Revisão
| Biochar from lignocellulosic biomass: a sustainable circular economy approach for removing organic and inorganic contaminants |
|
Rayanne O. Araujo; Otorvan C. dos Anjos; Luiz K. C. de Souza; Giovana A. Bataglion* Departamento de Química, Instituto de Ciências Exatas, Universidade Federal do Amazonas, 69077-000 Manaus – AM, Brasil Received: 05/09/2024 *e-mail: giovanabataglion@ufam.edu.br Metals, pharmaceuticals, personal care products, pesticides, and dyes constitute contaminants widely detected in aquatic ecosystems. Adsorption is an important technology for addressing these noxious contaminants in water systems. Its widespread employment stems from its uncomplicated design, environmentally benign nature, heightened sensitivity to contaminants, and mechanical robustness. These inherent advantages render it a highly promising solution for such challenges. Within this framework, biochar embodies key physicochemical properties akin to activated carbon, the foremost adsorbent globally recognized for its efficacy in water contaminant removal. Moreover, biochar derives from the repurposing of biomass waste, thereby presenting an opportunity to enhance the value chain of numerous agricultural byproducts across Brazil. Therefore, this review article aims to present the most commonly studied lignocellulosic residues from Brazil as adsorbents, the methods used to convert them into biochar, the factors influencing the adsorption of contaminants on biochar, and their environmental applications. The main results related to lignocellulosic biomass were presented, including sugarcane, soybean, corn, rice, cotton, coffee, açaí, tucumã, coconut, Brazil nut, cupuaçu, nuts, murumuru, orange, cassava, banana, malt, macaúba, and guava. It was concluded that chemical activation is more common than physical activation, especially using ZnCl2. Finally, the materials have been employed for the removal of metals and a few organic contaminants, mainly dyes, from synthetic solutions. INTRODUCTION Population growth and industrial and technological advancements have spurred a surge in the manufacturing and usage of everyday essentials. Consequently, this has resulted in the release of toxic contaminants, causing irreparable harm to aquatic ecosystems. Despite water covering approximately 70% of the Earth’s surface, only 3% of water resources adhere to potability standards stipulated in existing legislation. This alarming dissonance underscores the pressing need to confront and alleviate pollution issues, safeguarding the provision of clean, drinkable water for present and future generations.1 Metals, pharmaceuticals, personal care products, pesticides, and dyes are among the primary contaminants detected in water systems. Industrial activities contribute to the presence of metals such as arsenic (As), cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg), nickel (Ni), and zinc (Zn), among others. Certain organic substances, such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and organochlorine pesticides, are categorized as priority organic contaminants, while others, such as pharmaceuticals and personal care products, are classified as emerging contaminants. Though not yet included in routine monitoring programs, these substances pose a substantial risk to human health and the environment due to their potential ecotoxicological or toxicological effects, even at minuscule concentrations.2 A common technology employed for the environmental remediation of these toxic contaminants in water is adsorption. This technique is favored for its straightforward design, environmentally benign nature, heightened sensitivity to contaminants, and mechanical robustness. These inherent advantages position it as a highly promising solution for this purpose. Various adsorbents, including carbon materials like activated carbon, polymer resins, graphene oxide, metal-organic frameworks, and semi-carbonized materials, are utilized as raw materials in the production of these adsorbents.3 The adsorption capacity of carbon materials relies heavily on the development of specific textural properties, including the high ordering and uniformity of micro or mesopores. These properties contribute to a large surface area, facilitating rapid mass transport within the porous structure and ensuring high thermal and hydrothermal stability. Semi-carbonized materials, derived from lignocellulosic biomass residues and commonly referred to as biochar, possess these critical properties. In addition to resembling activated carbon, which stands as the foremost adsorbent utilized globally for removing various contaminants from water, biochar offers an environmentally friendly alternative with promising adsorption capabilities.4 The synthesis of semi-carbonized carbon materials can be accomplished through two primary methods: pyrolysis at temperatures exceeding 200 °C in an inert atmosphere, which produces biochar, or hydrothermal carbonization at temperatures below 250 °C under self-generated pressures within a sealed container, in which the product obtained is called hydrochar. Despite the low thermal stability (> 200 °C) and low porosity of hydrochar, which makes it difficult for the solid surface to interact with the structure of the contaminant under study, hydrochar has the advantages of being synthesized in a shorter time, low energy consumption, with reduced environmental risk (in most cases) and a high degree of functionalization on the surface. This last characteristic favors the capture of contaminants by the non-porous structure of hydrochar.5,6 Utilizing raw materials as adsorbents in both processes offers environmental benefits on two fronts. Firstly, it addresses the challenge of managing the substantial volume of fruit/grain processing waste. Inadequate waste disposal contributes to water body contamination and the proliferation of microorganisms. Secondly, employing waste as a precursor for biosorbents taps into a renewable source with physicochemical adsorption properties capable of effectively capturing contaminants in aqueous environments.6 Brazil is renowned for its extensive agricultural sector and serves as a significant exporter of agricultural commodities, contributing a remarkable 26.6% to the national economic production. This substantial figure underscores the paramount importance of agricultural activity to the Brazilian economy. Moreover, in 2020, the country secured its position as the world’s second-largest grain producer. Consequently, Brazil possesses a considerable abundance of biomass residues, including bark and seeds, which remain largely untapped and warrant further exploration regarding their potential utilization.7,8 Employing this waste for the preparation of biosorbents embodies the principles of the “circular economy”, characterized by the utilization of waste with substantial potential for diverse applications. The overarching objective is to move beyond the prevailing paradigm of the “linear economy”, wherein natural resources are exhausted, and underutilized waste is discarded into the environment, leading to adverse environmental impacts. In this context of abundant availability, underexploited resources, and economic viability, numerous studies9 have investigated the utilization of lignocellulosic biomass for environmental remediation applications, particularly through the adsorption of pharmaceuticals, personal care products, metals, and dyes from water. Considering the challenges and opportunities inherent in the application of these materials, this review seeks to assess and delineate the principal sources of lignocellulosic biomass that, ideally, do not compete with the food industry yet hold potential for adsorption purposes. The review is structured into five sections. Following this introduction, the subsequent section delves into lignocellulosic biomass residues as promising adsorbents, with a focus on their production and waste generation in Brazil, methods of biochar preparation, physicochemical factors influencing adsorption, and the adsorption mechanism. The third section underscores the environmental utility of biochar for eliminating both organic and inorganic contaminants, while the final section outlines the prospects and challenges associated with utilizing these biomasses as adsorbents.
LIGNOCELLULOSIC BIOMASS WASTE AS A PROMISING ADSORBENT Lignocellulosic biomass stands out as the sole renewable carbon source capable of synergistically yielding biochar, bio-oil, and biogas through thermal pretreatment methods such as pyrolysis, hydrothermal carbonization, torrefaction, or gasification. Biochar, in particular, has garnered considerable interest owing to its versatile and functional nature, characterized by distinctive physicochemical properties. These properties render biochar suitable for a myriad of applications across various fields, with environmental remediation standing out as a prominent area of focus. Availability Lignocellulosic biomass residues derived from agri-food industries contribute to an annual global production of approximately 140 billion tons. Since the 1990s, Brazil has played a significant role in the international trade of agri-food products, particularly focusing on the production and export of sugar, orange juice, and the soybean complex (grain, bran, and oil). Notably, an estimated one in every four agribusiness products circulating worldwide originates from Brazil. This prominence is attributed to the increasing demand from Asian countries, which forecast that by 2030, one-third of the globally traded products will originate from Brazil.10 In this context, Brazil emerges as a nation with extensive agricultural production, resulting in significant volumes of waste that necessitate proper disposal. The country boasts the capability to generate over 298 million tons annually of lignocellulosic waste, primarily stemming from the cultivation of rice, sugarcane, corn, soybeans, and wheat. While these residues have traditionally been utilized for bioenergy generation, there is growing interest in exploring their potential as adsorbents for various organic and inorganic contaminants, as evidenced by numerous research studies in this field.11 Based on the findings of the literature regarding the application of biochar as a promising adsorbent for environmental remediation, our study delved into the primary biomasses prevalent in Brazil. Existing research already recognizes these biomasses as effective adsorbents for removing metals, pharmaceuticals, personal care products, and dyes from various environmental matrices. Brazil is the world’s largest sugar cane producer, with a production volume reaching 724 million tons in 2022.12 Sugarcane serves as a raw material for sugar and ethanol production, with waste accounting for approximately 28% of the plant’s total output.13 Naga et al.14 conducted a study synthesizing biochar from sugar cane bagasse residue through chemical activation with ZnCl2 followed by pyrolysis at 500 °C. This preparation method significantly increased surface area (1145 m2 g–1) and pore volume (1.13 cm3 g–1), contributing to enhanced adsorption capabilities. The biochar exhibited notable adsorption capacity for diclofenac sodium, reaching 315 mg g–1. Jiang et al.15prepared a biochar from sugarcane bagasse modified with polyethyleneimine for the removal of Cu(II) from an aqueous solution. The adsorption performance of the material varied with pH, achieving a maximum adsorption capacity of 107.5 mg g–1. Another significant residual biomass abundantly produced in Brazil is coffee husk. In 2021, the country’s coffee bean production amounted to 3.4 million tons, with 45% of it constituting waste.13 Tran et al.16 conducted a study utilizing coffee husk residue to produce a biosorbent through hydrothermal carbonization followed by chemical activation with KOH, targeting the removal of methylene blue dye. The resulting material exhibited a notable surface area of 862 m2 g–1 and achieved a maximum adsorption capacity of 415.8 mg g–1, showcasing the potential of coffee husk residue for the production of activated carbon for environmental remediation purposes. Brazil consistently ranks among the top ten largest rice producers in the global rankings. In 2021, the country’s rice production amounted to 7.2 million tons of shelled grain. It is estimated that for every ton of rice produced, approximately 230 kg of rice husk waste is generated. Chaijak et al.17 conducted a study focusing on producing biochar from rice husk and straw to remove Pb(II) from water. The biochar exhibited an impressive adsorption capacity of 390 mg g–1 for Pb(II) and demonstrated high efficiency (96.10%) in contaminant removal. Similarly, Saad et al.18 highlighted the production of biochar from rice straw residue, activated with NaOH and KOH to create an adsorbent material. The biochar activated with KOH showcased a remarkable surface area of 2006 m2 g–1, while that activated with NaOH exhibited 322.81 m2 g–1. The maximum adsorption capacity for methylene blue was found to be 588.24 mg g–1 for the KOH-activated adsorbent and 232.56 mg g–1 for the NaOH‑activated adsorbent. In terms of corn production, Brazil ranks third globally, with production exceeding 105 million tons in 2021.19 The waste generated from corn production primarily consists of corn cob and stover, commonly utilized in field composting practices. It is estimated that the mass of corn cob corresponds to approximately 70% of the weight of the harvested grains, while corn stover accounts for approximately 15%.20 Song et al.21 conducted a study focusing on corn cob residue, wherein they produced a biosorbent through pyrolysis and chemical activation with KOH. The resulting biochar exhibited remarkable adsorption capacity for methylene blue dye, attributed to its high surface area (1600 m2 g–1) and pore volume (2 cm3 g–1). Brazil is also prominently featured in soy production, ranking among the top three producers globally with an output exceeding 131 million tons. This substantial production volume accounts for approximately one-third of the world’s total soybean production.19 The primary products derived from soybeans are oil and protein, with waste in soybean hulls as a common byproduct. Due to their nutritional composition and availability, soybean hulls are extensively utilized as animal feed.22 Soybean hull typically constitutes approximately 8-10% of the total grain mass. Despite its lower proportion relative to the grain, the high volume of soybean production generates a significant quantity of residue.23 Ma et al.24conducted a study utilizing soybean hull as an adsorbent for the removal of methylene blue dye. The material was activated with K2CO3, resulting in a surface area of 1455 m2 g–1 and a maximum adsorption capacity of 625 mg g–1. This research demonstrates the effectiveness of soybean hull as a promising adsorbent for dye removal applications. In 2021, Brazil produced approximately 6 million tons of cotton, securing the fourth position globally.19 Cotton, extensively utilized by the textile industry, generates stalk and shell waste.25 This waste, amounting to around 30-40% of the total product weight, finds reuse in various applications such as fertilizer production, biogas generation, and the production of adsorbents, among others. Sajednia et al.26conducted a study wherein cotton waste was repurposed to produce an adsorbent capable of removing cadmium sulfide nanoparticles from aquatic media. In addition to the agro-industrial residues derived from Brazil’s global leadership in agricultural production, regional biomass residues have significant potential for exploration. Some residues have already been studied and applied as adsorbents for removing contaminants. The Amazon region, in particular, stands out due to the abundance of native non-woody biomass sources available in extractive reserves. This availability of waste material presents opportunities for various applications, including the production of adsorbents for contaminant removal. Tucumã is widely consumed as a food source in the state of Amazonas. In 2017, an estimated 5 kilotons of fruit pulp were produced, resulting in peel and stone waste comprising over 70% of the total fruit. While the seeds are utilized in crafts, they are commonly discarded in trash cans.27 Literature sources (see Table 1) highlight the utilization of this residue as an adsorbent for contaminants.
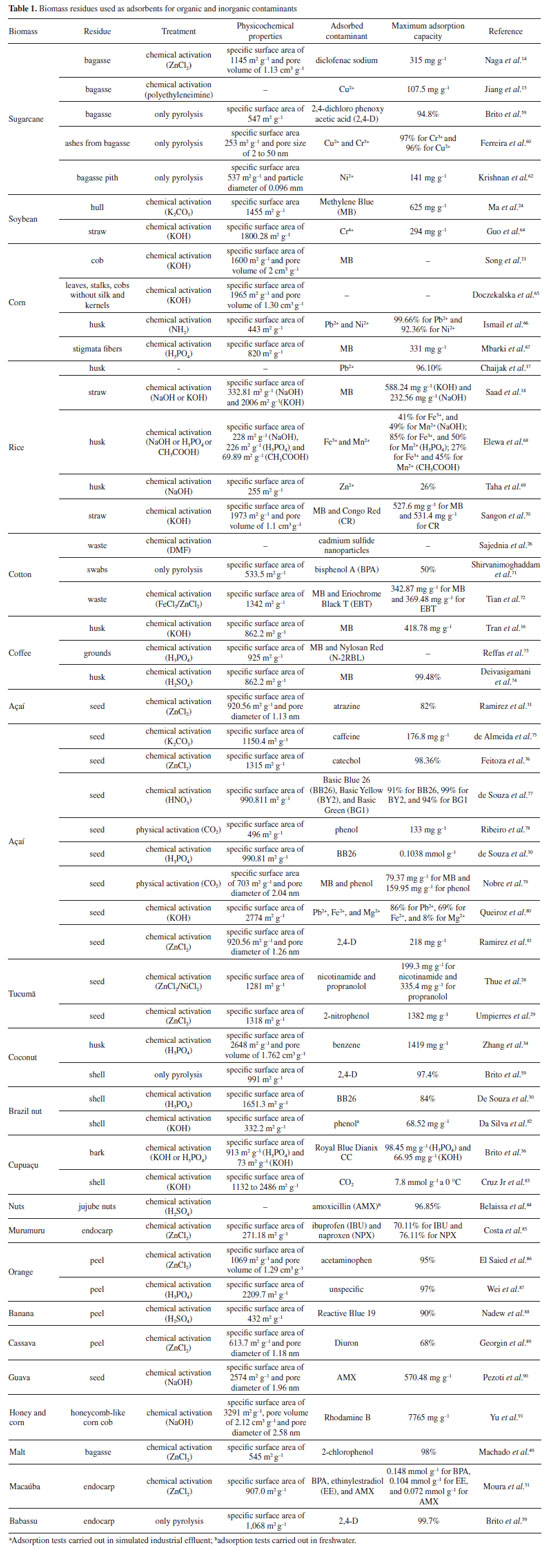
Thue et al.28 conducted a study wherein they produced biochar from tucumã seeds with a high surface area of 1281 m2 g–1 for the removal of synthetic effluents from pharmaceutical industries. Umpierres et al.29utilized tucumã seeds chemically activated with ZnCl2 to remove 2-nitrophenol from household fluids. The resulting adsorbent exhibited a surface area of 1318 m2 g–1 and a maximum adsorption capacity of 1382 mg g–1, demonstrating promising results in the remediation of this contaminant. Açaí is the primary fruit sold in the northern region of Brazil and is also exported to national and international markets. For every 100 tons of fruit, approximately 80 tons of açaí seeds are generated.30 Ramirez et al.31 emphasized the production of biochar from açaí seeds activated with ZnCl2, capable of removing up to 82% of herbicides from water. Another significant biomass consumed in the northern region is Brazil nut. The state of Pará is responsible for producing over 90% of Brazil nut almonds, with an average production of 19.6 kilotons per year, of which 90% constitutes Brazil nut shell residue. These statistics underscore the availability of residue for bio-adsorbent production.30 De Souza et al.30 highlighted the utilization of various biomass residues, including Brazil nut shells, as promising adsorbents for removing basic dyes, achieving contaminant removal rates of up to 84%. Coconut and cocoa are additional fruits widely consumed in Brazil, and their residues are utilized to produce biochar for contaminant adsorption purposes.32 Brazil produces more than 1 billion coconut fruits annually, each weighing an average of 900 g. Of this total, over 80% of the weight comprises the fibrous part.33 Zhang et al.34 utilized coconut shells to produce biochar chemically activated with H3PO4 for benzene removal, achieving a maximum contaminant removal of 1419 mg g–1. Cocoa production positions Brazil among the top ten largest producers globally. Increased production also results in a greater amount of waste, with this waste accounting for 80% of the fruit’s mass weight. In 2019, Brazil’s cocoa production reached 253 thousand tons.35 Cupuaçu finds diverse applications in the food and cosmetics industries, as well as in traditional medicine. The state of Pará is the largest producer of cupuaçu fruit, with an annual production of 28 thousand tons in 2018. Approximately 30% of this total corresponds to peel and seed residues. Literature reports35 indicate the utilization of cupuaçu waste in the production of adsorbents for environmental remediation purposes. Brito et al.36 highlighted the reuse of cupuaçu peel in the production of biochar chemically activated with KOH and H3PO4. The material was effectively applied for dye removal, achieving a maximum adsorption capacity of up to 99%. Here, we have presented some of the biomasses with the highest production and waste generation in Brazil, which have been the subject of application studies in the production of biochar for contaminant remediation. Figure 1 illustrates the proportion of waste generated after processing the fruit or grain. Additionally, Table 1 provides a summary of studies focusing on the application of Brazilian biomass waste as adsorbents for the remediation of organic and inorganic contaminants.
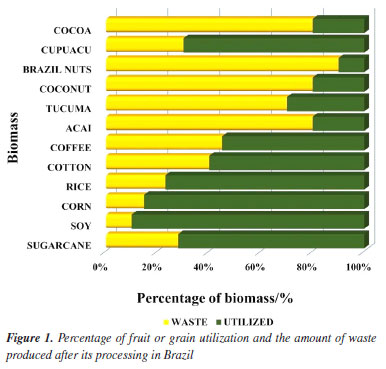
Preparation methods of biochar Biochar synthesis methods primarily involve hydrothermal treatment and pyrolysis, although other thermochemical processes, such as microwave synthesis and roasting, are also utilized. The yield of biochar is influenced by the raw material type and the thermochemical treatment method employed, which in turn determine the physicochemical properties of the resulting solid. These properties primarily include specific surface area, diameter, pore volume, and chemical functionalization of the biochar surface. Below, we outline the characteristics of each thermochemical technology. Hydrothermal carbonization (HTC) involves dissolving biomass in water at temperatures below 250 °C and self-generated pressures within a closed container. This process primarily entails hydrolysis reactions, resulting in the formation of biochar, along with the production of gases and liquids. The predominant non-condensable gaseous product (~90%) comprises CO2 and minor quantities of CO, H2, and light hydrocarbons like CH4. The liquid product consists of aqueous coproducts and reaction water.6,37 The biochar produced through HTC can achieve high yields, typically containing 55-90% of the initial mass of the raw material and retaining 80-95% of its energy potential. This indicates a significant conversion of biomass into biochar, rendering the HTC process highly efficient. Furthermore, unlike torrefaction, which is primarily a low-temperature pretreatment aimed at improving biomass’ thermal properties, hydrothermal treatment also effectively removes alkali metals from the biomass, enhancing the quality of the resulting biochar.38 The operational parameters of the HTC process play a crucial role in determining the mass and energy yields, as well as the physicochemical characteristics of the resulting biochar. Variations in biomass composition inherently lead to significant differences in decomposition behavior during heat treatment. However, residence time and temperature influence properties such as carbon content, surface area, pore structure, reactivity, calorific value, morphological structure, and functional groups. These properties are advantageous for various applications, including environmental remediation, which is the central focus of this revision.39 The second method of preparing biochar is pyrolysis, which involves heating biomass between 300-800 °C in the absence of oxygen to produce biochar, bio-oil, and biogas. Pyrolysis can generally be categorized into three types: slow, intermediate, and fast. In slow pyrolysis (around 450 °C), biochar is the primary product, with its yield directly influenced by the presence of ash and lignin in the biomass. In contrast, fast pyrolysis (450 to 800 °C) primarily produces bio-oil, which can substitute fuel oil in electricity generation. Intermediate pyrolysis (300-500 °C), compared to fast pyrolysis, yields liquids with lower tar content and viscosity.35 During the pyrolysis process, the main chemical components of biomass undergo thermodecomposition, including hemicellulose, cellulose, and lignin. Hemicellulose and cellulose degradation typically occurs in two stages. The initial mass loss stage involves the decomposition of hemicellulose at temperatures ranging from 240-290 °C, while the subsequent mass loss event corresponds to the decomposition of cellulose within the temperature range of 320‑360 °C. Lignin decomposition occurs during the final stage of mass loss, typically at temperatures above 500 °C.40 Several process parameters, including the type of raw material and particle size, type of pyrolytic reactor, temperature and residence time, heating rate, and flow rate of the gas atmosphere, influence the yield and properties of biochar produced via pyrolysis. Typically, biomass feedstock with higher lignin content is preferred for biochar production by pyrolysis, as it tends to yield materials with a well‑developed porous structure and high surface area.41 Pyrolysis can also be assisted by microwaves, offering an unconventional approach that provides an economical synthesis method in terms of energy usage and product selectivity. Several studies42 have reported that microwave-assisted pyrolysis produces biochar with superior yield and improved physicochemical characteristics compared to conventional pyrolysis methods. The success of the technique is attributed to its inherent advantages, including a high heating rate, which leads to shorter process times and rapid heat and mass transfer. Additionally, microwave-assisted pyrolysis offers uniform heating and creates an inert vacuum atmosphere, further enhancing its efficiency.43 In microwave-assisted pyrolysis, heat generation results from thermal energy converted by molecular friction. This energy transfer occurs rapidly from the core to the surface as microwaves propagate through the sample, leading to efficient heat generation within the material. In contrast, conventional pyrolysis involves heat and mass transfer occurring in opposite directions.44 The thermochemical technique demonstrates remarkable success, yielding biochar up to 83.2% by weight.34 Figure 2 provides a summary of the primary thermochemical methods and associated process conditions utilized in the preparation of biochar.
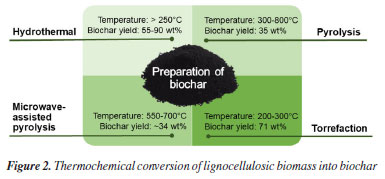
Factors affecting the adsorption of contaminants on biochar The adsorption efficiency of biochar is significantly influenced by its properties, which can be tailored to enhance the material’s versatility. The main factors directly impacting adsorption include the type of raw material used, the conditions of thermochemical processes, and structural modifications. Below, we delve into how these factors influence the adsorption of metals, pharmaceuticals, personal care products, and dyes in aqueous systems. The raw biomass’ inherent composition significantly shapes biochar’s adsorption capacity. This composition encompasses various organic components, including cellulose, hemicellulose, lignin, starch, proteins, pectins, and lipids, as well as inorganic matter such as mineral composition, moisture content, and volatile fractions unique to each biomass source. Among these components, lignin particularly stands out for its pivotal role in determining the yield and morphology of biochar.45,46 The synthetic conditions during thermochemical conversion alter the elemental composition and properties of biochar, with parameters such as temperature, residence time, and chosen thermal conversion technology playing pivotal roles. These factors also exert considerable influence on the efficiency of removing various contaminants. Bueno et al.4 synthesized insights from multiple studies in the literature regarding the application of different biomasses for contaminant removal from water. The findings revealed a notable increase in carbon content with rising pyrolysis temperature, accompanied by decreases in nitrogen, hydrogen, and oxygen contents as the treatment temperature increased. Consequently, these changes decreased molar ratios of H/C, O/C, and (O + N)/C.4 The H/C ratio (value of H/C < 0.7) is indicative of aromaticity and hydrophobicity, as the hydrogen content primarily originates from the organic matter composition of the biomass. Meanwhile, the O/C ratio (value of O/C < 0.2) indicates polar group presence, with lower O/C ratios suggesting a hydrophobic surface. Consequently, a lower O/C ratio justifies the presence of a more aromatic and less hydrophilic surface.4 Pyrolysis temperatures lead to the depletion of polar functional groups, such as −OH, −CH2, –CH3, C=O, and −CO. It is observed that temperatures below 500 °C typically disrupt hydrogen bonds and the oxidation of hydroxyl groups into carboxyl groups. Conversely, temperatures at or above 500 °C invariably trigger the dehydrogenation of −CH2, −CO, and −OH. A similar trend is observed in the decrease of the (O + N)/C ratio. In comparison to pyrolysis temperature, the heating rate and residence time have minimal impact on the physicochemical properties of biochar.47 As previously mentioned, thermal decomposition effectively breaks down unstable organic matter within biomass. However, stable carbon compounds, known as fixed carbon, remain largely unchanged, resulting in the formation of a more stable biochar that exhibits resistance to degradation. This resilience is attributed to the heightened degree of carbon polymerization induced by high temperatures during pyrolysis. Consequently, both carbon and ash content typically increase with rising pyrolysis temperatures.48 However, biochar with a high ash content is not recommended due to the presence of polycyclic aromatic hydrocarbons and metals, which can lead to elevated levels of atmospheric contaminant emissions. Rapid heating rates exceeding 200 °C min–1 can also pose challenges for biochar formation. Under such conditions, a significant portion of volatile matter decomposes rapidly, hindering the development of the desired porous structure. Instead of forming biochar, the volatile fraction decomposes into low molecular weight liquids and gases.48 The surface area of biochar typically increases with higher pyrolysis temperatures due to the volatilization of substances such as cellulose and hemicellulose. This process can lead to the formation of additional pores or even the unblocking of existing pores, increasing externally accessible surface areas. Moreover, elevating the treatment temperature may decrease pore size, promote the formation of internal pore structures, or enhance overall porosity.49 It is important to note that all the factors mentioned so far are outcomes of biochar production using the conventional pyrolysis method.47 However, the production of biochar via the hydrothermal method tends to favor the formation of oxygenated groups on the surface. Nevertheless, depending on the synthesis temperature conditions, surface area and pore structure development may be less significant compared to conventional pyrolysis.4 While pure biochar is often utilized as an adsorbent, it exhibits limitations in effectively adsorbing certain pharmacological contaminants. This is primarily attributed to the surface functional groups present after its preparation and its low capacity for mass exchange between biochar and contaminants.50 As a result, physical and chemical modifications to its structure have become necessary to enhance its performance. Physical modification of biochar is often regarded as more ecologically and economically viable since it avoids the use of chemicals during the modification process. However, it tends to be less effective compared to chemical activation methods. Common physical modifications typically involve reducing particle size and increasing specific surface area and surface functional groups.39 Particle size reduction can be achieved through physical grinding or ball-milling processes. Ball milling disrupts the chemical bonds of the involved molecules, increasing the surface area-to-volume ratio, surface reactivity, and adsorption capacity, particularly for nanometer-scale contaminants. Nanometer-scale particles offer enhanced availability for interactions, resulting in improved adsorption performance compared to larger particles. Additionally, mechanical action during these processes can shape biochar into cylindrical pellets or briquettes, creating a less bulky and more resilient material with favorable energy properties, thereby enhancing its suitability for the biomass supply chain.51 Physical activation methods, utilizing carrier gases such as steam, O2, and CO2, are employed to introduce oxygen-containing functional groups and enhance the specific surface area of biochar. However, this process may reduce the polarity and aromatization of the biochar. For instance, pyrolysis of biomass followed by CO2 activation primarily generates micropores. The selection of the activation atmosphere allows for the development of a pore structure tailored to accommodate the size of the adsorbed molecules on the biochar surface.52 Chemical modification involves treating biochar with acid, alkali, or metallic impregnation, which alters its surface chemistry, rendering it more reactive and enhancing pore structure and specific surface area. Acid or base activation involves the penetration of reagents into the biochar structure, leading to the formation of oxygenated functional groups. Acid treatment typically results in functional groups such as C–O–C and C=O, while base treatment yields –OH, C–O, and –COOH groups. Metallic impregnation occurs on the surface or within the pores of biochar, effectively increasing the number of reactive sites in the structure.50 Figure 3 provides an overview of the primary characteristics of lignocellulosic biomass that directly impact the thermochemical processes involved in biochar preparation. The selection of the synthesis method plays a crucial role in inducing structural changes, particularly on the surface of the biochar.
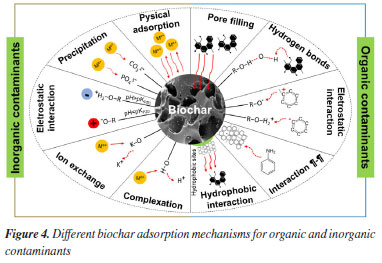
Adsorption mechanism As previously mentioned, the adsorption properties of biochar are influenced by various factors, including the type of raw material used, the conditions of thermochemical processes during its production, and modifications to its structure. Additionally, characteristics of the reaction medium, such as pH, biochar dosage, and temperature, play significant roles in determining its adsorption capacity. The pH of the solution is a critical parameter in optimizing the adsorption process. Its impact is closely linked to the presence of oxygen-containing functional groups on the surface of the adsorbent, which in turn affects the surface charge and degree of ionization. At low pH values, below the point of zero charge (pH < pHPZC), most of the functional groups on the biochar surface are protonated, which facilitates the adsorption of anionic contaminants. However, excess H+ and H3O+ ions can lead to increased competition with cationic contaminants for available adsorption sites. This competition can result in electrostatic repulsion between the ions, leading to reduced adsorption of metal ions at low pH levels.4 Increasing the pH (pH > pHPZC) promotes the deprotonation of functional groups on the biochar surface. This enhances the adsorption capacity of biochar for metals because there is a reduction in competition for active sites between metal ions and protons in the solution, resulting in more active sites available for metal-surface interaction. This principle also applies to the adsorption of ionic organic contaminants in wastewater. At alkaline pH levels, there is a stronger electrostatic interaction between the cationic dye molecules and the deprotonated surface of the biochar, facilitating the adsorption process. Conversely, in an acidic medium, the efficiency of biochar to adsorb organic dyes decreases due to the excess of H+ ions competing with the cationic dye molecules for available adsorption sites on the biochar.53 The adsorption capacity is also influenced by the amount of biochar used. Typically, increasing the dosage of biochar enhances the removal efficiency of both organic and inorganic contaminants. This is attributed to the greater availability of active sites in the reaction medium. However, once the saturation limit of active centers is reached, the efficiency levels off, even with higher dosages. Therefore, conducting a preliminary study to determine the optimal amount of adsorbent is crucial for cost reduction, particularly for large-scale applications of biochar.54 Regarding the temperature of the solution, the literature suggests that the adsorption process is endothermic, leading to an increase in adsorption capacity with rising temperature. As temperature increases, the system’s kinetic energy also rises, resulting in decreased viscosity, which facilitates the mobility of contaminant molecules and enhances diffusion rates within the pores on the biochar surface. Studies by Lin et al.55 and Azzam et al.56 have demonstrated the effectiveness of increasing system temperature for improved removal of dyes and antibiotics from effluents. So far, we have discussed the primary factors influencing the adsorption process. These factors include the choice of biochar precursor, the preparation method, and adjustments made to the reaction medium to optimize the interaction between biochar and contaminants. Now, let’s delve into understanding the adsorption mechanism. The principle of adsorption involves the interaction between the surface of the adsorbent and the adsorbate until equilibrium is achieved. This process typically progresses through several steps, including physical adsorption, precipitation and complexation, and filling of the pores. These steps occur within three distinct zones: the clean zone, where only physical interaction occurs without adsorption; the mass transfer zone, where adsorption is actively taking place; and the saturated zone, where equilibrium is reached, and the surface of the biochar becomes saturated with contaminating molecules or ions.46 However, it is important to note that the adsorption behavior of biochar may vary for different contaminants. The adsorption activity of biochar towards inorganic contaminants, such as metals, primarily relies on factors such as its specific surface area, porous structure, surface functional groups, and cation exchange capacity. The mechanism of action is complex and can vary, even for a specific metal. However, typically, the primary mechanism involves a combination of various interactions, including physical adsorption, electrostatic interaction, ion exchange, precipitation, and surface complexation.46 Physical adsorption occurs through the van der Waals force interaction between the biochar surface and the metal ion. This interaction is weak, and therefore, the process is considered reversible. When interacting with the surface, the metal ion becomes physically adsorbed or diffuses into the pores of the adsorbent. Thus, the biochar’s pore volume and surface area are relevant factors in studying the adsorption capacity of these contaminants. A greater specific surface area and pore volume allow for a larger contact area between the biochar and metal ions, enhancing the physical adsorption capacity, fixation, and passivation of the metal ion.57 Electrostatic interactions between the surface charges of biochar functional groups and those of metals are another mechanism involved in the remediation of metallic contaminants. The intensity of this interaction is related to several factors, including the pH of the solution, the valence state of the metal ions, the ionic radius, and the zero potential of the biochar. As previously stated, at pH > pHPZC, the biochar surface is deprotonated and negatively charged, facilitating electrostatic attraction with the metal cation. Greater electrostatic attraction is also observed with a higher valence state of the metal cation. Additionally, if two metals have the same charge, the one with the smaller radius interacts more strongly electrostatically with the biochar surface.58 The third proposed mechanism for heavy metal adsorption is ion exchange. This process involves reversible ion exchange reactions between the adsorbent and the adsorbate. Several adsorbents, including biochar, can be loaded with exchangeable ions of alkaline and alkaline earth metal salts (Na+, K+, Ca2+, and Mg2+). These ions are exchanged for metal cations in solution. However, this exchange will only be possible if the exchangeable ions of the biochar are insoluble in the medium in which the exchange is carried out. Furthermore, the adsorption capacity depends on the compatibility in the size of the ions that will be exchanged and the availability of the biochar cation linked to the surface functional groups.59,60 Surface precipitation is one of the main mechanisms used to remove inorganic contaminants. The process involves the formation of insoluble mineral precipitates in the solution or on the surface of the adsorbent. Biochar contains soluble phosphate and carbonate, which originate from the raw material used. For example, biochar of animal origin has a higher content of PO43– and CO32– than vegetable biochar. These anions precipitate with the metals in aqueous solution to form relatively stable minerals. For instance, Han et al.61 removed the Pb2+ metal ion in the form of the PbCO3 precipitate with biochar derived from cattle manures and rice husks. The largest amount of phosphorus adsorbed was in biochar of animal origin. The formation of the precipitate depends on the pyrolysis temperature and the pH of the solution. There is no ideal pyrolysis temperature; optimizing the heat treatment method that maintains the biochar with alkaline properties is necessary. In relation to the pH of the medium, it must be basic to favor the “lime effect”, in which metal removal occurs through the precipitation of metallic hydroxides, carbonates, and phosphates. This effect plays a role in the passivation of metal ions.62 The last proposed mechanism for the adsorption of metal ions is surface complexation. Similar to the precipitation mechanism, the removal of the metal cation in solution now occurs through the formation of a complex on the biochar surface instead of a precipitate. The covalent bond between surface oxygenated functional groups (hydroxyl, carbonyl, and carboxyl) and metal cations forms the complex. The reaction involving the formation of the complex is of the Lewis acid-base type, where the metal ion acts as an acid, accepting pairs of electrons donated by the oxygen of the functional groups, which serves as the Lewis base. The base will donate electrons due to the loss of hydrogen or other ions; therefore, complexation is affected by the pH of the solution because it involves the capture/release of the hydrogen ion.60 The adsorption mechanisms by which organic contaminants bind to biochar involve different interactions. In general, electrostatic interactions, hydrophobic interactions, hydrogen bonds, pore filling, and electron acceptor-donor interactions are the main mechanisms applied in the adsorption of these contaminants. As with heavy metal removal, the proposed mechanisms for organic contaminants are correlated with biochar properties, such as specific surface area, pore volume, functional groups, electronic interactions and attractions, and solution pH. Electrostatic interaction is the primary mechanism of adsorption for ionic organic compounds and ionizable organic compounds on the surface of biochar. This interaction occurs through the attraction between the opposite charges of the organic compound and the surface of the adsorbent. Similar to the adsorption of metals via electrostatic interaction, the magnitude of attraction also depends on the pH of the solution. At a pH lower than pHPZC, the surface of the adsorbent is protonated and positively charged in solution, leading to strong adsorption of anionic organic contaminants and electrostatic repulsion of cationic contaminants. Conversely, in solutions with a pH higher than the pHPZC, the surface of the adsorbent is deprotonated and negatively charged..60 Tang et al.63 investigated the adsorption of antibiotics on wheat straw biochar. They found that increasing the pH of the solution to 8 gradually enhanced the efficiency of tetracycline antibiotic removal. This enhancement is attributed to the negative surface of the biochar, which interacts electrostatically with the antibiotic in its cationic form. However, for pH values above 8, the removal efficiency gradually decreased due to the predominance of electrostatic repulsion between the biochar surface and the antibiotic. This is because tetracycline becomes anionic at pH > 7.8 while the biochar surface remains negative, as confirmed by zeta potential analysis. In hydrophobic or neutral organic compounds, adsorption occurs through hydrophobic interaction with the hydrophobic surface of biochar. The pyrolysis temperature and the solubility of the contaminants directly influence the strength of this interaction. Higher heat treatment temperatures enhance the hydrophobicity of biochar by decomposing surface polar groups. The solubility of organic compounds also plays a crucial role, as it depends on the presence of hydrophobic functional groups in their structures, such as methyl groups. These groups adhere to the biochar surface, facilitating the removal of contaminants.53 Hydrophobic interaction is the primary mechanism involved in the adsorption of organic contaminants such as benzoic acid, o-chlorobenzene acid, and p-chlorobenzene acid,46 as well as certain antibiotics like sulfamethoxazole, anisole, carbamazepine, ciprofloxacin, and sparfloxacin. The preference for this mechanism is attributed to the aromatic nature of the contaminants.64 Polar organic contaminants adhere to the surface of biochar through the formation of hydrogen bonds. This interaction occurs between a hydrogen atom, associated with the oxygenated functional groups on the biochar surface, and electronegative elements like oxygen, fluorine, nitrogen, and others present in the organic contaminants. Consequently, the strength of the π-H bond varies depending on the electronegativity of the atoms and steric hindrance of the organic molecule. The hydrogen bonds formed with antibiotics, dyes, organic sorbic acid, and 4-nitroaniline can take various forms, including C=O∙∙∙H–O, C–H∙∙∙O, N–H∙∙∙O=C.60 So far, mechanisms for removing organic contaminants have been presented through interactions between the biochar surface and organic groups available in the structure of the contaminants. However, the primary mechanism of interaction in the adsorption process is through the pore-filling effect. The effectiveness of this process hinges on the size of the adsorbed molecules relative to the diameter and volume of biochar pores. Therefore, a structure with a high surface area, well-defined porosity, and a significant volume of pores, particularly in the micro (< 2 nm) and mesopore (2‑50 nm) range, facilitates mass transport and the adsorption of specific contaminants.53 The development of surface area and pore volume increases with the pyrolysis temperature, resulting in reduced volatile material content in the biochar. This is a favorable parameter for the adsorption of organic compounds. The filling mechanism is rapid, leading to a higher initial removal rate. However, depending on the size and hydrophobicity of the organic contaminant molecules, pores may become blocked, deactivating the active sites present on the biochar surface. When this occurs, the biochar becomes saturated and requires regeneration for subsequent applications.65 The final mechanism of adsorption of organic compounds involves π-π interactions. This weak intermolecular interaction typically occurs between two aromatic rings, an electron-rich ring, and an electron-deficient ring, which is derived from the aromatic structures of the biochar and the organic contaminant. The electron density of the biochar depends on the pyrolysis temperature. If it is pyrolyzed below 500 °C, its aromatic π structure acts as an electron acceptor. Conversely, if the pyrolysis temperature exceeds 500 °C, the biochar acts as an electron donor.46 Some oxygen-containing functional groups, such as carboxylic acids, nitro groups, and ketones, when attached to the aromatic structure of biochar, cause it to act as an electron acceptor through π-π electron donor-acceptor interaction with the organic contaminant. Conversely, hydroxyl and amine groups render biochar as a π electron donor in this same type of adsorption mechanism.50 The organic and inorganic contaminant removal mechanisms described earlier have been schematized in Figure 4. In most cases, adsorption cannot be explained by just one mechanism but by the coexistence of two or more simultaneous adsorption mechanisms.
APPLICATIONS OF BIOCHAR FOR REMOVING ORGANIC AND INORGANIC CONTAMINANTS Both organic and inorganic contaminants in freshwater have surged due to heightened industrial activities, human consumption, and agricultural runoff. To address this issue, researchers globally have increasingly turned to eco-friendly methods, among which biochar adsorption stands out. Remarkably, biochar as an adsorbent demonstrates significant efficacy, capable of removing up to 40% of organic contaminants, 45% of metals, and 10% of nutrients such as phosphorus and nitrogen from aqueous environments. In comparison, other adsorbents typically achieve a maximum removal rate of only 5%.66 Organic contaminants constitute the predominant contaminants in some industrial effluents and wastewater, necessitating a more extensive removal process compared to metals and other inorganic contaminants. Their prevalence in freshwater adversely impacts dissolved oxygen levels and disrupts aquatic ecosystems. Among the organic contaminants are agrochemicals, dyes, pharmaceuticals, and industrial chemicals such as PAHs. Some of these compounds are classified as priority organic pollutants (POPs), while others are identified as contaminants of emerging concern (CECs).67 The proliferation of infectious diseases in recent times, coupled with advancements in medicine and the pharmaceutical industry, has led to heightened consumption of drugs like antibiotics, hormones, analgesics, and antidepressants. Concurrently, there has been a surge in the usage of personal care products such as fragrances, cosmetics, shampoos, and laundry items. These substances, categorized as CECs, may be excreted partially unchanged or as metabolites, some of which retain their bioactivity. Following excretion, they can enter sewage treatment facilities or be discharged directly into aquatic environments, posing potential risks.68 CECs are not currently included in routine monitoring programs. However, owing to their potential ecotoxicological or toxicological impacts, they may necessitate regulation in the future. While most CECs are fat-soluble, some are water-soluble, indicating that they can exert severe effects even at low concentrations. Consequently, remediating these contaminants poses a considerable challenge, compounded by the diverse chemical structures within their composition.69 Most CECs are polar and ionizable, containing functional groups such as hydroxyl, carboxyl, amine, or ketone in their structure. Electrostatic interactions, hydrogen bonds, pore filling, and electron donor-acceptor interactions are the main mechanisms involved in the adsorption of these contaminants. It is important to note that biochar has both hydrophobic and hydrophilic domains. Hydrophilic regions facilitate the interaction of antibiotics through the mechanisms mentioned above. Conversely, in more hydrophobic biochar, the adsorption process is primarily limited to the hydrophobic mechanism.70
PERSPECTIVE, CONSIDERATIONS AND CHALLENGES Modern life habits have significantly accelerated the production of both organic and inorganic waste, attributable to the expansion of industrial and technological sectors, alongside the fulfillment of fundamental human needs. However, this surge in waste generation precipitates numerous environmental repercussions, notably the escalation in contaminant levels in freshwater bodies. Consequently, a pressing necessity arises to remediate contaminated water through environmentally benign techniques. The proposition to mitigate such contaminants from water by employing biochar holds profound implications for the adsorption process, water quality enhancement, and the sustainable disposal of biomass waste. Indeed, the allure of biochar in contaminant adsorption predominantly stems from its cost-effectiveness. While the feedstock for biochar production varies widely, a substantial portion comprises lignocellulosic waste. Diverse physical and chemical methods are employed to tailor the structure of biochar to augment the adsorption efficacy of biochar, resulting in significant cost discrepancies among different modification processes. In certain instances, costly modifications may yield deleterious effects on contaminant removal, such as the generation of hazardous byproducts or secondary contamination, alongside the associated costs of managing these contaminants. Moreover, as biochar undergoes successive adsorption cycles, its absorptive capacity diminishes, posing challenges in the disposal of residual biochar waste. Consequently, no universal protocol exists for the production, characterization, or application of biochar. Each thermochemical synthesis method directly influences physicochemical properties and efficacy variations, consequently leading to performance disparities across diverse environmental applications. Additionally, comprehending the chemical structure of target contaminants is imperative to ascertain the optimal adsorption mechanism between the biochar surface and the contaminant. Finally, addressing the need for studies conducted on authentic samples is crucial. The vast majority of cited studies herein primarily conducted adsorption experiments on laboratory-prepared solutions. While acceptable for preliminary assessments of material adsorptive capacities vis-à-vis target contaminants, this approach inherently fails to capture the complexities of real-world samples, which comprise a myriad of additional substances alongside the target contaminant. Industrial and domestic effluents exhibit a plethora of potential interferents that necessitate consideration in advanced adsorption studies. In addition to prioritizing research on authentic samples, conducting studies at concentrations reflective of real-world environmental conditions is equally imperative.
CONCLUSIONS A total of 50 studies were found using lignocellulosic biomass from Brazil, including sugarcane (n = 5), soybean (n = 3), corn (n = 4), rice (n = 4), cotton (n = 3), coffee (n = 3), açaí (n = 10), tucumã (n = 2), coconut (n = 2), Brazil nut (n = 2), cupuaçu (n = 3), nuts (n = 1), murumuru (n = 1), orange (n = 2), cassava (n = 1), banana (n = 1), malt (n = 1), macaúba (n = 1), and guava (n = 1). Açaí seeds were the most studied lignocellulosic biomass for generating biochar to remove contaminants from water, having been tested for herbicides, caffeine, catechol, phenol, dyes, and metals. Other lignocellulosic biomasses from Brazil have also been predominantly applied to remove these contaminants, with particular emphasis on metals and dyes. Physical activation has been reported, but much less frequently than chemical activation. In this type of activation, bases, acids, and primarily ZnCl2 have been used. The adsorption capacity varies widely but is always within the mg g–1 range. The effectiveness of contaminant remediation by biochar is heavily influenced by its physical and chemical characteristics, which are contingent upon the conditions of the thermochemical process employed. Adsorption tests are predominantly conducted in synthetic solutions that do not reflect real environmental matrices and effluents, as well as at concentrations that are not consistent with environmental reality.
REFERENCES 1. Kumar, K.; Kumar, R.; Kaushal, S.; Thakur, N.; Umar, A.; Akbar, S.; Ibrahim, A. A.; Baskoutas, S.; Chemosphere 2023, 345, 140419. [Crossref] 2. Rangappa, H. S.; Herath, I.; Lin, C.; Ch, S.; Environ. Pollut. 2024, 343, 123140. [Crossref] 3. Osman, A. I.; El-Monaem, E. M. A.; Elgarahy, A. M.; Aniagor, C. O.; Hosny, M.; Farghali, M.; Rashad, E.; Ejimofor, M. I.; López-Maldonado, E. A.; Ihara, I.; Yap, P.-S.; Rooney, D. W.; Eltaweil, A. S.; Environ. Chem. Lett. 2023, 21, 2337. [Crossref] 4. Bueno, C. C.; Maia, A. A. D.; de Morais, L. C.; Rosa, A. H.; J. Braz. Chem. Soc. 2017, 28, 2202. [Crossref] 5. Anjum, A.; Mazari, S. A.; Hashmi, Z.; Jatoi, A. S.; Abro, R.; Bhutto, A. W.; Mubarak, N. M.; Dehghani, M. H.; Karri, R. R.; Mahvi, A. H.; Nasseri, S.; Heliyon 2023, 9, e15575. [Crossref] 6. Araujo, R. O.; Santos, V. O.; Ribeiro, F. C. P.; Chaar, J. S.; Falcão, N. P. S.; de Souza, L. K. C.; React. Kinet., Mech. Catal. 2021, 134, 199. [Crossref] 7. da Costa, G. G.; dos Santos, I. F. S.; Barros, R. M.; Tiago Filho, G. L.; Machado, G. O.; Barbedo, M. D. G.; J. Cleaner Prod. 2022, 349, 131466. [Crossref] 8. Bitencourt, G. F.; Machado, L. F. L.; Peixoto, B. S.; de Castro, L. F.; Veloso, M. C. C.; Romeiro, G. A.; Lima, T. M.; J. Braz. Chem. Soc. 2024, 35, e-20230182. [Crossref] 9. Mendoza Martinez, C. L.; Sermyagina, E.; Saari, J.; de Jesus, M. S.; Cardoso, M.; de Almeida, G. M.; Vakkilainen, E.; Biomass Bioenergy 2021, 147, 106004. [Crossref] 10. Forster-Carneiro, T.; Berni, M. D.; Dorileo, I. L.; Rostagno, M. A.; Resour., Conserv. Recycl. 2013, 77, 78. [Crossref] 11. da Silva, S. B.; Arantes, M. D. C.; de Andrade, J. K. B.; Andrade, C. R.; Carneiro, A. C. O.; Protásio, T. P.; Renewable Energy 2020, 147, 1870. [Crossref] 12. [s.d.], Instituto Brasileiro de Geografia e Estatística (IBGE), Produção de Cana-de-Açúcar, https://www.ibge.gov.br/explica/producao-agropecuaria/cana-de-acucar/br, accessed in October 2024. 13. da Cruz, M. A.; Casanova, R. F.; Boscardin, D.; Zanchet, A.; Rev. Mater. 2021, 26. [Crossref] 14. Naga, A. O. A.; El Saied, M.; Shaban, S. A.; El Kady, F. Y.; J. Mol. Liq. 2019, 285, 9. [Crossref] 15. Jiang, W.; Xing, Y.; Zhang, L.; Guo, X.; Lu, Y.; Yang, M.; Wang, J.; Wei, G.; J. Appl. Polym. Sci. 2020, 138, 49830. [Crossref] 16. Tran, T. H.; Le, A. H.; Pham, T. H.; Nguyen, D. T.; Chang, S. W.; Chung, W. J.; Nguyen, D. D.; Sci. Total Environ. 2020, 725, 138325. [Crossref] 17. Chaijak, P.; Michu, P.; Thipraksa, J.; Kongthong, A.; Pollution 2023, 9, 1666. [Crossref] 18. Saad, M. J.; Sajab, M. S.; Busu, W. N. W.; Misran, S.; Zakaria, S.; Chin, S. X.; Chia, C. H.; Sains Malaysiana 2020, 49, 2721. [Crossref] 19. Didonet, A. A.; Antoniassi, R.; Back, G. R.; de Faria-Machado, A. F.; Wilhelm, A. E.; Ferraz, I. D. K.; J. Am. Oil Chem. Soc. 2020, 97, 955. [Crossref] 20. Hadiya, V.; Popat, K.; Vyas, S.; Varjani, S.; Vithanage, M.; Gupta, V. K.; Núñez Delgado, A.; Zhou, Y.; Show, P. L.; Bilal, M.; Zhang, Z.; Sillanpää, M.; Mohanty, S. S.; Patel, Z.; Bioresour. Technol. 2022, 355, 127303. [Crossref] 21. Song, M.; Jin, B.; Xiao, R.; Yang, L.; Wu, Y.; Zhong, Z.; Huang, Y.; Biomass Bioenergy 2013, 48, 250. [Crossref] 22. Baskaran, P.; Abraham, M.; Sustainable Energy Technologies and Assessments 2022, 53, 102709. [Crossref] 23. Araujo, R. O.; Ribeiro, F. C. P.; Santos, V. O.; Lima, V. M. R.; Santos, J. L.; Vilaça, J. E. S.; Chaar, J. S.; Falcão, N. P. S.; Pohlit, A. M.; de Souza, L. K. C.; BioEnergy Res. 2022, 15, 834. [Crossref] 24. Ma, Z.; Zhang, Y.; Huang, Y.; Wang, R.; Wang, Z.; He, Y.; ChemistrySelect 2023, 8, e202301886. [Crossref] 25. Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E.; J. Anal. Appl. Pyrolysis 2015, 113, 551. [Crossref] 26. Sajednia, G.; Rahimi, E.; Alvand, N.; Karbassi, A.; Baghdadi, M.; SN Appl. Sci. 2019, 1, 1525. [Crossref] 27. Santos, V. O.; Queiroz, L. S.; Araujo, R. O.; Ribeiro, F. C. P.; Guimarães, M. N.; da Costa, C. E. F.; Chaar, J. S.; de Souza, L. K. C.; Bioresour. Technol. Rep. 2020, 12, 100553. [Crossref] 28. Thue, P. S.; Umpierres, C. S.; Lima, E. C.; Lima, D. R.; Machado, F. M.; dos Reis, G. S.; da Silva, R. S.; Pavan, F. A.; Tran, H. N.; J. Hazard. Mater. 2020, 398, 122903. [Crossref] 29. Umpierres, C. S.; Thue, P. S.; Lima, E. C.; dos Reis, G. S.; de Brum, I. A. S.; de Alencar, W. S.; Dias, S. L. P.; Dotto, G. L.; Environ. Technol. 2018, 39, 1173. [Crossref] 30. de Souza, T. N. V.; Vieira, M. G. A.; da Silva, M. G. C.; Brasil, D. S. B.; de Carvalho, S. M. L.; Environ. Sci. Pollut. Res. 2019, 26, 28533. [Crossref] 31. Ramirez, R.; Pinto, D.; Georgin, J.; de Oliveira, A. H. P.; Franco, D. S. P.; Wolff, D.; Carissimi, E.; Naushad, M.; Siva, L. F. O.; Lima, É. C.; Dotto, G. L.; J. Environ. Chem. Eng. 2023, 11, 109966. [Crossref] 32. Zhang, Y.; Cui, Y.; Liu, S.; Fan, L.; Zhou, N.; Peng, P.; Wang, Y.; Guo, F.; Min, M.; Cheng, Y.; Liu, Y.; Lei, H.; Chen, P.; Li, B.; Ruan, R.; Bioresour. Technol. 2020, 297, 122480. [Crossref] 33. Araujo, R. O.; Santos, J. L.; Colpani, D.; Pereira, B. R. S.; Falcão, N. P. S.; de Souza, L. K. C.; Encyclopedia of Renewable Energy, Sustainability and the Environment, vol. 1; Rahimpour, M. R., ed.; Elsevier, 2024, p. 509. [Crossref] 34. Zhang, Z.; Lei, Y.; Li, D.; Zhao, J.; Wang, Y.; Zhou, G.; Yan, C.; He, Q.; Renewable Energy 2020, 153, 1091. [Crossref] 35. Kim, W.-K.; Shim, T.; Kim, Y.-S.; Hyun, S.; Ryu, C.; Park, Y.-K.; Jung, J.; Bioresour. Technol. 2013, 138, 266. [Crossref] 36. Brito, M. J. P.; Borges, J. F.; de Oliveira, T. P.; Santos, M. P. F.; de Souza Júnior, E. C.; Santos, L. S.; Bonomo, R. C. F.; Veloso, C. M.; Desalin. Water Treat. 2020, 197, 424. [Crossref] 37. Xie, T.; Reddy, K. R.; Wang, C.; Yargicoglu, E.; Spokas, K.; Crit. Rev. Environ. Sci. Technol. 2015, 45, 939. [Crossref] 38. Du, L.; Ahmad, S.; Liu, L.; Wang, L.; Tang, J.; Sci. Total Environ. 2023, 858, 159815. [Crossref] 39. Liu, Y.; Weng, Z.; Han, B.; Guo, Z.; Tian, H.; Tang, Y.; Cai, Y.; Yang, Z.; J. Cleaner Prod. 2023, 421, 138495. [Crossref] 40. Zhao, Z.; Wang, B.; Theng, B. K. G.; Lee, X.; Zhang, X.; Chen, M.; Xu, P.; Biochar 2022, 4, 30. [Crossref] 41. Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H.; Sep. Purif. Technol. 2022, 300, 121925. [Crossref] 42. Jellali, S.; Khiari, B.; Usman, M.; Hamdi, H.; Charabi, Y.; Jeguirim, M.; Renewable Sustainable Energy Rev. 2021, 144, 111068. [Crossref] 43. Monga, D.; Shetti, N. P.; Basu, S.; Reddy, K. R.; Badawi, M.; Bonilla-Petriciolet, A.; Aminabhavi, T. M.; Fuel 2022, 311, 122510. [Crossref] 44. Marson, E. O.; Paniagua, C. E. S.; Gomes Júnior, O.; Gonçalves, B. R.; Silva, V. M.; Ricardo, I. A.; Starling, M. C. V. M.; Amorim, C. C.; Trovó, A. G.; Sci. Total Environ. 2022, 836, 155605. [Crossref] 45. Barquilha, C. E. R.; Braga, M. C. B.; Bioresour. Technol. Rep. 2021, 15, 100728. [Crossref] 46. Mukherjee, A.; Zimmerman, A. R.; Harris, W.; Geoderma 2011, 163, 247. [Crossref] 47. Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X.; J. Mol. Liq. 2016, 220, 432. [Crossref] 48. Barquilha, C. E. R.; Cossich, E. S.; Tavares, C. R. G.; Silva, E. A.; J. Cleaner Prod. 2017, 150, 58. [Crossref] 49. Machado, L. M. M.; Lütke, S. F.; Perondi, D.; Godinho, M.; Oliveira, M. L. S.; Collazzo, G. C.; Dotto, G. L.; J. Environ. Chem. Eng. 2020, 8, 104473. [Crossref] 50. Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H.; J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Crossref] 51. Moura, F. C. C.; Rios, R. D. F.; Galvão, B. R. L.; Environ. Sci. Pollut. Res. 2018, 25, 26482. [Crossref] 52. Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y.; Chemosphere 2022, 287, 132113. [Crossref] 53. Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S. J.; Du, J.; Qi, F.; Willett, I. R.; Sci. Total Environ. 2020, 744, 140714. [Crossref] 54. Jagadeesh, N.; Sundaram, B.; J. Hazard. Mater. Adv. 2023, 9, 100226. [Crossref] 55. Lin, R.; Liang, Z.; Yang, C.; Zhao, Z.; Cui, F.; J. Colloid Interface Sci. 2020, 573, 21. [Crossref] 56. Azzam, A. B.; Tokhy, Y. A.; El Dars, F. M.; Younes, A. A.; SSRN 2022. [Crossref] 57. Starling, M. C. V. M.; Amorim, C. C.; Leão, M. M. D.; J. Hazard. Mater. 2019, 372, 17. [Crossref] 58. Zhang, J.; Chen, Z.; Liu, Y.; Wei, W.; Ni, B.-J.; Chem. Eng. J. 2024, 479, 147615. [Crossref] 59. Brito, G. M.; Roldi, L. L.; Schetino Jr., M. Â.; Freitas, J. C. C.; Coelho, E. R. C.; J. Environ. Sci. Health, Part B 2020, 55, 767. [Crossref] 60. Ferreira, P. P. L.; Braga, R. M.; Teodoro, N. M. A.; Melo, V. R. M.; Melo, D. M. A.; Melo, M. A. F.; Ceramica 2015, 61, 435. [Crossref] 61. Han, L.; Qian, L.; Liu, R.; Chen, M.; Yan, J.; Hu, Q.; Sci. Rep. 2017, 7, 2264. [Crossref] 62. Krishnan, K. A.; Sreejalekshmi, K. G.; Baiju, R. S.; Bioresour. Technol. 2011, 102, 10239. [Crossref] 63. Tang, Y.; Li, Y.; Zhan, L.; Wu, D.; Zhang, S.; Pang, R.; Xie, B.; Sci. Total Environ. 2022, 805, 150158. [Crossref] 64. Guo, X.; Hu, W.; Gu, Z.; Li, J.; Xie, Z.; Fang, C.; Tao, H.; Water, Air, Soil Pollut. 2021, 232, 484. [Crossref] 65. Doczekalska, B.; Bartkowiak, M.; Łopatka, H.; Zborowska, M.; BioResources 2022, 17, 1794. [Crossref] 66. Ismail, M. S.; Yahya, M. D.; Auta, M.; Obayomi, K. S.; Heliyon 2022, 8, e09516. [Crossref] 67. Mbarki, F.; Selmi, T.; Kesraoui, A.; Seffen, M.; Ind. Crops Prod. 2022, 178, 114546. [Crossref] 68. Elewa, A. M.; Amer, A. A.; Attallah, M. F.; Gad, H. A.; Al-Ahmed, Z. A. M.; Ahmed, I. A.; Materials 2023, 16, 1251. [Crossref] 69. Taha, M. F.; Ibrahim, M. H. C.; Shaharun, M. S.; Chong, F. K.; AIP Conf. Proc. 2012, 1482, 252. [Crossref] 70. Sangon, S.; Hunt, A. J.; Attard, T. M.; Mengchang, P.; Ngernyen, Y.; Supanchaiyamat, N.; J. Cleaner Prod. 2018, 172, 1128. [Crossref] 71. Shirvanimoghaddam, K.; Czech, B.; Wójcik, G.; Naebe, M.; J. Colloid Interface Sci. 2019, 539, 425. [Crossref] 72. Tian, D.; Xu, Z.; Zhang, D.; Chen, W.; Cai, J.; Deng, H.; Sun, Z.; Zhou, Y.; J. Solid State Chem. 2019, 269, 580. [Crossref] 73. Reffas, A.; Bernardet, V.; David, B.; Reinert, L.; Lehocine, M. B.; Dubois, M.; Batisse, N.; Duclaux, L.; J. Hazard. Mater. 2010, 175, 779. [Crossref] 74. Deivasigamani, P.; Kumar, P. S.; Sundaraman, S.; Soosai, M. R.; Renita, A. A.; M, K.; Bektenov, N.; Baigenzhenov, O.; D, V.; J, A. K.; Environ. Res. 2023, 236, 116735. [Crossref] 75. de Almeida, A. S. V.; Vieira, W. T.; Bispo, M. D.; de Melo, S. F.; da Silva, T. L.; Balliano, T. L.; Vieira, M. G. A.; Soletti, J. I.; J. Environ. Chem. Eng. 2021, 9, 104891. [Crossref] 76. Feitoza, U. S.; Thue, P. S.; Lima, E. C.; dos Reis, G. S.; Rabiee, N.; de Alencar, W. S.; Mello, B. L.; Dehmani, Y.; Rinklebe, J.; Dias, S. L. P.; Molecules 2022, 27, 7570. [Crossref] 77. de Souza, T. N. V.; de Carvalho, S. M. L.; Vieira, M. G. A.; da Silva, M. G. C.; Brasil, D. S. B.; Appl. Surf. Sci. 2018, 448, 662. [Crossref] 78. Ribeiro, L. A. S.; Thim, G. P.; Alvarez-Mendez, M. O.; Coutinho, A. R.; de Moraes, N. P.; Rodrigues, L. A.; Int. J. Environ. Res. 2018, 12, 755. [Crossref] 79. Nobre, J. R. C.; Queiroz, L. S.; Castro, J. P.; Pego, M. F. F.; Hugen, L. N.; da Costa, C. E. F.; Pardauil, J. J. R.; do Nascimento, L. A. S.; da Rocha Filho, G. N.; Zamian, J. R.; de Souza, E. C.; Bianchi, M. L.; Heliyon 2023, 9, e17189. [Crossref] 80. Queiroz, L. S.; de Souza, L. K. C.; Thomaz, K. T. C.; Lima, E. T. L.; da Rocha Filho, G. N.; do Nascimento, L. A. S.; Pires, L. H. O.; Faial, K. C. F.; da Costa, C. E. F.; J. Environ. Manage. 2020, 270, 110868. [Crossref] 81. Ramirez, R.; Schnorr, C. E.; Georgin, J.; Netto, M. S.; Franco, D. S. P.; Carissimi, E.; Wolff, D.; Silva, L. F. O.; Dotto, G. L.; Molecules 2022, 27, 7781. [Crossref] 82. da Silva, M. C. F.; Schnorr, C.; Lütke, S. F.; Knani, S.; Nascimento, V. X.; Lima, É. C.; Thue, P. S.; Vieillard, J.; Silva, L. F. O.; Dotto, G. L.; Chem. Eng. Res. Des. 2022, 187, 387. [Crossref] 83. Cruz Jr., O. F.; Campello-Gómez, I.; Casco, M. E.; Serafin, J.; Silvestre-Albero, J.; Martínez-Escandell, M.; Hotza, D.; Rambo, C. R.; Carbon Lett. 2023, 33, 727. [Crossref] 84. Belaissa, Y.; Saib, F.; Trari, M.; React. Kinet., Mech. Catal. 2022, 135, 1011. [Crossref] 85. Costa, R. L. T.; do Nascimento, R. A.; de Araújo, R. C. S.; Vieira, M. G. A.; da Silva, M. G. C.; de Carvalho, S. M. L.; de Faria, L. J. G.; J. Mol. Liq. 2021, 343, 116980. [Crossref] 86. El Saied, M.; Shaban, S. A.; Mostafa, M. S.; El Naga, A. O. A.; Biomass Convers. Biorefin. 2024, 14, 2155. [Crossref] 87. Wei, Q.; Chen, Z.; Cheng, Y.; Wang, X.; Yang, X.; Wang, Z.; Colloids Surf., A 2019, 574, 221. [Crossref] 88. Nadew, T. T.; Keana, M.; Sisay, T.; Getye, B.; Habtu, N. G.; Water Practice and Technology 2023, 18, 947. [Crossref] 89. Georgin, J.; Pinto, D.; Franco, D. S. P.; Schadeck Netto, M.; Lazarotto, J. S.; Allasia, D. G.; Tassi, R.; Silva, L. F. O.; Dotto, G. L.; Molecules 2022, 27, 7574. [Crossref] 90. Pezoti, O.; Cazetta, A. L.; Bedin, K. C.; Souza, L. S.; Martins, A. C.; Silva, T. L.; Santos Júnior, O. O.; Visentainer, J. V.; Almeida, V. C.; Chem. Eng. J. 2016, 288, 778. [Crossref] 91. Yu, Y.; Qiao, N.; Wang, D.; Zhu, Q.; Fu, F.; Cao, R.; Wang, R.; Liu, W.; Xu, B.; Bioresour. Technol. 2019, 285, 121340. [Crossref]
Associate Editor handled this article: Eduardo H. S. Sousa |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access