Revisão
| Multiresidue determination of pesticides in exotic fruits: a review of the analytical strategies and residue occurrence |
|
Rafael Bel Prestes da Silva; Júlia Antunes de Oliveira; Cleusa Fátima Zanchin; Pimpernelli Jonco dos Santos; Osmar Damian Prestes; Renato Zanella Departamento de Química, Universidade Federal de Santa Maria, 97105-900 Santa Maria - RS, Brasil Received: 06/24/2024 *e-mail: renato.zanella@ufsm.br The growing awareness of health and well-being has led to increased consumption of healthy foods, such as exotic fruits, owing to their high nutritional value and global availability. However, the production and trade of exotic fruit presents significant challenges, particularly with regard to increasing supply and ensuring food safety concerning pesticide residues. This review examines modern analytical strategies employed to determine pesticide residues in exotic fruits. It is evident from the literature that ultra-high performance liquid chromatography and gas chromatography techniques, coupled with tandem mass spectrometry, remain the methods of choice for determining pesticide residues. For sample preparation, the QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method, renowned for its simplicity and efficiency, is extensively utilized in its original form as well as in acetate, citrate, and other modified versions to enhance its effectiveness. Developing these analytical methods is crucial for ensuring the safety of exotic fruits, protecting consumer health, and facilitating exports to global markets. Further advancements in this field are essential to overcome the challenges posed by producing and trading exotic fruits, thereby promoting food safety and public health. This review also discusses the occurrence of pesticides in exotic fruits. INTRODUCTION The concern for public health and well-being, aimed at enhancing the quality of life, has spurred an increased demand for healthy foods. Fruits, rich in nutrients, stand out due to their wide cultivation and global availability, making them highly sought after.1 The World Health Organization2 advocates for the regular consumption of fruits and vegetables as crucial elements in the prevention of chronic diseases, recommending a daily consumption of at least 400 g of fruits across a minimum of five servings. The burgeoning awareness of exotic fruits' nutritional and therapeutic benefits has led to a significant uptick in their production, trade, and consumption domestically and internationally.3 Small-scale fruits, known as exotic because of their smaller production volume compared to conventional fruits,4 have caught the interest of fruit producers. This is attributed to the increasing market demand where opportunities for marketing these fruits at local fairs, markets, hypermarkets, and even for export to various regions are expanding.5 Exotic fruits, renowned for their palatability and nutritional value, share similarities with traditional fruits but are distinguished by their unique sizes, shapes, colors, textures, fragrances, and flavors. Often produced in limited quantities per harvest, most species are still confined in their production, primarily due to their reliance on specific edaphoclimatic conditions.6 Exotic fruits belong to a vast and diversified category that encompasses varieties not commonly found in the regions where they are grown or consumed.7 From açai to acerola, through pithaya and starfruit, these fruits offer unique flavors and a wealth of essential nutrients for health. Beyond their flavors, exotic fruits often possess antioxidant properties, fiber, vitamins, and minerals that contribute to a balanced diet and promote overall well-being.8 European consumers are showing increasing interest in new varieties of fruits. This has increased the market value of exotic fruits, with most imports occurring in Northern Europe. Promoting the health benefits of exotic fruits and exploring more suitable transportation alternatives could help boost demand. Pomegranates, passion fruit, physalis, and pitahaya are already well-known by consumers and may see increased demand, but other exotic fruits, such as lychee, rambutan, and carambola, definitely have the potential for growth in terms of marketing and consumption.9 While some exotic fruits are cultivated on a large scale, like other fruits, they are generally perishable and seasonal, and their exports are limited. Others are not produced on a large scale; most are gathered and consumed by local people.1 Nonetheless, the nutritional value, changes in food consumption habits, and the prospects for income generation for small farmers contribute to the expansion of the consumption, production, and distribution of these fruits. Institutional statistics have yet to explore the production mapping of these fruits, which hinders the analysis of the progress of these cultures. Producers are increasingly investing in technology to boost productivity and maintain product quality. Different categories of pesticides, including insecticides, fungicides, and acaricides, are applied to protect crops against pests and diseases, thereby enhancing production quality and increase food production. These substances also contribute to increased food production, yielding progressively larger quantities.10,11 However, pesticide residues can reach consumers and potentially harm their health. Therefore, it is crucial to monitor the presence of pesticide residues, which must comply with the maximum levels of residues (MRLs) established by national and international regulations. Analytically, exotic fruits have received less attention than those more widely consumed in domestic and international markets. These MRL values are set by international organizations such as the Codex Alimentarius12 and the European Commission,13 while in Brazil, they are established by the National Health Surveillance Agency (ANVISA).14 The MRL values are determined through supervised field tests that start with minimal pesticides to achieve the required agricultural efficiency and ensure that the food's residue is as low as possible.15 Exotic fruits are considered minor crops and the MRLs are set by extrapolation using crop grouping system compiled by Codex Alimentarius12 and other legislations. Given the plethora of compounds used as pesticides, monitoring their residues is critical to ensure the quality of consumed products. This requires the application of reliable, highly selective, sensitive, and precise analytical methods capable of simultaneously detecting a large number of pesticides while considering the properties of the analytes of interest and the matrix composition of each fruit type. Laboratories can develop and validate methods for analyzing pesticide residues using various sample preparation methods, analytical techniques, and equipment. This review aims to provide an overview of the methods for determining pesticide residues in exotic fruits, focusing on the strategies employed by these methods and the occurrence of pesticide residues in these matrices.
METHODOLOGY A systematic literature review was conducted based on scientific articles selected from the databases SciFinder, Scopus, Science Direct, and Google Scholar using the keywords “pesticides” and “exotic fruit”, as well as “pesticides” and “minor tropical fruit”. The time limit for the review was 2010 to date and the language chosen was English. A total of 36 articles were found, 4 of which were excluded because they were not related to exotic fruits. Thus, 32 articles, the vast majority developed in Latin America, were considered for this study. This review provides an overview of the main analytical methods used to detect pesticide residues in exotic fruits, highlighting the methods' advantages and disadvantages. By reviewing the title, keywords, and abstract, studies that were relevant to the subject matter were selected, and a database for the research was established.
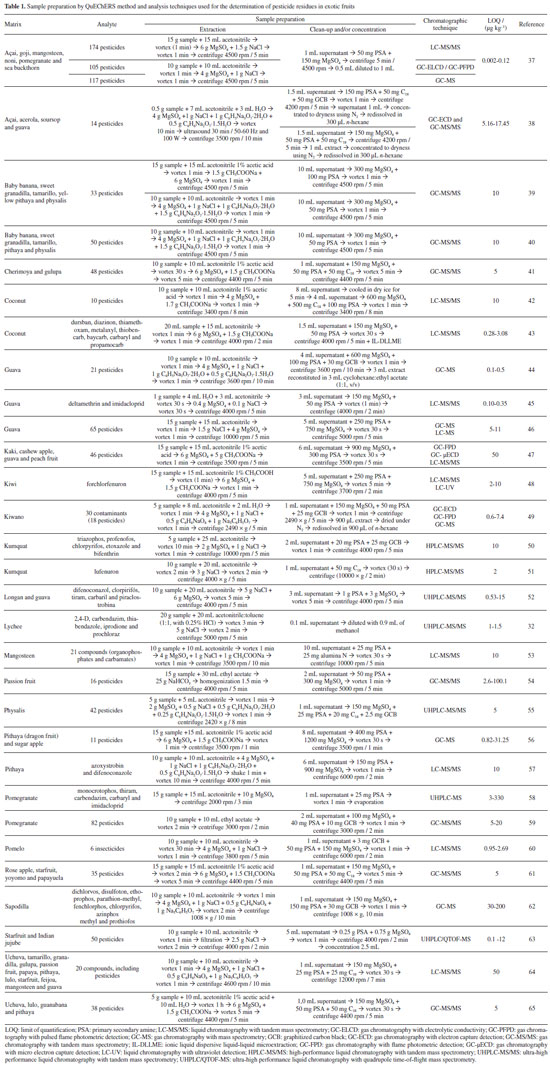
PESTICIDE RESIDUES DETERMINATION IN EXOTIC FRUITS Among the techniques used to identify pesticide residues in food samples, gas chromatography (GC) and liquid chromatography (LC) coupled with mass spectrometry (MS) continue to be the standard methods for this purpose, especially using tandem mass spectrometry, which allows for high selectivity and sensitivity even in complex matrices.16 Gas chromatography is founded on the varying distribution of the analytes between the stationary phase of a column and an inert gas as the mobile phase, facilitated by the programming of the oven temperature, enabling the analysis of a wide range of compounds that are volatile and thermally stable.17 Liquid chromatography employs a liquid mobile phase passing through a column containing the stationary phase and is one of the most widely used tools in separating compounds, from large biomacromolecules to smaller organic molecules.18 Ultra high-performance liquid chromatography (UHPLC), which operates with columns packed with particles below 2 μm at high pressures, is the predominant technique used today to determine pesticide residues.19 Regarding the scope of analysis, approximately 70% of pesticides are determined by UHPLC and around 30% by GC.20 The development of UHPLC was significantly influenced by the introduction of detectors capable of rapidly collecting and analyzing data to match the narrow peaks produced by these chromatographic analyses. A commonly employed detector is the mass spectrometer, which is generally used in tandem mode in the techniques UHPLC-MS/MS and GC tandem mass spectrometry (GC-MS/MS) that offer greater detection capability with high selectivity and sensitivity. Identifying pesticide residues in fruits using chromatographic techniques requires adequate sample preparation to reduce the majority of the interferences and obtain a suitable extract for analysis. Among the most employed techniques, the QuEChERS method, an acronym for “Quick, Easy, Cheap, Effective, Rugged, and Safe”, is the preferred choice for several reasons.21 It is characterized by the utilization of acetonitrile as an extraction solvent and the addition of different salts in the partitioning step to separate the organic solvent from the aqueous phase and enhance the recovery of polar pesticides through a quick and simple procedure.22 In addition to the QuEChERS method, the matrix solid phase dispersion (MSPD) technique was also used for the determination of pesticide residues in the exotic fruits açai23 and soursop.24 The pesticides bromuconazole, fenbuconazole, parathion-methyl, kresoxim-methyl and teflubenzuron were extracted from açai using alumina as sorbent and a mixture of cyclohexane:ethyl acetate (1:1, v/v). The extract was concentrated with nitrogen and determined by high-performance liquid chromatography with diode array detection (HPLC-DAD) with limit of quantification (LOQ) from 50 to 100 μg kg-1.23 For soursop the determination of thiamethoxam, thiacloprid, thiophanate-methyl, teflubenzuron and bifenthrin was performed by HPLC-DAD after the sample preparation by MSPD comparing mesoporous sorbents with Florisil, followed by the extraction with acetonitrile, concentration with nitrogen. The sorbents presented similar adequate performance and the LOQ values ranged from 50 to 100 μg kg-1.24
QuEChERS METHOD The complexity of fruit composition, as well as the low concentrations of pesticides present in them, has made monitoring these substances a challenge. The sample preparation stage tends to limit sample handling, lower the concentration levels detected and reduce the laboratory waste generated, as well as contributing to the speed of analysis and reliability in order to meet validation requirements.25,26 The choice of an analytical method is a critical step in laboratories that analyze residues and contaminants in various foods, and it is possible to use, for example, the QuEChERS method for pesticide extraction in fruit samples to verify compliance with specific food standards, and for other purposes.27 Anastassiades et al.28 first introduced the QuEChERS method for determination of pesticides in fruits and vegetables. This method has been widely investigated for various food matrices because it allows the determination of a large number of compounds with high percentages of analyte recovery (accuracy), removal of potential interferences, good precision and robustness, low cost, speed, ease, and safety.29 In addition, the method allows the use of less amounts of solvent compared with the previous methods, as well as ease of execution and simplicity of the clean-up step of the extracts, among other advantages, while exploring the possibilities given by modern analytical instrumentation. Originally, the QuEChERS procedure was proposed for pesticides residues analysis in high moisture fruits and vegetables, but more recently it is gaining significant popularity in the analysis of broad spectrum of analytes in huge variety of samples.30 QuEChERS has gained tremendous popularity for pesticide analysis in food samples and has the advantage of being a simple and fast procedure which minimize errors. It is considered very suitable and effective for sample preparation of a wide range of pesticides, especially considering the modifications developed recently.31 Some limitations of the QuEChERS method are that when extracting samples with a high fat or sugar content, the clean-up efficiency of the extract is not ideal.27 The QuEChERS method involves a combination of a pesticide extraction and a clean-up step to eliminate sugars, lipids, and organic acids from the matrices, including fruits. Other factors can affect the extraction effectiveness in these two steps.32 Following its initial applications, it was discovered that certain compounds showed stability and/or recovery problems based on the pH of the matrix, requiring optimizations involving the use of buffers (pH < 5) to increase recoveries, providing satisfactory (70-120%) results for pH-dependent compounds such as thiabendazole, pymetrozine, and imazalil.33,34 This method named “QuEChERS acetate” use acetonitrile acidified with acetic acid in the extraction step followed by the addition of sodium acetate in the partition step, resulting in a buffer effect with pH around 4.8. The method was adopted as an official method of the Association of Official Analytical Chemists (AOAC)35 for determination of pesticides residues in food. A modification of the QuEChERS method was made employing an extraction with pure acetonitrile and, in the partition step, a mixture of sodium citrate dihydrate and hydrogen citrate sesquihydrate as the buffering agents (pH 5.0-5.5), which was named “QuEChERS citrate”. This method was accepted as a reference method in the European Union in 2008.36
APPLICATION OF THE QuEChERS METHOD AND CHROMATOGRAPHIC TECHNIQUES IN THE DETERMINATION OF PESTICIDE RESIDUES IN EXOTIC FRUITS Although there are several options for sample preparation and devices that can be employed to evaluate pesticide residues in exotic fruits in the literature, the QuEChERS method is by far the most widely used. Table 1 lists the matrix, analytes, chromatographic technique, and limit of quantification achieved by using QuEChERS in its various versions.
MODIFICATIONS IN SAMPLE PREPARATION FOR DETERMINING PESTICIDES IN EXOTIC FRUITS In addition to the QuEChERS versions (original, acetate and citrate), several modifications in sample preparation for the determination of pesticide residues in general matrices, including fruits, have been proposed. These changes can be noticed in the extraction and/or clean-up steps. Meecharoen et al.53 presented an extraction step in which CH3COONa is introduced in the extraction step of the QuEChERS method in three experiments: (i) 4 g MgSO4 + 1 g NaCl; (ii) 4 g MgSO4 + 1 g NaCl + 1 g trisodium citrate dehydrate + 0.5 g disodium hydrogen citrate sesquihydrate; and (iii) 4 g MgSO4 + 1 g NaCl + 1 g anhydrous sodium acetate. Finally, it was verified that, for the analysis of pesticide residues in mangosteen (Garcinia mangostana), the use of set (iii) in the extraction presents greater recoveries. Bastidas et al.54 and Satpathy et al.59 performed the extraction of pesticides from passion fruit (Passiflora edulis) with replacement of acetonitrile by ethyl acetate. Aysal et al.66 pointed out that in the case of analysis by gas chromatography, ethyl acetate is a better solvent than acetonitrile, as it is less polar, provides a smaller volume of expansion from liquid to gas in the injection system, in addition to presenting greater stability to certain pesticides. One challenge that can be noticed during the analysis process of pesticide residues in complex matrices such as fruits is the variation in pH, which may affect the recovery of pesticides pH sensitive. In addition, Bastidas et al.54 proposed the inclusion of sodium bicarbonate (NaHCO3) in the extraction process to give a stable pH during extraction, independent of the pH of the original material. Li et al.32 described the use of a mixture of acetonitrile:toluene (1:1, v/v, including 0.25% HCl) as an option to maintain pH control in the extraction solvent, which aids to achieve adequate recoveries. In addition to this change, the authors presented modifications in the clean-up step, using methanol dilution rather sorbents materials. Certain compounds are difficult to detect when acetonitrile is used as the extraction solvent, so other solvents are used instead. Regarding the use of ultrasound, Paz et al.38 employed it in the extraction process with the aim of improving recoveries, as such action provides a better contact between the matrix and the extraction solvent via inductive stress caused by the propagation of ultrasonic waves in the liquid.67,68 Varela-Martínez et al.65 and Elbaz et al.45 demonstrated that adding ultrapure water to the dried fruits before the solvent extraction during the extraction process helps to produce greater recovery values of pesticides. In the cleaning step, it is possible to use alumina N associated with primary secondary amine (PSA) for effective removal of fatty acids in the matrix, maintaining good recoveries, as demonstrated by the work of Meecharoen et al.53 A modification already widely used and also found in several works, such as those by Botero-Coy et al.,64 Ferreira et al.,42 Paz et al.,38 Muñoz et al.,55 Varela-Martínez et al.41,61,65 and Li et al.51 deals with the addition of octadecylsilane (C18) in the clean-up step. This sorbent is added in the clean-up step due to its efficiency to remove interferences from extracts containing large amounts of lipids.69 Another very used modification found in the works made by Zhang et al.,60 Satpathy et al.,59 Guedes et al.,44 Paz et al.,38 Muñoz et al.,55 Alcântara et al.,62 Li et al.50 and Fernandez et al.49 is the use of graphitized carbon black (GCB) as a cleaning sorbent. Such modification takes place to remove extractable interferences, such as fatty acids and pigments that are present in several matrices and can decrease the recovery of pesticides and resulting in problems in the instrumentation of analysis.29,44 Ferreira et al.42 proposed a freezing step in dry ice that precedes the clean-up step for the extraction of pesticide residues in coconut (Cocos nucifera), in order to detect the aqueous layer, as well as the acetonitrile layer more easily. Lawal and Koki43 combined QuEChERS with ionic liquid-based dispersive liquid-liquid microextraction (IL-DLLME) in the extraction of pesticides in coconut (Cocos nucifera). Lawal et al.70 explain that such an association takes place with the aim of cleaning the matrix interferences as much as possible with less consumption of organic solvents, which improves the simplicity, speed, better detectability and selectivity of the targeted analytes.
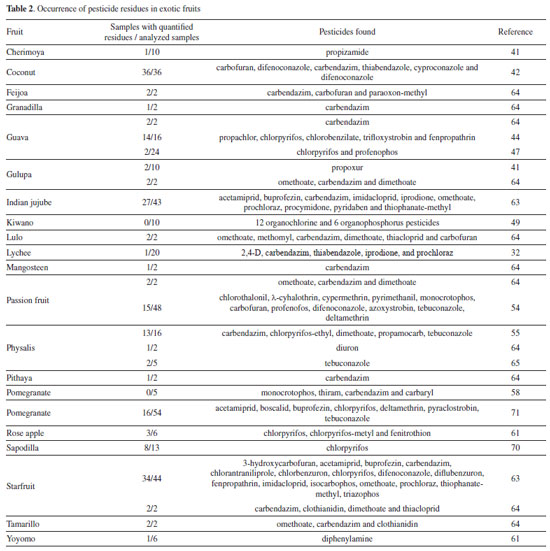
OCCURRENCE OF PESTICIDE RESIDUES IN EXOTIC FRUITS Few studies have focused on applying available methods to determine pesticide residues in exotic fruits. Table 2 shows the pesticides analyzed and the number of samples with residues in relation to the number of exotic fruit samples analyzed. The vast majority of studies have been carried out in Latin American countries and have been applied to samples from this region. The occurrence of residues indicates the need for increased monitoring to ensure the safety of these foods.
CONCLUSIONS Given that pesticides are compounds that can have detrimental effects on human health, attention should be paid to the consumption of foods that may contain pesticide residues above the maximum residue limits. Effective methods for analyzing pesticide residues in exotic fruits should be encouraged and further developed, keeping in mind the health implications for the population that consumes these fruits. It is recognized that most of the cultivation of these fruits originates from family farms; however, the use of pesticides in the production of exotic fruit is a reality, since analyses have indicated the occurrence of pesticide residues. Among these methods, the QuEChERS method and its adaptations stand out as the preferable approach for the proper analysis of pesticides in fruits. Evidence suggests that modifications to these methods have been proposed to enhance the efficiency of pesticide extraction and minimize the extraction of co-extractives from the matrix, aiming to achieve better recovery rates. Consequently, several authors have proposed various options regarding the use of different reagents and methods in the extraction and purification steps of the QuEChERS method for samples of exotic fruits. Hence, it is evident that studies such as those presented herein should be supported and expanded. Although there is a larger body of research on fruits of global consumption (e.g., oranges, lemons, strawberries, etc.), it is crucial to adapt these extraction methods to detect pesticides in exotic fruits. The presence of these chemical compounds in exotic fruits can pose a health risk to the local populations that consume these fruits, and it is noteworthy that many of these fruits are also potential candidates for export.
ACKNOWLEDGMENTS The authors are grateful to the financial support and fellowship grants from the Brazilian Funding Agencies CNPq and Fundação de Amparo à Pesquisa do Estado do Amazonas (01.02.016301.001624/2021-10-FAPEAM).
REFERENCES 1. Rodrigues, S.; Silva, E. O.; de Brito, E. S.; Exotic Fruits Reference Guide; Academic Press: London, 2018. [Link] accessed in October 2024 2. World Health Organization (WHO); Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO: Geneva, 2003. [Link] accessed in October 2024 3. Rufino, M. S. M.; Alves, R. E.; de Brito, E. S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. L.; Food Chem. 2010, 121, 996. [Crossref] 4. Watanabe, H. S.; de Oliveira, S. L.; Rev. Bras. Frutic. 2014, 36, 23. [Crossref] 5. Rodrigues, R.; Agroanalysis 2015, 35, 45. [Link] 6. de Oliveira Júnior, M. A.; Docema, M. L.; da Silva, M. S. C.; de Souza, M. W. R.; Research, Society and Development 2021, 10, e579101321377. [Crossref] 7. Marins, A. R.; Oliveira, A. M.; Gomes, R. L.; Feihrmann, A. C.; Gomes, R. G. In Compostos Bioativos e suas Aplicações; Nora, F. M. D., org.; Mérida Publishers: Canoas, 2021, ch. 15. [Crossref] 8. Bicas, J. L.; Molina, G.; Dionísio, A. P.; Barros, F. F. C.; Wagner, R.; Maróstic Jr., M. R.; Pastore, G. M.; Food Res. Int. 2011, 44, 1843. [Crossref] 9. Centre for the Promotion of Imports from Developing Countries (CBI) of The Netherlands Ministry of Foreign Affairs, The European Market Potential for Exotic Fruit, https://www.cbi.eu/market-information/fresh-fruit-vegetables/exotic-tropical-fruit/market-potential, accessed in October 2024. 10. Bandeira, D. D.; Munaretto, J. S.; Rizzetti, T. M.; Ferronato, G.; Prestes, O. D.; Martins, M. L.; Zanella, R.; Adaime, M. B.; Quim. Nova 2014, 37, 900. [Crossref] 11. Lorenz, J. G.; Costa, L. L. F.; Suchara, E. A.; Sant'Anna, E. S.; J. Braz. Chem. Soc. 2014, 25, 1583. [Crossref] 12. Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO), Codex Pesticides Residues in Food Online Database, https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/en/, accessed in October 2024. 13. European Commission, EU Legislation on MRLs, https://food.ec.europa.eu/plants/pesticides/maximum-residue-levels/eu-legislation-mrls_en, accessed in October 2024. 14. Agência Nacional de Vigilância Sanitária (ANVISA), Programa de Análise de Resíduos de Agrotóxicos em Alimentos - PARA, https://www.gov.br/anvisa/pt-br/assuntos/agrotoxicos/programa-de-analise-de-residuos-em-alimentos, accessed in October 2024. 15. Presidência da República, Casa Civil, Secretaria Especial para Assuntos Jurídicos; Lei No. 14.785 de 27 de dezembro de 2023, Dispõe sobre a Pesquisa, a Experimentação, a Produção, a Embalagem, a Rotulagem, o Transporte, o Armazenamento, a Comercialização, a Utilização, a Importação, a Exportação, o Destino Final dos Resíduos e das Embalagens, o Registro, a Classificação, o Controle, a Inspeção e a Fiscalização de Agrotóxicos, de Produtos de Controle Ambiental, de seus Produtos Técnicos e Afins; Revoga as Leis Nos. 7.802, de 11 de Julho de 1989, e 9.974, de 6 de Junho de 2000, e Partes de Anexos das Leis Nos. 6.938, de 31 de Agosto de 1981, e 9.782, de 26 de Janeiro de 1999; Diário Oficial da União (DOU), Brasília, Brazil, 2023. [Link] accessed in October 2024 16. Hernández-Mesa, M.; Moreno-González, D.; Separations 2022, 9, 148. [Crossref] 17. Collins, C. H.; Braga, G. L.; Bonato, P. S.; Fundamentos de Cromatografia, 2nd ed.; Unicamp: Campinas, 2009. 18. Rusli, H.; Putri, R. M.; Alni, A.; Molecules 2022, 27, 907. [Crossref] 19. Lee, J.; Shin, Y.; Lee, J.; Lee, J.; Kim, B. J.; Kim, J.-H.; Chemosphere 2018, 207, 519. [Crossref] 20. Kornas, P.; Klink, T.; Quantitation of Over 1,000 Pesticide Residues in Tomato According to SANTE 11312/2021 Guideline, https://www.agilent.com/cs/library/applications/an-pesticide-quant-tomato-5994-6895en-agilent.pdf, accessed in October 2024. 21. Sampaio, M. R. F.; Tamasini, D.; Cardoso, L. V.; Caldas, S. S.; Primel, E. G.; J. Braz. Chem. Soc. 2012, 23, 197. [Crossref] 22. Prestes, O. D.; Friggi, C. A.; Adaime, M. B.; Zanella, R.; Quim. Nova 2009, 32, 1620. [Crossref] 23. Froés, M. B. R.; Santos, L. F. S.; Navickiene, S.; Food Anal. Methods 2013, 6, 328. [Crossref] 24. Santos, L. F. S.; de Jesus, R. A.; Costa, J. A. S.; Gouveia, L. C. T.; de Mesquita, M. E.; Navickiene, S.; Inorg. Chem. Commun. 2019, 101, 45. [Crossref] 25. Fenik, J.; Tankiewicz, M.; Biziuk, M.; TrAC, Trends Anal. Chem. 2011, 30, 814. [Crossref] 26. Borges, K. B.; Pereira, A. C.; Mano, V. In Preparo de Amostras para Análise de Compostos Orgânicos; Borges, K. B.; Figueiredo, E. C.; Queiroz, M. E. C., eds.; LTC: Rio de Janeiro, 2015, ch. 1. 27. Zanella, R.; Prestes, O. D.; Adaime, M. B.; Martins, M. L. In Preparo de Amostras para Análise de Compostos Orgânicos; Borges, K. B.; Figueiredo, E. C.; Queiroz, M. E. C., eds.; LTC: Rio de Janeiro, 2015, ch. 24. 28. Anastassiades, M.; Lehotay, S. J.; Štajnbaher, D.; Schenck, F. J.; J. AOAC Int. 2003, 86, 412. [Crossref] 29. Prestes, O. D.; Adaime, M. B.; Zanella, R.; Sci. Chromatogr. 2011, 3, 51. [Crossref] 30. Rejczak, T.; Tuzimski, T.; Open Chem. 2015, 13, 980. [Crossref] 31. Musarurwa, H.; Chimuka, L.; Pakade, V. E.; Tavengwa, N. T.; J. Food Compos. Anal. 2019, 84, 103314. [Crossref] 32. Li, M.; Dai, C.; Wang, F.; Kong, Z.; He, Y.; Huang, Y. T.; Fan, B.; Sci. Rep. 2017, 7, 42489. [Crossref] 33. Lehotay, S. J.; de Kok, A.; Hiemstra, M.; van Bodegraven, P.; J. AOAC Int. 2005, 88, 595. [Crossref] 34. Lehotay, S. J.; Maštovská, K.; Lightfield, A. R.; J. AOAC Int. 2005, 88, 615. [Crossref] 35. Association of Official Analytical Chemists (AOAC); Method 2007.01: Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate; AOAC: Rockville, 2007. [Link] accessed in October 2024 36. European Committee for Standardization (CEN); BS EN 15662:2008: Foods of Plant Origin - Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE - QuEChERS-Method; CEN: Brussels, 2008. [Link] accessed in October 2024 37. Tran, K.; Eide, D.; Nickols, S. M.; Cromer, M. R.; Sabaa-Srur, A.; Smith, R. E.; Food Chem. 2012, 134, 2398. [Crossref] 38. Paz, M.; Correia-Sá, L.; Vidal, C. B.; Becker, H.; Longhinotti, E.; Domingues, V. F.; Delerue-Matos, C.; J. Environ. Sci. Health, Part B 2017, 52, 48. [Crossref] 39. España Amórtegui, J. C.; Guerrero Dallos, J. A.; Food Addit. Contam.: Part A 2014, 31, 676. [Crossref] 40. España Amórtegui, J. C.; Guerrero Dallos, J. A.; Food Chem. 2015, 182, 14. [Crossref] 41. Varela-Martínez, D. A.; González-Curbelo, M. Á.; González-Sálamo, J.; Hernández-Borges, J.; Microchem. J. 2020, 157, 104950. [Crossref] 42. Ferreira, J. A.; Ferreira, J. M. S.; Talamini, V.; Facco, J. F.; Rizzetti, 42. M.; Prestes, O. D.; Adaime, M. B.; Zanella, R.; Bottoli, C. B. G.; Food Chem. 2016, 213, 616. [Crossref] 43. Lawal, A.; Koki, I. B.; ChemSearch J. 2019, 10, 87. [Link] accessed in October 2024 44. Guedes, J. A. C.; Silva, R. O.; Lima, C. G.; Milhome, M. A. L.; do Nascimento, R. F.; Food Chem. 2016, 199, 380. [Crossref] 45. Elbaz, G. A.; Zaazaa, H. E.; Abd El Halim, L. M.; Monir, H. H.; Microchem. J. 2023, 185, 108218. [Crossref] 46. Mandal, S.; Poi, R.; Ansary, I.; Hazra, D. K.; Bhattacharyya, S.; Karmakar, R.; SN Appl. Sci. 2020, 2, 188. [Crossref] 47. Jardim, A. N. O.; Mello, D. C.; Goes, F. C. S.; Frota Junior, E. F.; Caldas, E. D.; Food Chem. 2014, 164, 195. [Crossref] 48. Negre, M.; Passarela, I.; Vindrola, D.; Baglieri, A.; J. AOAC Int. 2014, 97, 938. [Crossref] 49. Fernandes, V. C.; Podlasiak, M.; Vieira, E. F.; Rodrigues, F.; Grosso, C.; Moreira, M. M.; Delerue-Matos, C.; Foods 2023, 12, 993. [Crossref] 50. Li, Z.; Su, X.; Dong, C.; Zhou, J.; An, W.; Wang, C.; Jiao, B.; Ecotoxicol. Environ. Saf. 2021, 228, 112958. [Crossref] 51. Li, K.; Chen, W.; Deng, P.; Luo, X.; Xiong, Z.; Li, Z.; Ning, Y.; Liu, Y.; Chen, A.; J. Food Compos. Anal. 2022, 112, 104643. [Crossref] 52. Zhao, J.; Pu, J.; Wu, X.; Chen, B.; He, Y.; Zhang, Y.; Han, B.; Microchem. J. 2021, 168, 106375. [Crossref] 53. Meecharoen, W.; Tayaputch, N.; Pitiyont, V.; Leepipatpiboon, N.; Thai J. Agric. Sci. 2011, 44, 168. 54. Bastidas, D. A.; Guerrero, J. A.; Wyckhuys, K.; Rev. Colomb. Quim. 2013, 42, 39. [Link] accessed in October 2024 55. Muñoz, N. C.; Floriano, L.; de Souza, M. P.; Bandeira, N. M. G.; Prestes, O. D.; Zanella, R.; Food Anal. Methods 2017, 10, 320. [Crossref] 56. Coello-Villanueva, J. M.; Aceretto-Escoffié, O. M.; Barrón-Zambrano, J. A.; Muñoz-Rodríguez, D.; J. Mex. Chem. Soc. 2017, 61, 290. [Link] accessed in October 2024 57. Noegrohati, S.; Hernadi, E.; Asviastuti, E.; Bull. Environ. Contam. Toxicol. 2018, 100, 821. [Crossref] 58. Bilehal, D. C.; Chetti, M. B.; Sung, D. D.; Goroji, P. T.; J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1633. [Crossref] 59. Satpathy, G.; Tyagi, Y. K.; Gupta, R. K.; Am. J. Food Sci. Technol. 2014, 2, 53. [Crossref] 60. Zhang, F.; Li, Y.; Yu, C.; Pan, C.; Bull. Environ. Contam. Toxicol. 2012, 88, 885. [Crossref] 61. Varela-Martínez, D. A.; González-Curbelo, M. Á.; González-Sálamo, J.; Hernández-Borges, J.; Food Chem. 2019, 280, 221. [Crossref] 62. Alcântara, D. B.; Paz, M. S. O.; Rodrigues, T. H. S.; Fernandes, T. S. M.; Barbosa, P. G. A.; Loiola, A. R.; Grinberg, P.; Zocolo, G. J.; de Brito, E. S.; do Nascimento, R. F.; J. Braz. Chem. Soc. 2018, 29, 2180. [Crossref] 63. Yang, X.; Luo, J.; Duan, Y.; Li, S.; Liu, C.; Food Chem. 2018, 241, 188. [Crossref] 64. Botero-Coy, A. M.; Marín, J. M.; Serrano, R.; Sancho, J. V.; Hernández, F.; Anal. Bioanal. Chem. 2014, 407, 3667. [Crossref] 65. Varela-Martínez, D. A.; González-Curbelo, M. Á.; González-Sálamo, J.; Hernández-Borges, J.; J. Sep. Sci. 2020, 43, 929. [Crossref] 66. Aysal, P.; Ambrus, Á.; Lehotay, S. J.; Cannavan, A.; J. Environ. Sci. Health, Part B 2007, 42, 481. [Crossref] 67. Tadeo, J. L.; Sánchez-brunete, C.; Albero, B.; García-Valcárcel, A. I.; J. Chromatogr. A 2010, 1217, 2415. [Crossref] 68. Santos Júnior, D.; Krug, F. J.; Pereira, M. G.; Korn, M.; Appl. Spectrosc. Rev. 2006, 41, 305. [Crossref] 69. Lee, S. W.; Choi, J.-H.; Cho, S.-K.; Yu, H.-A; El-Aty, A. M. A.; Shim, J.-H.; J. Chromatogr. A 2011, 1218, 4366. [Crossref] 70. Lawal, A.; Wong, R. C. S.; Tan, G. H.; Abdulra'uf, L. B.; Anal. Lett. 2019, 52, 231. [Crossref] 71. Balkan, T.; Yılmaz, Ö.; Gida - The Journal of Food 2023, 48, 993. [Crossref]
Associate Editor handled this article: Eduardo M. Richter |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access