Artigo
| Triflic acid-promoted regio and stereoselective synthesis of polyfunctionalized enaminones from dibenzylidene ketones and organic azides |
|
Lucas Souza PratesI I. Instituto de Química, Universidade Federal da Bahia, Campus de Ondina, 40170-290 Salvador - BA, Brasil Received: 06/04/2024 *e-mail: silviodc@ufba.br A stereoselective synthesis of α-aryl Z enaminones was developed by TfOH-promoted condensation of dibenzylidene ketones with organic azides. The cascade reactions involve the sequence of 1,3-dipolar cycloaddition of alkyl azides with the enone, aziridine formation, and 1,2-aryl migration via phenonium ion, whose stereochemical control by a strong N-H...O=C intramolecular hydrogen bond affords Z isomer exclusively. The readily available starting materials and ease of handling of TfOH are experimental advantages that give diversely substituted enaminones with total control of functionalization along the N-C=C-C=O conjugated system. INTRODUCTION Enaminone presents the conjugated system N-C=C-C=O, which confers diverse reactive sites and many applications in organic synthesis1-7 and medicinal chemistry.8 There are a multitude of methodologies to obtain this class of compounds, and the simplest is the reaction of 1,3-dicarbonyl compounds with amines (Figure 1, type 1).1,8 The access of type 1 enaminones was boosted by the substitution of 1,3-dicarbonyl compounds as starting materials to several other substrates such as phenacyl sulfoxides,9 alkynyl derivatives,10-13 vinyl azides,14 among others.15-17
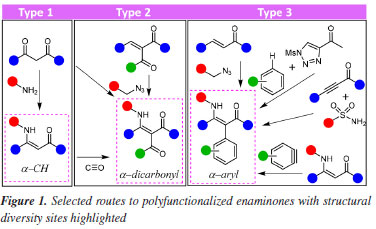
Synthetic approaches to α-substituted type 218-21 and type 3 enaminones,22-26 functionalized with additional sites of structural diversity (highlighted in Figure 1 by colored circles), are scarce.18-26 α-Aryl enaminones (type 3) are strategic in synthesizing indole derivatives,1,2 however, the synthetic route is limited to a few reported methods.22-26 Among these, the reaction of β-aryl enones and benzyl azide promoted by BF3.OEt2 is the simplest one but provides a mixture of isomeric acyclic E/Z enaminones, corresponding to 1,2-aryl migration from β-aryl enone to α-aryl enaminone.26 However, the Lewis acid BF3.OEt2 as a promotor demands tedious manipulation before each enaminone preparation and during the reaction progress. Azides have been a successful building block to the nitrogenated group for enaminone synthesis,14,15,18,20,26-30 and herein we amplify this strategy by the stereoselective synthesis of polyfunctionalized Z enaminones from dibenzylidene ketones with organic azides promoted by triflic acid (TfOH, trifluoromethanesulfonic acid) at room temperature. In addition, the simple reaction conditions associated with easy TfOH manipulation in comparison to other Lewis acid such as BF3.OEt227 and substrates preparation open a way to introduce structural diversity along all components of enaminone's conjugated system type 3 (Figure 1).
RESULTS AND DISCUSSION For the synthesis of substituted enaminones from dibenzylidene ketones and organic azides, the starting point was the conditions of our previous studies26 concerning the use of BF3.OEt2, which afforded a mixture of E/Z isomers. In the search for a practical promotor to develop a stereoselective synthesis of α-aryl substituted enaminones, benzyl azide 1a and easily prepared dibenzalacetone 2a were chosen as model substrates and different reaction parameters were investigated (Table 1).
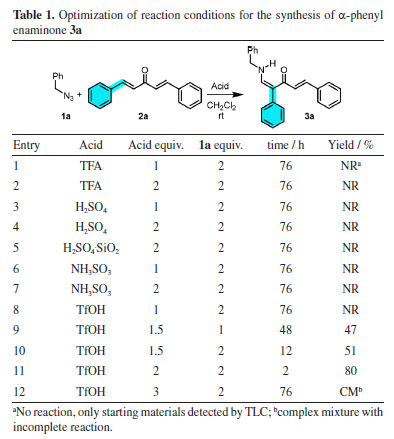
Our previous work26 and Gao and co-workers15 study have concluded that CH2Cl2 is the best solvent for condensation of organic azides with α,β-unsaturated carbonyl compound. Thus, a CH2Cl2 solution of one and two equivalents of trifluoroacetic acid (TFA) as promotor was tested with two equivalents of 1a. However, only starting materials were detected, even at long reaction times (entries 1 and 2, Table 1). A similar result was observed for sulfuric acid, sulfuric acid-supported silica gel (entries 3-5), and sulfamic acid (entries 6-7). The result was unsatisfactory when 1 equivalent of TfOH was tested (entry 8). However, when the amount of TfOH was increased, desirable enaminone 3a was isolated (entries 9-12) except for 3 equivalents. The condition highlighted in entry 11 of Table 1 was the best one corresponding to the synthesis of polyfunctionalized enaminone from dibenzylidene ketones, because only one previous synthesis was reported,26 but low yield and opposite E isomer as major product. With optimal condition determined with TfOH as promotor, the scope of dibenzylidene ketones 2a-2o and three organic azides 1a-1c were evaluated, and the formation of enaminones 3a-3j is presented in Scheme 1.
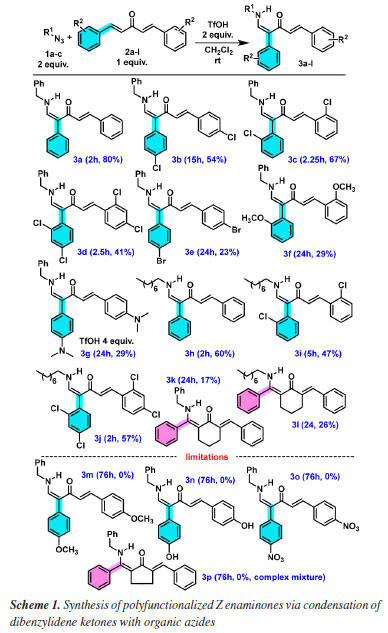
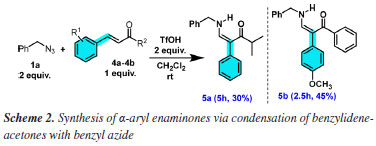
While benzyl azide 1a and octyl azide 1b reacted, no reaction was observed with phenyl azide 1c (2a was recovered). Nonetheless, contrary to the previous method with BF3.OEt2, Z isomers were exclusively formed, as shown by 1H nuclear magnetic resonance (NMR) analyses, which permitted the structural assignment of polyfunctionalized enaminones with one olefinic moiety on the right side and with the 1,2-aryl migration26 in the left side of carbonyl group as indicated for 3a-3j. These structural features of enaminones were deduced due to the disappearing of one olefinic CHα from 2a-2g and the presence of a CHβ-NH 3J coupling in 3a-3j due to the incorporation of the RCH2NH moiety from azides 1a-1b, besides the presence of typical H/H trans coupling constant integrated to only one olefinic group. For all acyclic 2a-2g tested substrates, regioselective α-aryl substituted enaminones were formed. Besides, despite two equivalents of organic azide being necessary in relation to dibenzylidene ketones 2a-2h, only one olefinic moiety of 2a-2h was transformed (Scheme 1). For 2,6-dibenzylidenecyclohexanone 2h without α-hydrogen reacting with azides 1a-1b, no 1,2-aryl migration26 nor ring contraction/expansion was observed,28 and β-aryl substituted Z enaminones 3k-3l were formed. A mechanistic explanation of this dual behavior for acyclic dibenzylidene ketones 2a-2g and cyclic 2h is discussed later. To compare the performance of the two acids (TfOH and BF3.OEt2), simple enones 4a-4b were submitted to TfOH-promoted conditions, but the yields were inferior to those obtained with BF3.OEt2,26 albeit enaminones 5a-5b were still stereoselectivity formed (Scheme 2). However, for all benzylidene ketones, the easy handling of TfOH in comparison to BF3.OEt2 is an advantage to be considered in the overall experimental context, avoiding the previous tedious BF3.OEt2 purification with this Lewis acid before each novel synthetic run. Besides, the superacid TfOH has several characteristics that intensify its use in organic synthesis in relation to BF3.OEt2: it is inexpensive and insensitive to moisture and air.27 Moreover, NMR analysis proved that TfOH afforded enaminones 3a-3j and 5a-5b as single isomers, revealing that this acid is adequate to the stereoselective synthesis of α-phenyl-substituted enaminones, in contrast to BF3.OEt2 that led to 5a-5b as a mixture of E/Z isomers.26 In this way, results of Schemes 1 and 2 indicate that TfOH-promoted synthesis is regio (exclusive acyclic α-aryl 3a-3j or cyclic β-aryl 3k-3l substituted enaminones) and stereoselective (only isomer Z) for all tested mono and dibenzylidene ketones.
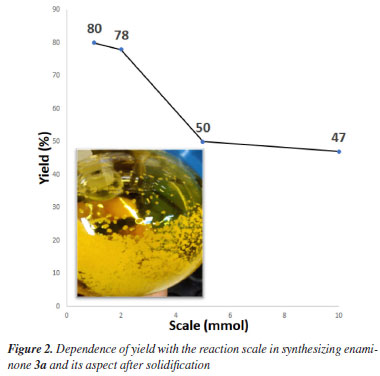
The scalability of the synthesis of enaminone 3a was investigated, and it was increased from 1 to 10 mmol, as shown in Figure 2. A significant difference was noticed in yields from 1-2 to 5-10 mmol scales, with yield decreasing to approximately half, but no pronounceable difference was observed among 1-2 mmol or 5-10 mmol. However, the 1-2 mmol scale reaction time was 2 h, and 24 h was necessary for 5-10 mmol scale to complete the reaction. After purification by column chromatography, 3a was obtained as a yellow oil, solidifying on standing (Figure 2). The decrease in yields by scale increase may be associated with inefficient mixing of the reaction media under magnetic stirring due to the formation of viscous liquid during the reaction progress.
To rationalize the regio and stereoselective formation of Z isomer for all obtained α-phenyl-substituted enaminone 3a-3j, a mechanism pathway is proposed in Scheme 3, which was inspired by our previous synthesis of α-aryl enaminones through reactions of β-aryl enones with benzyl azide.26 However, different intermediates were invoked to explain the observed stereoselective reaction. A 1,3-dipolar cycloaddition between alkyl azides 1 and one olefinic moiety of the protonated benzylideneacetone 2' gives the dihydro triazole 6a which, after proton shift to 6b, suffers ring opening to the diazonium 7a, which is in equilibrium with the rotamer 7b (pathway highlighted in pale blue), wherein alkyl-NH2 and N2+ are antiperiplanar. Favorable 3-exo-tet ring closure by the alkylamino attack and molecular nitrogen extrusion forms protonated aziridine 8 stabilized by a strong N-H...O=C intramolecular hydrogen bond, which suffers ring opening and ring closure via phenonium ion 9, preserving the hydrogen bond with nitrogen RCH2N-H and carbonyl groups at the same face, driving to the observed regio and stereoselectivity. Aromatization of phenonium ion 9 is assisted by nitrogen electrons lone pair leading to the 1,2-aryl migration intermediate 10, which thus yields Z enaminones 3a-3j after hydrogen abstraction by triflate ion. When cyclic benzylidene ketone 2h is the substrate (Scheme 3) the steric crowding by the bulky six-membered ring and absence of α-hydrogen prevents phenonium ion formation, and thus no 1,2-aryl migration is observed. The β-aryl Z enaminones 3k-3l are formed by the same initial reaction pathway of acyclic 2a-2g until corresponding intermediate 7a', which now is in equilibrium with rotamer 7b', wherein benzylic C-H and C-N2+ groups are antiperiplanar, which gives Z enaminones 3k-3l after hydrogen abstraction by triflate ion (see box in Scheme 3).
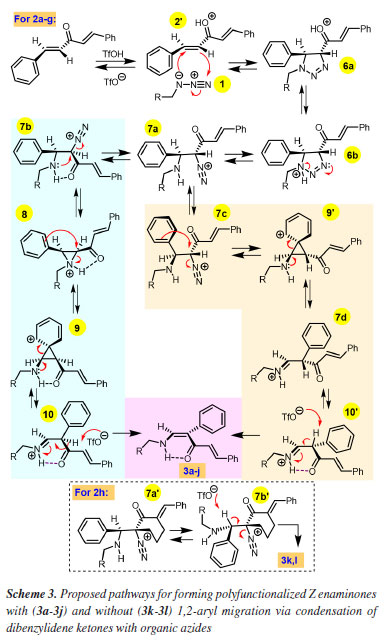
Alternatively, it would be possible that, instead of the aziridinium ion formation (7b to 8, Scheme 3), a direct attack of the phenyl ring on the diazonium might occur via 7c (pathway highlighted in pale orange), which the phenyl ring is antiperiplanar to the diazonium. In this case, the phenonium ion 9' would be directly formed, bypassing the formation of the aziridinium ion 8. In this alternative pathway, aromatization of phenonium ion 9' is assisted by nitrogen electrons lone pair leading to the 1,2-aryl migration intermediate 7d, which rotate to more stable isomer 10' due to the intramolecular H-bond. The hydrogen abstraction by triflate ion affords 3. As one can see, the two possible pathways are degenerated route to the same compound.
CONCLUSIONS Triflic acid was an efficient promotor of the regio and stereoselective synthesis of polyfunctionalized Z enaminones through the condensation of dibenzylidene ketones with organic azides. The readily available dibenzylidene ketones and the practical handling of TfOH are experimental advantages in the context of the cascade reactions sequence, which gives diversely substituted enaminones with total control of functionalization along the N-C=C-C=O conjugated system.
EXPERIMENTAL The reactions were monitored by thin-layer chromatography (TLC) and were performed on pre-coated plates of silica gel 60F254/0.2 mm supported on aluminum (Merck). Visualization in TLC was achieved using UV light (254 and 356 nm) or iodine. The obtained products were separated by column chromatography, which was carried out using Merck silica gel (60 mesh). The appropriate eluent mixtures are described in the following sections. NMR spectra were obtained on a Bruker Avance III spectrometer. The 500 MHz instrument was used for 1H NMR, and the 125 MHz instrument was used for 13C NMR in CDCl3 with tetramethylsilane (TMS) as the internal standard. Chemical shifts are expressed in ppm, and coupling constants are given in Hz. The 1H NMR data are described as follows: chemical shift (d, ppm), multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad signal), coupling constant (Hz). The 13C NMR data are described in terms of chemical shift (d, ppm). The melting points were determined using a Microquímica MQAPF 301 apparatus and are not corrected. Elemental analyses were performed on a PerkinElmer 2401 Elemental Analysis. Azides 1a-1b,29,30 dibenzylidene ketones 2a-2h,31-34 and enones 4a-4b35,36 were synthesized by known procedures. All reagents used were from Aldrich (purchased from representatives in the country; São Paulo, SP, Brazil), and the solvents were from Quimidrol (Joinville, Santa Catarina, Brazil). All equipment was purchased by representatives in the country (São Paulo, SP, Brazil). Caution: TfOH is a very strong acid and can cause severe burnings and is corrosive.37 All reactions were performed with a recent opened bottle of TfOH. General procedure for the synthesis of enaminones To a solution of 1.0 mmol of benzylidene ketones 2a-2o in 1.5 mL of CH2Cl2 under ice bath and under magnetic stirring, was slowly added 2.0 mmol of triflic acid dissolved in 1.5 mL of CH2Cl2. Then, 2.0 mmol of azide 1a-1c was added. The mixture was stirred for 15 min and then removed from the ice bath and kept under magnetic stirring at room temperature for the indicated time in each case. At the end of the reaction, confirmed by TLC, the mixture was diluted in CH2Cl2, and the organic layer was washed with 20 mL of water, 20 mL of brine, dried with anhydrous MgSO4, filtered, and the solvent was evaporated. The obtained material was purified as indicated in each case. (1Z,4E)-1-(Benzylamino)-2,5-diphenylpenta-1,4-dien-3-one (3a) Following the general procedure using 2a (234.9 mg, 1 mmol), benzyl azide 1a (250 µL, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 2 h. Column chromatography purification (hexane/EtOAc 9:1) afforded 3a (271 mg, 80%) as a yellow solid, mp 90-92 ºC. 1H NMR (500 MHz, CDCl3) d 11.13 (s, 1 H), 7.57 (d, J 15.5 Hz, 1H), 7.33-7.19 (m, 15H), 7.03 (m, 1H), 6.82 (d, J 15.5 Hz, 1H), 4.45 (d, J 6 Hz, 2H); 13C NMR (125 MHz, CDCl3) d 155.6, 139.3, 137.7, 136.0, 130.5, 129.3, 129.0, 128.8, 128.5, 128.1, 128.0, 127.4, 126.3, 53.1. Anal. calcd. for C24H21NO: C, 84.92%; H, 6.24%; N, 4.13%; found: C, 85.55%; H, 6.99%; N, 3.89%. Synthesis of enaminones 3a in different scales Following the general procedure using 2a (470 mg, 2 mmol), benzyl azide (516 mmL, 4 mmol), triflic acid (353 µL, 4 mmol), for 2 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3a (530 mg, 78%) as a yellow solid, mp 90-92 ºC. Following the general procedure using 2a (1.1720 g, 5 mmol), benzyl azide (1.3 mL, 10 mmol), triflic acid (882 µL, 10 mmol), for 24 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3a (849 mg, 50%) as a yellow solid. Following the general procedure using 2a (2.3654 mg, 10 mmol), benzyl azide (2.6 mL, 20 mmol), triflic acid (1.8 mL, 20 mmol), for 24 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3a (1.5954 g, 47%) as a yellow solid. (1Z,4E)-1-(Benzylamino)-2,5-bis(4-chlorophenyl)penta-1,4-dien-3-one (3b) Following the general procedure using 2b (304.5 mg, 1 mmol), benzyl azide 1a (250 µL, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 15 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3b (220.4 mg, 54%) as a yellow solid, mp 80-82 ºC. 1H NMR (500 MHz, CDCl3) d 11.14 (m, 1H), 7.51 (d, J 15.5 Hz, 1H), 7.34-7.21 (m, 11H), 7.13-7.11 (m, 2H), 7.02 (m, 1H), 6.72 (d, J 15.5 Hz, 1H), 4.47 (m, 2H); 13C NMR (125 MHz, CDCl3) d 155.6, 138.3, 137.4, 135.3, 134.4, 131.8, 129.3, 129.1, 129.1, 128.7, 198.1, 127.4, 126.2, 53.2. Anal. calcd. for C24H19Cl2NO: C, 70.60%; H, 4.69%; N, 3.43%; found: C, 70.99%; H, 5.02%; N, 3.21%. (1Z,4E)-1-(Benzylamino)-2,5-bis(2-chlorophenyl)penta-1,4-dien-3-one (3c) Following the general procedure using 2c (303.6 mg, 1 mmol), benzyl azide 1a (250 µL, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 2 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3c (273 mg, 67%) as a yellow solid, mp 105-107 ºC. 1H NMR (500 MHz, CDCl3) d 11.24 (m, 1H), 7.99 (d, J 16 Hz, 1H), 7.47-7.11 (m, 15H), 7.02 (d, J 13 Hz, 1H), 6.53 (d, J 16 Hz, 1 H), 4.50 (s, 2H); 13C NMR (125 MHz, CDCl3) d 186.3, 156.1, 137.7, 137.6, 136.1, 135.3, 135.1, 134.4, 133.7, 130.1, 130.0, 129.8, 129.1, 128.6, 128.2, 128.0, 127.7, 127.5, 127.0, 126.9, 108.5, 53.2. Anal. calcd. for C24H19Cl2NO: C, 70.60%; H, 4.69%; N, 3.43%; found: C, 70.99%; H, 5.02%; N, 3.21%. (1Z,4E)-1-(Benzylamino)-2,5-bis(2,4-dichlorophenyl)penta-1,4-dien- 3-one (3d) Following the general procedure using 2d (372.7 mg, 1 mmol), benzil azyde 1a (250 µL, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 2 h 30 min. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3d (195.6 mg, 41%) as a yellow oil. 1H NMR (500 MHz, CDCl3) d 11.15-11.12 (m, 1H), 7.80 (d, J 15.5 Hz, 1H), 7.38 (m, 1H), 7.28-7.10 (m, 9H), 7.04-7.02 (m, 1H), 6.88 (d, J 12.5 Hz, 1H), 6.36 (d, J 5.5 Hz, 1H), 4.40 (m, 1H); 13C NMR (125 MHz, CDCl3) d 185.7, 156.1, 137.3, 136.8, 136.2, 135.6, 135.3, 134.4, 134.3, 133.7, 132.8, 129.9, 129.6, 129.1, 128.4, 128.1, 127.5, 127.4, 127.3, 107.3, 53.2. Anal. calcd. for C24H17Cl4NO: C, 60.41%; H, 3.59%; N, 2.94%; found: C, 60.71%; H, 3.98%; N, 2.57%. (1Z,4E)-1-(Benzylamino)-2,5-bis(4-bromophenyl)penta-1,4-dien-3-one (3e) Following the general procedure using 2e (395.0 mg, 1 mmol), benzyl azide 1a (250 µL, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 24 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3e (114.4 mg, 23%) as a yellow solid, mp 75.3-76.9 ºC. 1H NMR (500 MHz, CDCl3) d 11.22-11.19 (m, 1H), 7.54-7.24 (m, 12H), 7.12-7.04 (m, 3H), 6.79 (d, J 15.5 Hz, 1H), 4.51 (d, J 6 Hz, 2H); 13C NMR (125 MHz, CDCl3) d 186.3, 155.6, 138.4, 137.4, 134.8, 132.1, 132.0, 131.6, 129.5, 129.1, 128.1, 127.4, 126.4, 123.6, 120.4, 53.2. Anal. calcd. for C24H19Br2NO: C, 57.97%; H, 3.85%; N, 2.82%; found: C, 60.13%; H, 4.21%; N, 2.42%. (1Z,4E)-1-(Benzylamino)-2,5-bis(2-methoxyphenyl)penta-1,4-dien-3-one (3f) Following the general procedure using 2f (296.8 mg, 1 mmol), benzyl azide 1a (250 µL, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 24 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3f (115.8 mg, 29%) as a yellow oil. 1H NMR (500 MHz, CDCl3) d 11.12 (s, 1H), 7.91 (d, J 16 Hz, 1H), 7.38-7.17 (m, 9H), 7.01-6.92 (m, 3H), 6.85-6.82 (m, 2H), 6.77 (d, J 16 Hz, 1H), 4.48 (d, J 5.5 Hz, 2H), 3.76 (s, 2 × 3H); 13C NMR (125 MHz, CDCl3) d 153.8, 140.3, 138.9, 138.3, 132.2, 130.0, 129.8, 129.1, 125.0, 124.7, 123.8, 121.2, 119.3, 114.8, 114.5, 50.3, 33.6, 26.0, 25.3. Anal. calcd. for C26H25NO3: C, 78.17%; H, 6.31%; N, 3.51%; found: C, 78.48%; H, 6.64%; N, 3.44%. (1Z,4E)-1-(Benzylamino)-2,5-bis(4-(dimethylamino)phenyl)penta-1,4-dien-3-one (3g) Following the general procedure using 2g (320.8 mg, 1 mmol), benzyl azide 1a (250 µL, 2 mmol), triflic acid (353 µL, 4 mmol), for 48 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3g (85.1 mg, 20%) as an orange solid, mp 90-92 ºC. 1H NMR (500 MHz, CDCl3) d 11.02-10.99 (m, 1H), 7.56 (d, J 15.5 Hz, 1H), 7.37-7.28 (m, 7H), 7.15 (m, 2H), 6.98 (d, J 12.5 Hz, 1H), 6.79 (m, 1H), 6.73 (d, J 15.5 Hz, 1H), 6.62 (d, J 8.5 Hz, 1H), 4.47 (d, J 5 Hz, 2H), 2.99 (s, 6H), 2.97 (s, 6H); 13C NMR (125 MHz, CDCl3) d 188.2, 154.6, 151.3, 139.7, 138.3, 131.5, 129.7, 128.9, 127.7, 127.4, 124.4, 122.0, 112.1, 52.9, 40.4, 29.8. Anal. calcd. for C28H31N3O: C, 78.17%; H, 6.31%; N, 3.51%; found: C, 79.29%; H, 6.79%; N, 3.19%. (1Z,4E)-1-(Octylamino)-2,5-diphenylpenta-1,4-dien-3-one (3h) Following the general procedure using 2a (235.7 mg, 1 mmol), octyl azide 1b (310.5 mg, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 2 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3h (216 mg, 60%) as a yellow oil. 1H NMR (500 MHz, CDCl3) d 10.99 (s, 1H), 7.61 (d, J 15.5 Hz, 1H), 7.39-7.38 (m, 4H), 7.29-7.23 (m, 6H), 7.02 (m, 1H), 6.86 (d, J 15.5 Hz, 2H), 3.32-3.28 (q, J 6.5 Hz, 2H), 1.66-1.60 (m, 2H), 1.40-1.27 (m, 10H), 0.90-0.87 (m, 3H); 13C NMR (125 MHz, CDCl3) d 156.0, 138.7, 136.1, 130.4, 129.1, 128.6, 128.3, 127.9, 126.3, 126.0, 49.6, 31.8, 31.0, 29.2, 26.7, 22.6, 14.1. Anal. calcd. for C25H31NO: C, 83.06%; H, 8.64%; N, 3.87%; found: C, 83.16%; H, 8.88%; N, 3.88%. (1Z,4E)-2,5-Bis(2-chlorophenyl)-1-(octylamino)penta-1,4-dien-3-one (3i) Following the general procedure using 2c (303.2 mg, 1 mmol), octyl azide 1b (310.5 mg, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 5 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3i (202.3 mg, 47%) as a yellow oil. 1H NMR (500 MHz, CDCl3) d 11.00 (m, 1H), 7.99 (d, J 15.5 Hz, 1H), 7.48-7.10 (m, 10H), 6.51 (d, J 15.5 Hz, 1H), 3.33 (m, 2H), 1.67-1.61 (q, J 6.5 Hz, 2H), 1.41-1.25 (m, 10H), 0.89-0.87 (m, 3H); 13C NMR (125 MHz, CDCl3) d 135.9, 135.0, 134.2, 133.7, 131.8, 130.0, 129.8, 127.6, 127.2, 126.9, 126.7, 49.7, 47.2, 31.8, 31.0, 29.2, 29.1, 26.6, 22.6, 14.1. Anal. calcd. for C25H29Cl2NO: C, 69.76%; H, 6.79%; N, 3.25%; found: C, 69.86%; H, 7.17%; N, 2.78%. (1Z,4E)-2,5-Bis(2,4-dichlorophenyl)-1-(octylamino)penta-1,4-dien-3-one (3j) Following the general procedure using 2d (372.0 mg, 1 mmol), octyl azide 1b (310.5 mg, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 2 h. Flash column chromatography purification (hexane/EtOAc 8:2) afforded 3j (284.6 mg, 57%) as a yellow solid, mp 49-50 ºC. 1H NMR (500 MHz, CDCl3) d 10.98 (s, 1H), 7.55-7.42 (m, 5H), 7.26 (d, J 9 Hz, 2H), 7.10 (d, J 8 Hz, 2H), 6.77 (d, J 15.5 Hz, 1H), 3.33 (m, 2H), 1.66-1.61 (m, 2H), 1.38-1.11 (m, 12H), 0.90-0.87 (t, J 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) d 199.7, 163.6, 158.2, 144.1, 136.4, 130.6, 130.5, 130.2, 130.1, 129.7, 127.1, 125.1, 118.0, 114.5, 114.1, 113.9, 113.9,113.8, 55.6, 55.2, 48.4, 45.2, 44.7. Anal. calcd. for C25H27Cl4NO: C, 60.14%; H, 5.45%; N, 2.81%; found: C, 59.89%; H, 5.39%; N, 2.88%. (Z)-2-((Benzylamino)(phenyl)methylene)-6-((E)-benzylidene)cyclohexan-1-one (3k) Following the general procedure using 2h (275.3 mg, 1 mmol), benzyl azide 1a (250 µL, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 24 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3k (64.5 mg, 17%) as a yellow solid, mp 142-144 ºC. 1H NMR (500 MHz, CDCl3) d 13.19 (m, 1H), 7.82 (s, 1H), 7.53-7.26 (m, 16H), 4.29 (s, 2H), 2.82 (s, 2H), 2.14 (s, 2H), 1.69 (s, 2H); 13C NMR (125 MHz, CDCl3) d 185.8, 166.6, 138.6, 137.7, 137.4, 134.3, 132.3, 130.1, 128.8, 128.3, 127.6, 127.5, 127.4, 127.2, 103.1, 48.9, 28.5, 28.1, 24.3. Anal. calcd. for C27H25NO: C, 85.45%; H, 6.64%; N, 3.69%; found: C, 85.49%; H, 6.69%; N, 3.46%. (Z)-2-((E)-Benzylidene)-6-((octylamino)(phenyl)methylene)cyclohexan-1-one (3l) Following the general procedure using 2h (276.1 mg, 1 mmol), octyl azide 1b (310.5 mg, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 24 h. Flash column chromatography purification (hexane/EtOAc 9:1) afforded 3l (104.4 mg, 26%) as a yellow solid, mp 72-74 ºC. 1H NMR (500 MHz, CDCl3) d 12.89 (m, 1H), 7.72 (m, 1H), 7.47-7.33 (m, 7H), 7.24-7.23 (m, 1H), 7.20-7.18 (m, 2H), 2.98-2.91 (m, 2H), 2.72-2.69 (m, 2H), 2.02-1.99 (m, 2H), 1.59-1.48 (m, 4 H), 1.30-1.18 (m, 10 H), 0.86 (t, J 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) d 184.9, 166.9, 137.9, 137.5, 137.0, 136.2, 136.0, 134.5, 131.4, 130.4, 129.9, 128.8, 128.6, 128.6, 128.4, 128.1, 127.3, 102.2, 45.1, 31.8, 30.7, 29.2, 29.1, 28.5, 28.4, 27.9, 26.8, 24.2, 22.6, 14.1. Anal. calcd. for C28H35NO: C, 85.45%; H, 6.64%; N, 3.69%; found: C, 85.85%; H, 6.89%; N, 3.34%. (Z)-1-(Benzylamino)-4-methyl-2-phenylpent-1-en-3-one (5a) Following the general procedure using 4a (172.3 mg, 1 mmol), benzyl azide 1a (250 µL, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 5 h. Flash column chromatography purification (hexane/EtOAc 8:2) afforded 5a (83.8 mg, 30%) as a yellow oil. 1H NMR (500 MHz, CDCl3) d 10.63 (m, 1H), 7.43-7.22 (m, 11H), 6.89 (d, J 12.5 Hz, 1H), 4.44 (d, J 6 Hz, 2H), 2.92-2.82 (m, 1H), 1.03 (s, 3H), 1.02 (s, 3H); 13C NMR (125 MHz, CDCl3) d 204.0, 154.3, 140.4, 137.9, 130.8, 128.9, 128.4, 127.8, 127.5, 126.2, 109.8, 52.9, 35.5, 19.6. (Z)-3-(Benzylamino)-2-(4-methoxyphenyl)-1-phenylprop-2-en-1-one (5b) Following the general procedure using 4b (238.9 mg, 1 mmol), benzyl azide 1a (250 µL, 2 mmol), triflic acid (176.5 µL, 2 mmol), for 2 h 30 min. Flash column chromatography purification (hexane/EtOAc 8:2) afforded 5b (154 mg, 45%) as a yellow solid, mp 73.2-74.6 ºC. 1H NMR (500 MHz, CDCl3) d 10.79 (m, 1H), 7.46 (m, 1H), 7.46-7.07 (m, 14H), 6.89 (m, 3H), 6.68 (m, 2H), 4.47 (m, 2H), 3.75 (s, 3H); 13C NMR (125 MHz, CDCl3) d 193.3, 157.7, 155.9, 152.8, 141.4, 137.8, 133.1, 131.8, 131.1, 129.8, 129.5, 129.0, 128.8, 127.9, 27.6, 127.4, 127.2, 55.3, 53.0.
SUPPLEMENTARY MATERIAL NMR spectra of all products can be found at http://quimicanova.sbq.org.br, as PDF file, with free access.
ACKNOWLEDGMENTS The authors gratefully acknowledge the financial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, financial code 001). We also thank Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), and L. Antonelli by manuscript reading and suggestions.
REFERENCES 1. Yang, K.; Wu, L.; Fu, X.; Chen, W.; Liu, Z.; Peng, X.; Tetrahedron 2024, 156, 133948. [Crossref] 2. Oliveira, M.; de Santana, L. L. B.; Serafim, J. C.; Santos, A. O.; Quintino, M. P.; Correia, J. T. M.; Damasceno, F.; Sabino, J. R.; Pires, T. R. C.; Coelho, P. L. C.; Lopes, G. P. F.; Ulrich, H.; Costa, S. L.; Cunha, S.; Invest. New Drugs 2020, 38, 1257. [Crossref] 3. Cunha, S.; Serafim, J. C.; de Santana, L. L. B.; Damasceno, F.; Correia, J. T. M.; Santos, A. O.; Oliveira, M.; Ribeiro, J.; Amparo, J.; Costa, S. L.; J. Heterocycl. Chem. 2017, 54, 3700. [Crossref] 4. Cunha, S.; Santos, A. O.; Correia, J. T. M.; Sabino, J. R.; Tetrahedron 2014, 70, 3284. [Crossref] 5. Cunha, S.; de Santana, L. L. B.; J. Braz. Chem. Soc. 2014, 25, 1311. [Crossref] 6. Cunha, S.; dos Santos Filho, R. F.; Saraiva, K. H.; Azevedo-Santos, A. V.; Menezes, D.; Tetrahedron Lett. 2013, 54, 3366. [Crossref] 7. Klintworth, R.; Morgans, G. L.; Scalzullo, S. M.; de Koning, C. B.; van Otterlo, W. A. L.; Michael, J. P.; Beilstein J. Org. Chem. 2021, 17, 2543. [Crossref] 8. Amaye, I. J.; Haywood, R. D.; Mandzo, E. M.; Wirick, J. J.; Jackson-Ayotunde, P. L.; Tetrahedron 2021, 83, 131984. [Crossref] 9. Dong, J.; Zhang, T.; Chen, Y.; Sheng, C.; Wang, Y.; Zhang, X.; J. Org. Chem. 2024, 89, 2800. [Crossref] 10. Hsu, Y.-C.; Lai, J.-H.; Liu, R.-S.; Chem. Commun. 2017, 53, 6009. [Crossref] 11. Wang, L.; Ma, J.; Chen, X.; Zhou, X.; Synlett 2017, 28, 2680. [Crossref] 12. Kawade, R. K.; Tseng, C.-C.; Liu, R.-S.; Chem. - Eur. J. 2014, 20, 13927. [Crossref] 13. Yu, D.; Sum, Y. N.; Ean, A. C. C.; Chin, M. P.; Zhang, Y.; Angew. Chem., Int. Ed. 2013, 52, 5125. [Crossref] 14. Ning, Y.; Zhao, X.-F.; Wu, Y.-B.; Bi, X.; Org. Lett. 2017, 19, 6240. [Crossref] 15. Zhang, D.; Zheng, H.; Zeng, L.; Nie, L.; Fan, Y.; Rao, W.; Gao, L.; Org. Lett. 2023, 25, 179. [Crossref] 16. Nguyen, K. X.; Pham, P. H.; Nguyen, T. T.; Yang, C.-H.; Pham, H. T. B.; Nguyen, T. T.; Wang, H.; Phan, N. T. S.; Org. Lett. 2020, 22, 9751. [Crossref] 17. Quiñones, R. E.; Glinkerman, C. M.; Zhu, K.; Boger, D. L.; Org. Lett. 2017, 19, 3568. [Crossref] 18. Rengasamy, R.; Raj, J. P.; Vijayalakshmi, K.; Punitha, N.; Kesavan, M.; Vajjiravel, M.; Elangovan, J.; Eur. J. Org. Chem. 2022, e202101470. [Crossref] 19. Chen, M.; Yu, L.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H.; Chem. Commun. 2017, 53, 6243. [Crossref] 20. Yan, Z.-m.; Wu, N.; Liang, D.; Wang, H.-s.; Pan, Y.-m.; Org. Lett. 2014, 16, 4048. [Crossref] 21. Koduri, N. D.; Wang, Z.; Cannell, G.; Cooley, K.; Lemma, T. M.; Miao, K.; Nguyen, M.; Frohock, B.; Castaneda, M.; Scott, H.; Albinescu, D.; Hussaini, S. R.; J. Org. Chem. 2014, 79, 7405. [Crossref] 22. Miura, T.; Zhao, Q.; Murakami, M.; Angew. Chem., Int. Ed. 2017, 56, 16645. [Crossref] 23. Chen, M.; Peng, J.; Mao, T.; Huang, J.; Org. Lett. 2014, 16, 6286. [Crossref] 24. Ramtohul, Y. R.; Chartrand, A.; Org. Lett. 2007, 9, 1029. [Crossref] 25. Rabet, P. T. G.; Boyd, S.; Greaney, M. F.; Angew. Chem., Int. Ed. 2017, 56, 4183. [Crossref] 26. Cunha, S.; Gomes, A. T.; Tetrahedron Lett. 2012, 53, 6710. [Crossref] 27. Li, H.; Shan, L.; Liu, C.; Liu, N.; Wang, X.; Hu, Y.; Org. Lett. 2023, 25, 4656. [Crossref] 28. Reddy, D. S.; Judd, W. R.; Aubé, J.; Org. Lett. 2003, 5, 3899 [Crossref]; for reaction of azides with other enones, see: Mahoney, J. M.; Smith, C. R.; Johnston, J. N.; J. Am. Chem. Soc. 2005, 127, 1354 [Crossref]; Casey, M.; Donnelly, J. A.; Ryan, J. C.; Ushioda, S.; Arkivoc 2003, 7, 310. [Link] accessed in October 2024; Donald, A. S. R.; Marks, R. E.; J. Chem. Soc. C 1967, 1188. [Crossref] 29. For compound 1a: Sá, M. M.; Ramos, M. D.; Fernandes, L.; Tetrahedron 2006, 62, 11652. [Crossref] 30. For compound 1b: Balkenende, D. W. R.; Cantekin, S.; Duxbury, C. J.; van Genderen, M. H. P.; Meijer, E. W.; Palmans, A. R. A.; Synth. Commun. 2012, 42, 563. [Crossref] 31. For compounds 2a and 2h: Hathaway, B. A.; J. Chem. Educ. 1987, 64, 367. [Crossref] 32. For compounds 2b and 2c: Vander Jagt, D.; Deck, L.; Abcouwer, S.; Orlando, R.; Royer, R.; Weber, W.; Bobrovnikova-Marjon, E.; Hunsaker, L.; US pat. 0060644A1 2007. (CAN: 146:337624) 33. For compounds 2d, 2f and 2g: Snyder, J. P.; Daves, M. C.; Adams, B.; Shoji, M.; Liotta, D. C.; Ferstl, E. M.; Sunay, U. B.; WO pat. 01/40188A1 2001. (CAN: 135:19497) 34. For compound 2e: Hiroyuki, S.; Yoshiharu, I.; Hisatsugu, O.; Hiroyuki, Y.; Yuichi, K.; WO pat. JP312806 2006. (CAN: 146:115005) 35. For compound 4a: Garcia-Raso, A.; Campaner, B.; Sinisteua, J. V.; Marino, J. M.; An. Quim., Ser. C 1982, 78, 113. 36. For compound 4b: Sivakumar P. M.; Seenivasan, S. P.; Kumar, V.; Doble, M.; Bioorg. Med. Chem. Lett. 2007, 17, 1695. [Crossref] 37. For further information on physical properties and proper handling of TfOH, please check the following: ChemCAS, http://www.chemcas.com/material/cas/archive/1493-13-6.asp, accessed in October 2024; Wiley Online Library, Trifluoromethanesulfonic Acid, https://onlinelibrary.wiley.com/doi/10.1002/047084289X.rt246.pub2, accessed in October 2024.
Associate Editor handled this article: Giovanni W. Amarante |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access






