Artigo
| Greenhouse gas emissions from constructed wetlands for anaerobic sludge treatment: effect of seasonality, plant species type, and sludge loading - a preliminary analysis |
|
Diogo A. P. da SilvaI; Mateus P. MatosII; José Antônio Ribeiro de AraújoI; Thiago de A. NevesI,* I. Departamento de Engenharia Sanitária e Ambiental, Universidade Federal de Minas Gerais, 31270-010 Belo Horizonte - MG, Brasil Received: 04/02/2024 *e-mail: thiago@desa.ufmg.br At sewage treatment plants, sludge management remains a bottleneck, requiring efficient and cost-effective solutions for treating this solid byproduct. It is known that the presence of plants can accelerate the sludge drying process, as a result, vertically flowed constructed wetlands (VFCWs) have been evaluated as alternatives to traditional drying beds. Though, for better applicability of this technology, this study premised on evaluating greenhouse gas (GHG) emissions in VFCWs constructed for anaerobic sludge treatment. To compare GHG emissions across different seasonal conditions, three configurations were tested: four units planted with Tifton-85 grass (Cynodon spp.), four units planted with elephant grass (Cenchrus purpureus), four control units without vegetation presence, with four sludge loading rates in each configuration: 75, 100, 150, and 200 kg TS (total solids) m-2 year-1. Higher sludge loading rates led to increased methane emissions, with emissions escalating during the rainy season. Methane emissions decreased towards the end of the resting period. The presence of vegetation reduced methane emissions mainly during the resting period due to increased aeration of the units. The conditions that would provide lower GHG emissions would be with loads of up to 100 kg m-2 year-1, in units planted with Tifton grass. INTRODUCTION The disposal and management of sludge in sewage treatment plants (WWTPs) account for approximately 60% of the total operating costs.1 In Brazil, annual sludge production has already exceeded 80 million tons2 and continues to increase due to population growth and the upward trend in sewage treatment rates. Therefore, effective methods for final disposal are necessary, as well as the adaptation of the sludge's physical, chemical, and microbiological properties to meet the standards established by Brazilian legislation and ensure proper disposal.3,4 Whenever possible, it is ideal to adopt treatment technologies that provide low operating costs, are compatible with local conditions, are economically viable, and adhere to principles of environmental sustainability. In biological sewage treatment technologies, excess sludge has a high moisture content (around 98%), making it challenging to adopt a low-cost treatment technology.2 The most used processes for sludge treatment involve solid/liquid separation for dewatering, along with stabilization, sanitation, conditioning, and dehydration techniques. These methods aim to reduce the content of biodegradable organic matter, decrease the sludge volume, and mitigate health risks associated with soil application (if this solution is chosen).5 An economical option is the use of drying beds. However, drying beds require a large area due to the long drying time, necessitate the removal of dry sludge every 14 days, and do not stabilize or sanitize the solid waste. Additionally, there is a risk, although low if properly operated, of odor release.6,7 An alternative technology that combines some advantages of drying beds with additional benefits is constructed wetlands (CWs) or constructed wetlands with vertical flow (VFCW). These treatment units have gained significant recognition in sludge treatment due to their lower energy demand, reduced operating costs, absence of chemical additives, and minimal environmental impact. Studies8-10 have shown that vertical wetlands for sludge treatment produce material rich in organic matter with low water content. Additionally, the biosolids resulting from this treatment can be disposed of in agricultural areas, and the plant biomass produced can be used for animal feed.8-10 This full-scale sludge treatment system involves a series of units that are fed sequentially, with alternating periods of feeding and resting between beds. This process promotes drying, stabilization, sanitization, and mineralization of the sludge. The units receive sludge continuously over a period of 10 to 20 years without removal, leading to the accumulation of an organic residue layer.11 In constructed wetlands, each bed is lined to prevent percolated water from leaching into the soil. A layer of filtering material is then applied over the lining, facilitating the cultivation of fast-growing plants that are resilient to the bed's operational conditions. This feature distinguishes constructed wetlands from other alternatives and helps in both drying and treating solid materials while draining liquids. Pipes are installed for feeding sludge into the units, and a piping system collects percolated water, which also serves to aerate the units internally.12 The stabilization of accumulated organic matter is facilitated by microorganisms, which engage in processes such as fermentation, methanogenesis, sulfate reduction, denitrification, and aerobic respiration.13 However, the mineralization of organic material through biochemical processes results in the production of greenhouse gases (GHGs), primarily CO2, CH4, and N2O. This presents an environmental concern that requires careful study before widespread adoption of the technology.14 Given that sludge treatment in wetlands is relatively new and GHG emissions from these treatment systems are a recent concern, many questions remain unanswered. Ongoing studies15,16 aim to refine the operational conditions of planted sludge treatment beds to minimize potential environmental impacts. It is recognized that in sewage treatment, higher organic loads and elevated temperatures can intensify gas production, contributing to both odor generation and GHG emissions that contribute to global warming.15,16 In this study, we aimed to preliminarily evaluate CH4 emissions from the mineralization process of sludge accumulated in vertical wetlands on a pilot scale. The study spanned a 15-month period and included assessments of annual seasonality (dry and rainy periods) with two sampling campaigns for each, as well as varying loads of applied sludge.
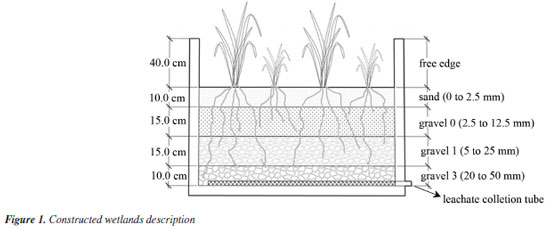
EXPERIMENTAL Experimental area and pilot units The research was conducted in Belo Horizonte, MG, specifically at the Sanitation Research and Training Center (CEPTS/UFMG), located within the Ribeirão Arrudas Sewage Treatment Plant (ETE-Arrudas). Operated by the Minas Gerais Sanitation Company (COPASA), this plant receives primarily sanitary sewage. It is situated at the geographic coordinates 19º53'42" S and 43º52'42" W. Twelve pilot-scale vertical wetland units, each with a volume of 0.9 m3 (height = 0.9 m, area = 1 m2), were installed at CEPTS (Figure 1). Each bed was filled with a 0.50 m layer of support medium, consisting of four vertical layers: 10 cm of crushed sand (0 to 2.5 mm), 15 cm of gravel 0 (2.5 to 12.5 mm), 15 cm of gravel 1 (5 to 25 mm), and 10 cm of gravel 3 (20 to 50 mm), arranged from top to bottom as described. Above the bed, a 40 cm space was allocated for the accumulation of sludge. A PVC drainage pipe (40 mm) with 8 mm holes was installed at the bottom of each bed to collect leachate.
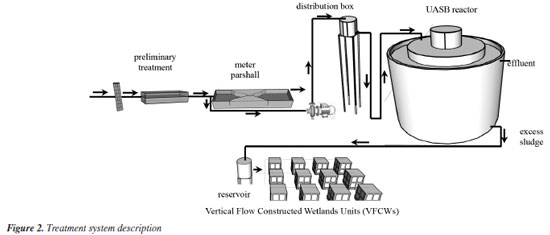
Constructed wetlands operational conditions As depicted in Figure 2, domestic sewage entering the Arrudas WWTP undergoes preliminary treatment before flowing into the Parshall flume. At this juncture, a portion of the effluent is gravitationally diverted to CEPTS. Upon arrival at CEPTS, the sewage is pumped to a distribution box using a pump with a flow rate of 30 m3 d-1, regulated by a frequency inverter, and subsequently directed to feed the upflow anaerobic sludge blanket (UASB) reactor.
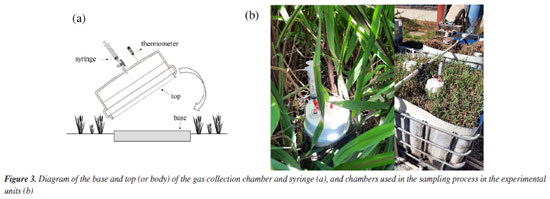
The anaerobic sludge from the UASB reactor was gravity-fed into a feed reservoir (0.250 m3), which also supplied the vertical wetland units by gravity across their bed surfaces. The volume was controlled using a sheet thickness differential (where each 1 cm of sheet equals 0.00407 m3), regulated by valves in the reservoir. Upon loading, most of the free water in the sludge drained rapidly through the support medium, with higher flow rates observed during the initial four hours. Meanwhile, solids remained retained on the surface of the units. Initially, the units were loaded with sludge twice weekly during the first four months (acclimation period). Subsequently, the loading frequency reduced to once every two weeks (15 days) for the remainder of the monitoring period. To assess greenhouse gas emissions performance among the twelve units, three configurations were utilized: four units planted with Tifton-85 grass (Cynodon spp.), four with elephant grass (Cenchrus purpureus), and four control units without vegetation. Sludge loading rates varied: 75, 100, 150, and 200 kg TS (total solids) m-2 year-1. Each elephant grass, Tifton-85 grass, and vegetation-free wetland unit received one of these rates. These rates were chosen based on guidelines like those from Stefanakis and Tsihrintzis,10 recommending up to 75 kg TS m-2 year-1 for wetland sludge treatment. They also encompass values comparable to those in drying beds (which operate differently with sludge removal), typically around 15 kg TS m-2 (NBR 12.209/1992).17 The highest rate, 200 kg m-2 year-1, equals approximately 273.75 kg TS per m2 annually. Intermediate rates of 100 and 150 kg m-2 year-1 were also included for thorough comparison. The units began operating with sludge after an acclimatization period, from November 2022 to February 2024, during which a sludge layer accumulated with thicknesses varying according to the loading rate. On-site temperature monitoring was conducted using a portable sensor. Samples of the accumulated material were collected randomly from different points across the beds and throughout the accumulated layer to assess water content. This sampling was carried out concurrently with the methane gas collection period, as described below. Methane sampling Four gas sampling campaigns were conducted: two during the dry season (May and August 2023) and two during the rainy season (November 2023 and February 2024). The sampling method used was the statistical chamber method (Figures 3a and 3b), similar to that proposed in the literature.18,19 Each sampling period included four gas collections spaced over a fifteen-day interval between sludge loadings. The first collection was immediately after loading (at time zero), the second at 48 h (2 days), the third at 96 h (4 days), the fourth at 168 h (7 days), and the final collection at 336 h (14 days after loading).
The static chamber consists of two independent parts: the base (diameter = 15 cm, height = 10 cm) and the top (diameter = 15 cm, height = 9 cm), both made of polyvinyl chloride (PVC) material. To perform gas sampling, the base collar was first inserted into the surface of the units at a depth of 5 cm immediately after loading. Subsequently, the top chamber was attached to the upper part of the collar. The top chamber featured two interfaces: one for gas collection, equipped with a three-way valve to connect syringes (20 mL capacity) for collecting gas samples, and another interface where a thermometer was inserted to record the chamber temperature. To monitor weather conditions during sampling, a PRO2 meteorological station installed in the experimental area provided relevant data. During the gas collection process after coupling the upper part, four sampling times were considered: 0, 30, 60, 90, and 120 min. This procedure was repeated for each collection day (0, 2, 4, 7, and 14 days). The initial sampling (0 min) was conducted immediately after coupling, typically between 8 am and 12 pm. Samples were transported in the syringes themselves and stored in a cooled container (Styrofoam with thermal gel) in the laboratory, refrigerated at 4 ºC. Methane concentrations were analyzed using a gas chromatograph (GC-2014, Shimadzu) coupled with flame ionization detector as soon as possible after collection. Methane flux The concentration of methane in the samples collected from the chambers was determined by calculating the ratio of the peak areas of the samples, obtained from the chromatogram to the areas of standard gas concentrations. Following the determination of concentrations in the chamber samples, the rate of gas increase over time was calculated using a linear regression model. Once the best fit was determined, the flux can be calculated using the equation 1: 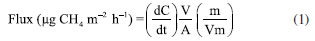 where: dC/dt represents the rate of change of the gas inside the chamber per unit of time (ppm hour-1); m is the molecular weight (g); V and A are the volume (L) and area of the chamber (m2), respectively; Vm is the molecular volume of the gas also in L, which needs to be corrected according to the temperature inside the chamber during sampling (1 mole of gas occupies 22.4 L under normal temperature and pressure conditions - CNTP), simply multiply 22.4 by (273 + T)/273, where T is the average temperature inside the chamber in degrees Celsius.
RESULTS AND DISCUSSION Methane fluxes and seasonal variation The hourly averages of precipitation and temperature during the study period are depicted in Figure 4. In the dry season, air temperatures ranged from 10 to 35 ºC, accompanied by low precipitation rates, whereas during the rainy season, temperatures averaged between 20 and 38 ºC. Changes in meteorological conditions can significantly impact the biogeochemical dynamics of wetland ecosystems and influence gas emissions.19
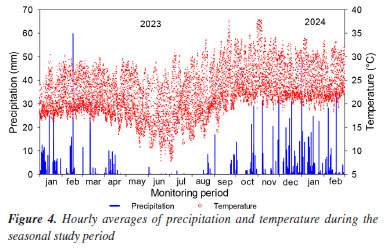
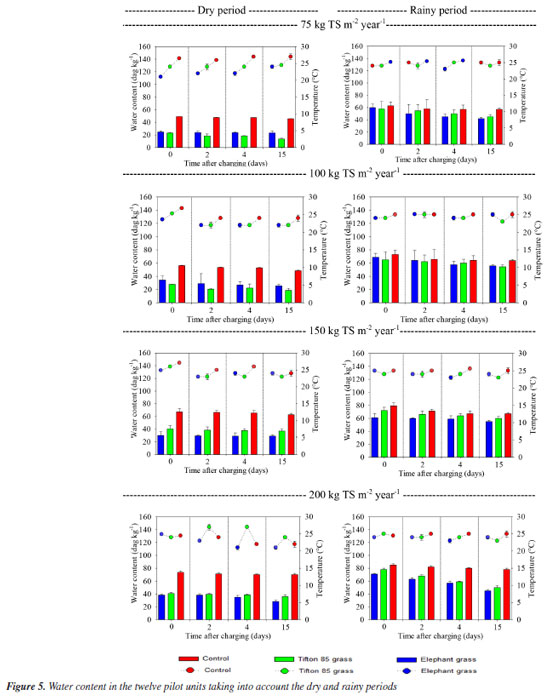
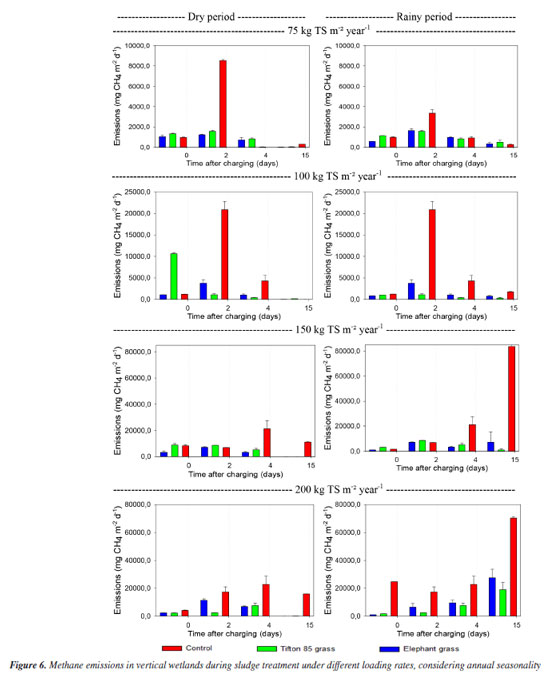
Seasonal variations included lower rainfall rates during the dry season and more pronounced effects of rainfall from November 2023 to February 2024. Studies indicate that water content plays a crucial role in gas emissions from wetlands used for sludge treatment, as it affects microbial activity rates within the units.16,20 This variability suggests that wastewater and solid waste treatment plants may exhibit seasonal contributions to the release of gases that contribute to climate change. In this study, temperature variations ranging from 22 to 27 ºC were observed in the sludge residue layer accumulated within the units (Figure 5). These fluctuations were less pronounced than those seen in the air but still significantly influenced the dynamics within the bed. Higher temperatures generally lead to increased gas emissions due to heightened microbial activity and accelerated mineralization of organic matter.21,22 The elevated temperatures are particularly conducive to greater methane (CH4) production by affecting carbon availability, microbial community composition, and methanogenic activity rates.14-16
According to several authors, temperature may contribute to higher emissions during the rainy season, potentially stimulating microbial activity and organic matter mineralization. Therefore, temperature and water content are critical variables that impact both biogas generation and greenhouse gas emissions, necessitating further investigation.20 In addition to depicting sludge temperature variations (as previously mentioned), Figure 5 illustrates changes in the water content of the accumulated material in the study units, while Figure 6 highlights the methane emission from each monitored bed.
In general, higher water content was observed during the rainy season and with higher sludge loads. However, there was a decrease in water content at the end of the rest period, which was less pronounced in units receiving solid application rates of 150 and 200 kg TS m-2 year-1. These units maintained the sludge moister, with water contents ranging between 40 and 90%. This phenomenon can be attributed to the higher organic matter content present in wetlands receiving heavier loads (data not presented in this study), which helps retain moisture in the environment for longer periods.23-26 In contrast, the decrease in water content was less evident in non-planted units, possibly due to lower rates of mineralization in such environments. Throughout the study, water content in the units increased with each loading event. After the rest period, when accumulated layers underwent refeeding with new nutrients and organic matter, this increase supported the hypothesis that the accumulation of organic carbon influences water retention in the environment. Lower water content values were also observed in planted units compared to control units. The literature suggests that plants play a crucial role in the dehydration and mineralization processes of organic material on wetland surfaces. This role is attributed to factors such as transpiration (which enhances evaporation), influence on microbial communities, and the creation of pathways that facilitate liquid percolation.27,28 To investigate how water retention and loading influenced methane emissions, we conducted a thorough analysis using Figure 6. The results revealed that the highest gas emissions occurred during the rainy season under the highest loadings. This finding supports previous studies29,30 that have observed increased CH4 emissions in conditions of elevated humidity, which promote both methane production and oxidation processes. Conversely, during the dry season, the units exhibited lower water contents and lower temperatures, resulting in decreased emissions. This trend is consistent with findings in the literature and is attributed to variations in microbial activity rates and the redox potential of the environment.31 These results can be explained by the higher water content observed in control beds, as discussed earlier, and the environmental conditions facilitated by plants. As mentioned previously, plant species aid in dehydration processes and create more aerobic environments by enhancing atmospheric oxygen intake and establishing preferential flow pathways.32 Regarding the influence of plants on methane emissions, it was observed that there were lower emissions in the planted units, contrary to some studies33,34 that have demonstrated an increase in CH4 release with the presence of vegetation. Bringing values into discussion, variations in emissions during the seasonal periods were noted. The highest daily rates in the control units during the dry periods (May and August) were 8533.92, 20890.00, 21287.29, and 22691.50 mg m-2 day-1 for loadings of 75, 100, 150, and 200 kg TS m-2 year-1, respectively. In contrast, during the rainy periods (November 2023 and January 2024), these rates were 3349.54, 20893.03, 83618.28, and 70387.50 mg m-2 day-1, respectively. For units planted with Tifton-85 grass, peak rates during the dry periods were 1576.17, 10635.12, 8796.51, and 1576.00 mg m-2 day-1, while during the rainy periods, they were 1589.81, 1089.60, 42363.98, and 18020.00 mg m-2 day-1 for loadings of 75, 100, 150, and 200 kg TS m-2 year-1, respectively. Finally, units planted with elephant grass showed peak rates during the dry periods of 1235.10, 3765.23, 7133.43, and 11246.04 mg m-2 day-1, and during the rainy periods of 1644.91, 3037.32, 7266.65, and 27649.23 mg m-2 day-1, respectively, for loadings of 75, 100, 150, and 200 kg TS m-2 year-1. In summary, considering the observations presented above, it was found that the emission rates at all four loading levels were higher during the rainy season compared to the dry season. This finding aligns with studies conducted in China and Europe,29,31 which also reported increased CH4 emission rates in vertical wetland units for sludge treatment during warmer, rainier seasons. Other authors35 support these findings, suggesting that the higher temperatures enhance anaerobic microbial activities of methanogenic bacteria, thereby promoting increased CH4 emissions. During both the dry and rainy seasons, emission rates in most units followed a decreasing order: control > elephant grass > Tifton-85 grass, respectively, for loadings of 75, 100, 150, and 200 kg TS m-2 year-1. However, an exception occurred during the dry season where the order was control > Tifton-85 grass > elephant grass, particularly for loadings of 100 and 150 kg TS m-2 year-1. It can be observed that control units without vegetation exhibited the highest emissions throughout both loading and resting periods. Among the plant species, there was variability in which one contributed the most to CH4 emissions at different times. The role and contributions of plants in gas emissions remain contentious. On one hand, plants facilitate the intake of atmospheric oxygen through aerenchyma in their aerial parts and assimilate CO2, thereby reducing emissions. On the other hand, the production of exudates in the root zone contributes to the release of CO2, CH4, and N2O into the atmosphere.36 There appears to be a delicate balance between gas retention and emissions by different plant species. The extent of GHG removal often outweighs emissions, which is influenced by the crop type and plant development. Greater biomass production typically leads to enhanced GHG removal, resulting in lower measured values by active or passive samplers installed in wetlands. Research suggests that under heavier loads, Tifton 85 grass, characterized by rhizomatous and stoloniferous growth with roots close to the surface, tends to exhibit more robust plant development compared to species with deeper, fasciculate root systems like vetiver grass and elephant grass.37,38 In most treatments, Tifton-85 grass generally exhibited conditions conducive to robust development, as suggested by visual analyses and productivity data (which are not detailed in this study), except in two loading scenarios during the dry period where elephant grass showed lower emissions. Throughout the seasonal cycle, methane (CH4) emission rates typically showed an initial increase shortly after loading, peaking within the first two days, followed by a decline until the final resting period of the bed. Methanogenesis, an anaerobic process, primarily occurs under moist conditions with limited oxygen supply, typical of environments with accumulated sludge.29 A different emission pattern was observed in units loaded with 150 and 200 kg TS m-2 year-1 during the rainy period, where emission rates increased and persisted until the end of the resting period. These findings are consistent with previous studies that indicate higher sludge loads provide more fresh organic matter, enhancing anaerobic decomposition processes such as methanogenesis. Additionally, water content significantly influences methane emissions; periods of frequent precipitation increase water content, thereby affecting GHG emissions accordingly.22,39
CONCLUSIONS This study evaluated methane emissions from constructed wetland systems used to treat anaerobic sludge from a UASB reactor. Based on the results obtained, higher sludge loading rates were directly linked to increased methane emissions. These emissions peaking during the rainy season were due to elevated moisture content in the sludge. Methane release was highest immediately after sludge loading, gradually tapering off until the next cycle. Notably, the presence of vegetation, particularly Tifton-85 grass, significantly reduced emissions during resting periods between loadings. Optimal conditions for minimizing greenhouse gas emissions were observed at loading rates up to 100 kg TS m-2 year-1 in vegetated wetlands. These findings highlight the effectiveness of constructed wetlands as a sustainable solution for mitigating methane emissions while treating anaerobic sludge. The results suggest that, especially in tropical climates, vertical flow wetlands with vegetation can significantly contribute to reducing greenhouse gas emissions, offering an environmentally friendly approach to sludge management and supporting broader environmental conservation efforts.
ACKNOWLEDGMENTS To the Minas Gerais State Research Support Foundation (FAPEMIG) for the scholarship granted, to CNPq (437130/2018-5) for financing part of the research and to the Postgraduate Program in Sanitation, Environment and Water Resources (SMARH/UFMG).
REFERENCES 1. von Sperling, M.; Andreoli, C. V. In Lodos de Esgotos: Tratamento e Disposição Final; Andreoli, C. V.; Sperling, M. V.; Fernandes, F., eds.; Editora UFMG: Belo Horizonte, 2014, ch. 1. 2. von Sperling, M.; Gonçalves, R. F. In Lodos de Esgotos: Tratamento e Disposição Final; Andreoli, C. V.; Sperling, M. V.; Fernandes, F., eds.; Editora UFMG: Belo Horizonte, 2014, ch. 2. 3. Ministério do Meio Ambiente (MMA); Resolução CONAMA 498, de 19 de agosto de 2020, Define Critérios e Procedimentos para Produção e Aplicação de Biossólido em Solos, e Dá Outras Providências; DOU, Brasília, 2020. [Link] accessed in October 2024 4. Tsutiya, M. T. In Biossólidos na Agricultura; Tsutiya, M. T.; Comparini, J. B.; Sobrinho, A. P.; Hespanhol, I.; Carvalho, P. C. T.; Melfi, A. J., eds.; Sabesp: São Paulo, 2001, ch. 4. 5. Rulkens, W. H.; Water Sci. Technol. 2004, 49, 11. [Crossref] 6. Basamykina, A.; Kharlamova, M.; Mada, S. Y.; E3SWeb of Conferences 2020, 169, 02008. [Crossref] 7. Soares, S. R. A.; de Matos, Z. M. R.; Bernardes, R. S.; Revista Brasileira de Engenharia Agrícola e Ambiental 2001, 5, 313. [Crossref] 8. Uggetti, E.; Ferrer, I.; Nielsen, S.; Arias, C.; Brix, H.; García, J.; Ecol. Eng. 2012, 40, 210. [Crossref] 9. Lopes, B. C.; Zumalacarregui, J. A. G.; Matos, M. P.; Matos, A. T.; von Sperling, M.; Water Practice and Technology 2020, 15, 598. [Crossref] 10. Stefanakis, A. I.; Tsihrintzis, V. A.; Chem. Eng. J. 2011, 172, 430. [Crossref] 11. Magri, M. E.; Francisco, J. G. Z.; Sezerino, P. H.; Philippi, L. S.; Ecol. Eng. 2016, 95, 316. [Crossref] 12. Nielsen, S.; Peruzzi, E.; Macci, C.; Doni, S.; Masciandaro, G.; Water Sci. Technol. 2014, 69, 539. [Crossref] 13. Nielsen, S.; Larsen, J. D.; Water Sci. Technol. 2016, 74, 1793. [Crossref] 14. Uggetti, E.; García, J.; Lind, S. E.; Martikainen, P. J.; Ferrer, I.; Water Res. 2012, 46, 1755. [Crossref] 15. Wang, S.; Ma, F.; Ma, W.; Wang, P.; Zhao, G.; Lu, X.; Water 2019, 11, 133. [Crossref] 16. Zhang, C.; Sun, Y.; Cao, T.; Wang, W.; Huo, S.; Liu, Z.-H.; Int. J. Hydrogen Energy 2022, 47, 32849. [Crossref] 17. Associação Brasileira de Normas Técnicas (ABNT); Norma Brasileira NBR 12.209: Projeto de Estações de Tratamento de Esgoto Sanitário; ABNT: Rio de Janeiro, 1992. [Link] accessed in October 2024 18. Collier, M. S.; Ruark, M. D.; Oates, L. G.; Jokela, W. E.; Dell, C. J.; J. Vis. Exp. 2014, 90, e52110. [Crossref] 19. Liang, J.; Cui, Y.; Zhang, M.; Chen, Z.; Wang, S.; Li, X.; Ecol. Eng. 2021, 159, 106124. [Crossref] 20. Wu, J.; Wang, H.; Li, G.; Wu, J.; Gong, Y.; Wei, X.; Ecol. Eng. 2022, 174, 106461. [Crossref] 21. Paterson, E.; Sim, A.; Global Change Biol. 2013, 19, 1562. [Crossref] 22. Zhao, M.; Han, G.; Li, J.; Song, W.; Qu, W.; Eller, F.; Wang, J.; Jiang, C.; J.Cleaner Prod. 2020, 269, 122316. [Crossref] 23. Minasny, B.; Mc Bratney, A. B.; Eur. J. Soil Sci. 2018, 69, 39. [Crossref] 24. Libohova, Z.; Seybold, C.; Wysocki, D.; Schoeneberger, P.; Williams, C.; Lindbo, D.; Stott, D.; Owens, P. R.; J. Soil Water Conserv. 2018, 73, 411. [Crossref] 25. Santos, J. A.; Gonzaga, M. I. S.; de Almeida, A. Q.; da Silva, A. J.; Santos, J. C. J.; Lima, I. S.; Research, Society and Development 2022, 11, e48411528360. [Crossref] 26. Matos, M. P.; von Sperling, M.; Matos, A. T.; Miranda, S. T.; Souza, T. D.; Costa, L. M.; Ecol. Eng. 2017, 106, 588. [Crossref] 27. Chen, Z.; Hu, S.; Hu, C.; Huang, L.; Liu, H.; Vymazal, J.; Environ. Sci. Pollut. Res. 2016, 23, 11957. [Crossref] 28. Hu, S.; Lv, Z.; Zuo, X.; Liu, H.; Vymazal, J.; Chen, Z.; Sci. Total Environ. 2020, 730, 139142. [Crossref] 29. Teiter, S.; Mander, Ü.; Ecol. Eng. 2005, 25, 528. [Crossref] 30. Zhang, Y.; Hou, W.; Chi, M.; Sun, Y.; An, J.; Yu, N.; Zou, H.; CATENA 2020, 194, 104677. [Crossref] 31. Larsen, J. D.; Nielsen, S.; Scheutz, C.; Ecol. Eng. 2017, 106, 279. [Crossref] 32. Zhang, J.; Yan, Q.; Bai, G.; Guo, D.; Chi, D.; Li, B.; Yang, L.; Ren, Y.; Environ. Res. 2023, 239, 117377. [Crossref] 33. Suntti, C.; Magri, M. E.; Philippi, L. S.; Eng. Sanit. Ambiental 2011, 16, 63. [Crossref] 34. Tanner, C. C.; Water Sci. Technol. 2001, 44, 9. [Crossref] 35. Wang, S.; Cui, Y.; Li, A.; Wang, D.; Zhang, W.; Chen, Z.; J. Environ. Manage. 2019, 240, 231. [Crossref] 36. Yin, X.; Jiang, C.; Xu, S.; Yu, X.; Yin, X.; Wang, J.; Maihaiti, M.; Wang, C.; Zheng, X.; Zhuang, X.; Water 2023, 15, 2871. [Crossref] 37. Teixeira, D. L.; Matos, A. T.; Matos, M. P.; Miranda, S. T.; Teixeira, D. V.; J. Environ. Sci. Health, Part A 2021, 56, 248. [Crossref] 38. Matos, M. P.; Matos, A. T. In Wetlands Construídos como Ecotecnologia para o Tratamento de Águas Residuárias: Experiências Brasileiras; Sezerino, P. H.; Pelissari, C., eds.; Brazil Publishing: Curitiba, 2021, ch. 2. 39. Zhou, G.; Gao, S.; Xu, C.; Dou, F.; Shimizu, K.; Cao, W.; Geoderma 2019, 361, 114071. [Crossref]
Associate Editor handled this article: Lucia Mascaro |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access