were estimated in interstitial water employing the equilibrium partitioning approach described elsewhere39 and presented in Equation 2.  The estimated PBDE concentration in the pore water is represented by cpw (ng L-1), whereas cBDE is the measured sediment concentration, foc is the fraction of organic carbon in the sediment sample according to the ECQ model, level I (0.04), and KOC is the partition coefficient for sediment organic carbon, predicted using the Advanced Chemistry Development software (ACD/Labs Percepta Platform). The values of all parameters used to calculate HQ in this study are shown in Table 4S, in the Supplementary Material.
RESULTS AND DISCUSSION Distribution and sources of PBDEs in the sediments Table 1 summarizes the individual concentrations of nine PBDEs analyzed in the sediments of Paranoá Lake, along with the sums of the concentrations of the nine congeners at each sampling point (∑9PBDE), the sum of the concentrations of tri, tetra, and pentaBDE congeners (∑tri, ∑tetra, and ∑penta, respectively), and the ratio between the concentrations of tetra and pentaBDEs (∑tetra/∑penta).
Only six out of the nine investigated congeners were found in the sediments of Paranoá Lake (BDE-28, -47, -66, -85, -99, and -100), with positive concentrations ranging from 0.14 ± 0.01 ng g-1 dw (BDE‑28) to 8.1 ± 0.4 ng g-1 dw (BDE-66). PBDEs were found in four out of the seven sampling points, precisely those experiencing direct impacts whether from WWTP effluents (SP2 and SP4) or surface runoff discharges (SP3 and SP5). At these sampling points, all six PBDEs were detected, with BDE-28 showing positive concentrations only at the points impacted by the WWTPs. The highest concentrations for all congeners were recorded in the sample collected sampling point SP4, which is highly impacted by both the BS-WWTP and the potentially polluted waters from the Riacho Fundo tributary. In addition to the direct contribution of effluents from the two WWTPs into the lake waters, the occurrence of PBDE in sediments was also influenced by diffuse sources associated with urban drainage, where potentially polluting loads are discharged through storm drainage systems, which may be affected by domestic wastewaters interconnections. The total concentration of PBDE (∑9PBDE) observed in this study (3.9 ± 0.2 to 19 ± 1 ng g-1 dw), considering only the positive results, is consistent with other reports.40-48 Otherwise specified, for comparison purposes, only the sum of tri to hepta PBDEs investigated elsewhere will be considered, as the most prevalent congener is often the decabrominated BDE-209, not investigated in this study. The levels observed in Paranoá Lake are higher than those reported in the Guarani recharge point in Ribeirão Preto, Brazil (∑9PBDE up to 5.4 ± 0.2 ng g-1 dw), in the Baiyangdian Lake (∑10PBDE from 0.05 to 5.03 ng g-1 dw), and in the Fuhe River (∑10PBDE from 0.13 to 6.39 ng g-1 dw), both in North China.40 Also in China, the concentration (∑13PBDE) in the Yellow River ranged from 0.02 to 0.61 ng g-1 dw during the dry season and from 0.11 to 1.06 ng g-1 dw during the rainy season.41 Lower concentrations were also reported in sediments from Murchison Bay in Lake Victoria, Uganda (∑11PBDE from 0.06 to 0.18 ng g-1 dw),42 in urban lakes of Hanoi, Vietnam (∑23PBDEs 0.38 to 5.60 ng g-1 dw),43 in Thane creek, Mumbai, India (∑14PBDE 2.7 to 6.4 ng g-1 dw),44 and in the Prédecelle River (∑7PBDE between 0.88 and 4.61 ng g-1 dw) a small suburban river flowing in the southern part of the Paris conurbation in France.45 Concentrations in Paranoá Lake were similar to those found in the Niagara River, Canada (∑6PBDE from 0.02 to 23 ng g-1 dw),46 Awash River Basin, Ethiopia (∑7PBDE from 3.71 to 18.95 ng g-1 dw),47 and in sediments from Chaohu Lake, China (∑37PBDE mono to hepta from 0.87 to 23.5 ng g-1 dw).48 It is noteworthy that the overall levels of PBDEs can vary within the same aquatic body depending on the positioning of sampling sites and the selection of congeners during the implementation of analytical methods. For instance, in Lake Taihu, China, slightly different concentration ranges have been reported in various studies.49-52 In this case, lower concentrations relative to those identified in Paranoá Lake were reported by Yin et al.51 (∑8PBDE from 0.07 to 0.92 ng g-1 dw), whereas higher values were observed by Chen et al.52 (∑10PBDE between 0.37 and 41.7 ng g-1 dw). In addition to typical anthropogenic sources found in urbanized environments, such as the discharge of wastewater, stormwater runoff, and industrial effluents, PBDE levels in various environmental compartments, including aquatic sediments, have been notably impacted by activities related to e-waste management. In Lagos, Nigeria, high ∑7PBDE concentrations, between 51.4 ± 22.2 to 85.8 ± 23.9 ng g-1 dw, were noticed particularly during the wet season in sediments samples from e-waste dumpsites.53 In southern China, ∑13PBDE levels varied between 0.26 to 5,444 ng g-1 dw in sediments samples within a typical electronic waste dismantling region. In river sediments collected in the Guiyu region, also in China, the concentrations of ∑14PBDE ranged from 4,434 to 16,088 ng g-1 dw.54 Notably, BDE-209 comprised only 0.4% of the total levels, underscoring the influence of other PBDE commercial formulations present in materials processed at nearby e-waste facilities. In sediments from a typical e-waste dismantling region in southern China, concentrations up to 1,530; 1,540; 2,460; and 15,400 ng g-1 dw were noticed for the individual congeners BDE-47, -99, -153, and -183, respectively. These congeners are typical in the penta and octaBDE commercial formulations.55 The congeners most commonly found in environmental samples, including indoor dust, water, air, particulate matter, human tissues, sediments, and soil are BDE-47, -99, and -100.56 In the present study, these three congeners were detected in sediments exhibiting PBDE contamination, accounting for 64 to 73% of the concentrations at sampling points SP2, SP3, and SP5. However, at sampling point SP4, the sum of these three congeners was responsible for only 47% of the total PBDE concentration, with BDE-66 alone accounting for 43%, which is uncommon in studies involving environmental contamination of aquatic sediments. Numerous studies aim to assess the distribution of different congeners in environmental samples in order to establish correlations with the main commercial formulations used as flame retardants in materials. In the present study, the evaluation of PBDE concentration profiles in relation to bromination levels was performed by comparison between Σtetra (BDE-47 and BDE-66), and Σpenta (BDE‑85, BDE‑99, and BDE-100), as shown in Table 1. The profiles indicate higher relative concentrations of pentabrominated congeners at SP2, SP3, and SP5. In addition, at these sampling points ∑tetra/∑penta ratios between 0.41 ± 0.04 and 0.58 ± 0.05 suggest not only the use of pentaBDE formulations in consumer good of Brazil, but the possible major influence of the American formulation DE-71 compared to the European Bromkal 70-5 DE. In this case, BDE-47/-99 ratios of 0.8 and 1.0 in the commercial products, respectively, have been used to differentiate them in environmental samples.18,57 In the samples from Paranoá Lake, these ratios ranged from 0.49 ± 0.04 to 0.84 ± 0.04, thus being closer to that corresponding to the American formulation. However, it is worth mentioning that the PBDE profile observed at point SP4 requires greater attention due to the prevalence of congener BDE-66, as pointed out earlier. The congener BDE-66 is a product of pentaBDE formulations, but some studies suggest that it may also arise in environmental samples due to microbial or photolytic degradation of high brominated BDEs, such as BDE-209.58,59 PBDEs undergo reductive debromination by anaerobic microorganisms, leading to the formation of less brominated species.60 One of the major debromination sequences progresses from BDE-138 to BDE-85, followed by BDE-85 to BDE‑66, and finally from BDE-66 to BDE‑28.61,62 Given that BDE-138 was not detected in the sediments of Paranoá Lake, it is plausible that debromination originated from BDE-85, thereby leading to the formation of BDE-66. Additionally, at the sampling points where BDE-28 was detected with positive values (SP2 and SP4), concentrations of BDE-66 exceeded those of BDE-85, providing further support for the occurrence of debromination of the latter as a potential source of BDE-66. As these two sampling points are impacted by effluents from the two WWTPs discharging directly into the lake, a mixed influence of materials enriched with commercial penta and decabrominated flame retardants is suggested, either through domestic production or as a result of importing consumer goods. Finally, there are also reports suggesting the prevalence of BDE-66 in sediments due to industrial and/or e-wastes activities.4,63 Considering that the industrial sector is incipient in FD, representing only 4% of the local gross domestic product,64 contamination due to industries is unlikely. Consequently, there is an urgent need for a deeper understanding of e-wastes activities, especially in the region served by the BS-WWTP. Modeling PBDE distribution in adjacent compartments In this study, two sequential modeling approaches were employed to assess the distribution of PBDEs in the environment. Initially, PBDE emissions were estimated based on the global market for the pentaBDE commercial formulation in 2006, which was the final year of production before the ban imposed by the Stockholm Convention and American producers. Then, based on the PBDEs distribution in each adjacent compartment, data on their occurrence in the investigated samples were incorporated, and a new modeling approach provided estimated data for the environment under study. This approach was previously applied in a case study examining the fate of PBDEs using the EQC model.65 Firstly, considering that the pentaBDE product comprises approximately 0.2% tribrominated congeners, 23% tetrabrominated congeners, and 55% pentabrominated congeners,66 the emissions for each congener were estimated based on their average proportion in the commercial product. According to this preliminary assessment, the mass percentage distribution of PBDEs in each compartment suggests that soil receives the majority of emissions (approximately 97%), followed by sediments (around 2%). The estimated relative distribution of all congeners across the compartments is provided in Table 5S of the Supplementary Material. The proportions of each congener attributed to the sediments were used to estimate PBDE loads impacting each sampling point in Paranoá Lake, as shown in Equation 3, where EBDE is the emission for each congener, V and r are the volume and density of sediment, respectively (Table 3S), cBDE is the congener concentration determined at each sampling point (Table 1), and f represents the percentage fraction of each congener attributed to the sediment (Table 5S). 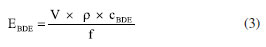 Using Equation 3, corrected emissions were finally employed as input parameters in the ECQ model to assess the distribution of PBDEs in the study area, as well as to determine potential concentrations in adjacent compartments. The results obtained for SP4 are presented in Table 2.
In Table 2 it is possible to observe that the corrected emissions for each congener result in a total PBDE emission of approximately 209 tons influencing sampling point SP4. The concentrations of each congener in the adjacent compartments were also estimated. Sediment levels align with those empirically obtained, suggesting that the other estimates were made in accordance with the characteristics of the investigated sampling point. In this case, presenting the results in terms of concentration is more suitable for understanding PBDE distribution, especially if such information is used to guide decision-making regarding environmental health. As an illustration, the estimated concentration of BDE-47 for soil (1.4 ng g-1) was about half of that found for sediment (2.85 ng g-1), despite the mass percentage distribution of congeners indicating soil as the predominant environment for PBDE accumulation. Clearly, the volumes and densities of each compartment used in modeling are crucial to standardizing the concentrations. Likewise, it is noteworthy that the estimated concentration of BDE-47 for biota (8.7 ng g-1 in fish) holds significance and can be used for comparison with studies dedicated to this specific part of the environment. Relatively high concentrations of all congeners are observed in aquatic suspended solids. This is a significant outcome, as these solids serve as precursors for sediment enrichment and as sources of mobility for hydrophobic substances like PBDEs in aquatic ecosystems. These values may be somehow overestimated because the parameters for the EQC standard environment used in the model may differ from the lentic lacustrine environment investigated in this study. However, given the accumulation of solids in Paranoá Lake, which may include algae from the vicinity of the WWTPs, as well as various particles from urban runoff and WWTP effluents, the estimates were deemed satisfactory. The distribution of PBDEs in the other sampling points is shown in Tables 6S to 8S of the Supplementary Material. The combined emissions from each sampling point suggest a total load of 430 tons of PBDEs entering Paranoá Lake. As limited studies have explored the presence of PBDEs in water due to their low solubility, the approach based on the use of partitioning models can be an alternative for estimating such low concentrations. For instance, the overall results indicate PBDE levels in Paranoá Lake waters ranging from 0.06 to 0.35 ng L-1. These concentrations are indeed low and may fall below the detection limits of the majority of analytical instruments available for water monitoring in Brazil, except those installed in public universities, which end up being responsible for providing the few available monitoring data, even though this is not their main responsibility. Other outcomes revealed concentrations ranging from 0.2 to 5.6 ng m-3 in air, from 0.01 to 9.6 ng m-3 in atmospheric aerosols, from 19 to 93 ng g-1 in suspended solids, from 11 to 57 ng g-1 in biota (fishes), and between 1.9 and 9.5 ng g-1 in soils. Preliminary ecological risk assessment Figure 3 presents the risk quotients calculated with PBDE concentrations estimated in the pore water. Usually, when HQ values are lower than 1, the target substance is considered to be unlikely to pose adverse effects. Conversely, if the HQ value exceeds 1, it may indicate a potential ecological risk. According to Figure 3, all HQ values are below 1, suggesting that PBDEs pose no risk to the studied environment. Nevertheless, it is noteworthy to emphasize the values exceeding 0.1 for the congeners BDE-47, -66, and -99, all calculated for the SP4 sampling point, which was identified as the most impacted in this study.
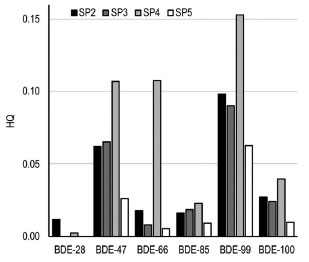 Figure 3. Ecological risk assessment of PBDEs in sediment from Paranoá Lake
Komori et al.,67 in their investigation of the presence of pharmaceuticals in water, propose an alternative criterion for ecological risk assessment, wherein an HQ below 0.1 is considered “acceptable”, values between 0.1 and 1 indicate the need for “further survey”, and values equal to or greater than 1 indicate the need for “detailed evaluation”. Based on this criterion, investigations regarding the presence of PBDEs in Paranoá Lake warrant further survey. This consideration appears to be more cautious, taking into account that it is a preliminary risk assessment, influenced not only by inherent uncertainties in concentration and risk estimates, but also by the intrinsic characteristics of PBDEs. In this particular case, in addition to their persistence and low solubilities in aquatic bodies, the risks to aquatic biota should also incorporate important ecological processes such as bioaccumulation and biomagnification. Strengths and limitations This study had two strengths. It is one of the few exploring the presence of PBDEs in freshwater sediments in Brazil, a topic lacking information considering the persistence, potential danger, and sources associated with the use, disposal, and recycling of various materials typical of everyday life in large cities, particularly electronic products. It also confirmed the influence of both point and diffuse sources on the input of these substances, emphasizing not only the necessity for dedicated studies in WWTPs, which are equally underexplored in Brazil, but also for investigating the role of storm drainage systems in water contamination. Two main limitations were also identified in this study. Firstly, only selected tri to hexa PBDE congeners were assessed, while BDE-209, commonly investigated in sediment samples worldwide, was not included due to limited access to a suitable analytical standard during the investigation period. Moreover, there were limited sediment samples measured in Paranoá Lake and the absence of additional sampling campaigns, especially during different seasons, due to difficulties in obtaining logistical support.
CONCLUSIONS This study provided a comprehensive assessment of PBDE levels in Paranoá Lake sediments, highlighting their widespread occurrence and distribution, with significant concentrations in areas influenced both by point sources (effluents from WWTPs) and diffuse contributions form stormwater drainage systems. Six out of the nine investigated congeners were detected in the samples, all tri and hexabrominated, with concentrations consistent with various reports in the literature that also highlight the role of wastewater and urban drainage on PBDE levels. Analysis of PBDE congeners suggested a predominant influence of the commercial pentaBDE formulation, especially DE-71, although prevalence of BDE-66 was observed in points nearby the wastewater treatment plants, indicating possible reductive debromination reactions of highly brominated congeners, probably BDE-209. The use of the EQC model, level I, allowed estimation of PBDE distribution in the environment, emphasizing the importance of considering not only concentrations in the water column, but also in biota and sediment materials. Preliminary ecological risk assessments indicate no risks associated with the PBDE congeners, but also underscore the necessity for further surveys. Future research should address the gaps identified in this study, including a more comprehensive analysis of all PBDE congeners, especially BDE-209, and a more detailed investigation of PBDE transport and transformation processes in Paranoá Lake. This information is essential for a better understanding the impact of PBDEs on environmental health and for the development of effective mitigation and management strategies for these contaminants.
SUPPLEMENTARY MATERIAL Supplementary data are available free of charge at http://quimicanova.sbq.org.br as PDF file.
ACKNOWLEDGMENTS The authors would like to thank the Analytical Center of the Institute of Chemistry (CAIQ) at the University of Brasília for providing the instruments required for this work. The authors also thank the National Institute for Advanced Analytical Science and Technology (INCTAA), grant CNPq para 465768/2014-8 for financial support.
REFERENCES 1. Annunciação, D. L. R.; Almeida, F. V.; Hara, E. L. Y.; Grassi, M. T.; Sodré, F. F.; Quim. Nova 2018, 41, 782. [Crossref] 2. Pieroni, M. C.; Leonel, J.; Fillmann, G.; Quim. Nova 2017, 40, 317. [Crossref] 3. Dorta, D. J.; de Souza, A. O.; Pereira, L. C.; Pazin, M.; de Oliveira, G. A. R.; de Oliveira, D. P. In Advances in Environmental Research; Daniels, J. A., ed.; Nova Science Publishers: New York, 2013, ch. 2. 4. Ohoro, C. R.; Adeniji, A. O.; Okoh, A. I.; Okoh, O. O.; Environ. Geochem. Health 2022, 44, 3409. [Crossref] 5. Wu, Z.; Han, W.; Yang, X.; Li, Y.; Wang, Y.; Environ. Sci. Pollut. Res. 2019, 26, 23219. [Crossref] 6. La Guardia, M. J.; Hale, R. C.; Harvey, E.; Environ. Sci. Technol. 2006, 40, 6247. [Crossref] 7. Ge, J.; Yun, X.; Liu, M.; Yang, Y.; Zhang, M.; Wang, J.; Ecotoxicology 2014, 23, 978. [Crossref] 8. Yu, G.; Bu, Q.; Cao, Z.; Du, X.; Xia, J.; Wu, M.; Huang, J.; Chemosphere 2016, 150, 479. [Crossref] 9. Ok, G.; Shirapova, G.; Matafonova, G.; Batoev, V.; Lee, S. H.; Polycyclic Aromat. Compd. 2013, 33, 173. [Crossref] 10. Rauert, C.; Lazarov, B.; Harrad, S.; Covaci, A.; Stranger, M.; Atmos. Environ. 2014, 82, 44. [Crossref] 11. Liagkouridis, I.; Cousins, I. T.; Cousins, A. P.; Sci. Total Environ. 2014, 491-492, 87. [Crossref] 12. Cristale, J.; Belé, T. G. A.; Lacorte, S.; de Marchi, M. R. R.; Environ. Pollut. 2018, 237, 695. [Crossref] 13. Gasperi, J.; Sebastian, C.; Ruban, V.; Delamain, M.; Percot, S.; Wiest, L.; Mirande, C.; Caupos, E.; Demare, D.; Kessoo, M. D. K.; Saad, M.; Schwartz, J. J.; Dubois, P.; Fratta, C.; Wolff, H.; Moilleron, R.; Chebbo, G.; Cren, C.; Millet, M.; Barraud, S.; Gromaire, M. C.; Environ. Sci. Pollut. Res. 2014, 21, 5267. [Crossref] 14. Xiang, N.; Chen, L.; Meng, X. Z.; Li, Y. L.; Liu, Z.; Wu, B.; Dai, L.; Dai, X.; Sci. Total Environ. 2014, 487, 342. [Crossref] 15. Komolafe, O.; Bowler, B.; Dolfing, J.; Mrozik, W.; Davenport, R. J.; Anal. Methods 2019, 11, 3474. [Crossref] 16. Wang, X.; Xi, B.; Huo, S.; Deng, L.; Pan, H.; Xia, X.; Zhang, J.; Ren, Y.; Liu, H.; Chemosphere 2013, 93, 1624. [Crossref] 17. North, K. D.; Environ. Sci. Technol. 2004, 38, 4484. [Crossref] 18. Xiang, N.; Zhao, X.; Meng, X. Z.; Chen, L.; Sci. Total Environ. 2013, 461-462, 391. [Crossref] 19. Rayne, S.; Ikonomou, M. G.; J. Environ. Eng. Sci. 2005, 4, 353. [Crossref] 20. Deng, D.; Chen, H.; Tam, N. F. Y.; Sci. Total Environ. 2015, 502, 133. [Crossref] 21. Komolafe, O.; Mrozik, W.; Dolfing, J.; Acharya, K.; Vassalle, L.; Mota, C. R.; Davenport, R.; J. Environ. Manage. 2021, 287, 112286. [Crossref] 22. Song, M.; Chu, S.; Letcher, R. J.; Seth, R.; Environ. Sci. Technol. 2006, 40, 6241. [Crossref] 23. Quinete, N.; Lavandier, R.; Dias, P.; Taniguchi, S.; Montone, R.; Moreira, I.; Mar. Pollut. Bull. 2011, 62, 440. [Crossref] 24. Lavandier, R.; Quinete, N.; Hauser-Davis, R. A.; Dias, P. S.; Taniguchi, S.; Montone, R.; Moreira, I.; Chemosphere 2013, 90, 2435. [Crossref] 25. Dorneles, P. R.; Lailson-Brito, J.; Dirtu, A. C.; Weijs, L.; Azevedo, A. F.; Torres, J. P. M.; Malm, O.; Neels, H.; Blust, R.; Das, K.; Covaci, A.; Environ. Int. 2010, 36, 60. [Crossref] 26. Lavandier, R.; Arêas, J.; Quinete, N.; de Moura, J. F.; Taniguchi, S.; Montone, R.; Siciliano, S.; Moreira, I.; Environ. Pollut. 2016, 208, 442. [Crossref] 27. Vidal, L. G.; de Oliveira-Ferreira, N.; Torres, J. P. M.; Azevedo, A. F.; Meirelles, A. C. O.; Flach, L.; Domit, C.; Fragoso, A. B. L.; Silva, F. J. L.; Carvalho, V. L.; Marcondes, M.; Barbosa, L. A.; Cremer, M. J.; Malm, O.; Lailson-Brito, J.; Eljarrat, E.; Sci. Total Environ. 2023, 905, 167704. [Crossref] 28. Rodrigues, E. M.; Ramos, A. B. A.; Cabrini, T. M. B.; Fernandez, M. A. S.; Int. J. Environ. Health 2015, 7, 247. [Crossref] 29. Ferrari, R. S.; de Souza, A. O.; Annunciação, D. L. R.; Sodré, F. F.; Dorta, D. J.; Water 2019, 11, 1601. [Crossref] 30. Cristale, J.; Belé, T. G. A.; Lacorte, S.; de Marchi, M. R. R.; Environ. Res. 2019, 168, 420. [Crossref] 31. Nunes, G.; Minoti, R. T.; Koide, S.; Hydrology 2020, 7, 85. [Crossref] 32. Fonseca, F. O.; Olhares sobre o Lago Paranoá, 1ª ed.; Secretaria do Meio Ambiente e Recursos Hídricos: Brasília, 2001. 33. Sodré, F. F.; Freire, D. J. S.; Alcântara, D. B.; Maldaner, A. O.; Frontiers in Analytical Science 2022, 2, 930480. [Crossref] 34. Tsuji, T. M.; Costa, M. E. L.; Koide, S.; Water Sci. Technol. 2019, 79, 1912. [Crossref] 35. ADASA, https://www.adasa.df.gov.br/images/storage/programas/PIRHFinal/Volume_I.zip, accessed in July 2024. 36. Annunciação, D. L. R.; Almeida, F. V.; Sodré, F. F.; Microchem. J. 2017, 133, 43. [Crossref] 37. Mackay, D.; Di Guardo, A.; Paterson, S.; Cowan, C. E.; Environ. Toxicol. Chem. 1996, 15, 1627. [Crossref] 38. ECOSAR software, v 2.2; USEPA, USA, 2022. 39. Di Toro, D. M.; Zarba, C. S.; Hansen, D. J.; Berry, W. J.; Swartz, R. C.; Cowan, C. E.; Pavlou, S. P.; Allen, H. E.; Thomas, N. A.; Paquin, P. R.; Environ. Toxicol. Chem. 1991, 10, 1541. [Crossref] 40. Hu, G.; Xu, Z.; Dai, J.; Mai, B.; Cao, H.; Wang, J.; Shi, Z.; Xu, M.; J. Environ. Sci. 2010, 22, 1833. [Crossref] 41. Pei, J.; Yao, H.; Wang, H.; Li, H.; Lu, S.; Zhang, X.; Xiang, X.; Mar. Pollut. Bull. 2018, 129, 106. [Crossref] 42. Ssebugere, P.; Sillanpää, M.; Wang, P.; Li, Y.; Kiremire, B. T.; Kasozi, G. N.; Zhu, C.; Ren, D.; Shang, H.; Zhang, Q.; Jiang, G.; Sci. Total Environ. 2014, 500-501, 1. [Crossref] 43. Hoang, A. Q.; Duong, H. T.; Trinh, H. T.; Kadokami, K.; Takahashi, S.; Environ. Sci. Pollut. Res. 2023, 30, 31436. [Crossref] 44. Tiwari, M.; Sahu, S. K.; Bhangare, R. C.; Ajmal, P. Y.; Pandit, G. G.; Environ. Geochem. Health 2018, 40, 2587. [Crossref] 45. Labadie, P.; Tlili, K.; Alliot, F.; Bourges, C.; Desportes, A.; Chevreuil, M.; Anal. Bioanal. Chem. 2010, 396, 865. [Crossref] 46. Richman, L. A.; Kolic, T.; MacPherson, K.; Fayez, L.; Reiner, E.; Chemosphere 2013, 92, 778. [Crossref] 47. Dirbaba, N. B.; Li, S.; Wu, H.; Yan, X.; Wang, J.; PLoS One 2018, 13, e0205026. [Crossref] 48. Chen, G.; Deng, X.; Wang, J.; Environ. Monit. Assess. 2022, 194, 631. [Crossref] 49. Wang, J.; Jia, X.; Gao, S.; Zeng, X.; Li, H.; Zhou, Z.; Sheng, G.; Yu, Z.; Environ. Sci. Pollut. Res. 2016, 23, 10361. [Crossref] 50. Yuan, X.; Wang, Y.; Tang, L.; Zhou, H.; Han, N.; Zhu, H.; Uchimiya, M.; Environ. Monit. Assess. 2020, 192, 309. [Crossref] 51. Yin, G.; Zhou, Y.; Strid, A.; Zheng, Z.; Bignert, A.; Ma, T.; Athanassiadis, I.; Qiu, Y.; Environ. Sci. Pollut. Res. 2017, 24, 7740. [Crossref] 52. Chen, J.; Wang, P. F.; Wang, C.; Liu, J. J.; Gao, H.; Wang, X.; Environ. Pollut. 2018, 232, 200. [Crossref] 53. Oloruntoba, K.; Sindiku, O.; Osibanjo, O.; Weber, R.; Emerging Contam. 2022, 8, 206. [Crossref] 54. Luo, Q.; Cai, Z. W.; Wong, M. H.; Sci. Total Environ. 2007, 383, 115. [Crossref] 55. Ling, S.; Zhou, S.; Tan, J.; Lu, C.; Fu, M.; Peng, C.; Zhang, W.; Hu, S.; Lin, K.; Zhou, B.; Sci. Total Environ. 2022, 824, 153813. [Crossref] 56. Ohoro, C. R.; Adeniji, A. O.; Okoh, A. I.; Okoh, O. O.; J. Environ. Health Sci. Eng. 2021, 19, 1229. [Crossref] 57. Vane, C. H.; Ma, Y. J.; Chen, S. J.; Mai, B. X.; Environ. Geochem. Health 2010, 32, 13. [Crossref] 58. Fang, L.; Huang, J.; Yu, G.; Wang, L.; Chemosphere 2008, 71, 258. [Crossref] 59. He, J.; Robrock, K. R.; Alvarez-Cohen, L.; Environ. Sci. Technol. 2006, 40, 4429. [Crossref] 60. Tokarz, J. A.; Ahn, M. Y.; Leng, J.; Filley, T. R.; Nies, L.; Environ. Sci. Technol. 2008, 42, 1157. [Crossref] 61. Huang, H. W.; Chang, B. V.; Lee, C. C.; Int. Biodeterior. Biodegrad. 2014, 87, 60. [Crossref] 62. Lee, L. K.; He, J.; Appl. Environ. Microbiol. 2010, 76, 794. [Crossref] 63. Muenhor, D.; Moon, H. B.; Lee, S.; Goosey, E.; J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 1284. [Crossref] 64. Agência Brasília, https://www.agenciabrasilia.df.gov.br/2024/03/10/setor-de-servicos-e-responsavel-por-95-da-economia-do-df/, accessed in July 2024. 65. Palm, A.; Cousins, I. T.; Mackay, D.; Tysklind, M.; Metcalfe, C.; Alaee, M.; Environ. Pollut. 2002, 117, 195. [Crossref] 66. Chen, Y.; Li, J.; Liu, L.; Zhao, N.; J. Environ. Manage. 2012, 113, 22. [Crossref] 67. Komori, K.; Suzuki, Y.; Minamiyama, M.; Harada, A.; Environ. Monit. Assess. 2013, 185, 4529. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access