Artigo
| Phytochemical profile, antioxidant and cytotoxic activities of leaf extract of Dipteryx lacunifera Ducke |
|
Krislany A. L. Guedes AraujoI; Herbert G. SousaI; João P. da Silva GomesI I. Departamento de Química, Universidade Federal do Piauí, 64049-550 Teresina - PI, Brasil Received: 05/29/2024 *e-mail: mariana@ufpi.edu.br; magela@ufpi.edu.br The Dipteryx genus, a member of the Fabaceae family with approximately 14 species, is found in Brazil's Amazon Rainforest, Northeast and Central regions, and Central America. Plants of this genus are used in popular medicine and contain coumarins, flavonoids, terpenoids, and fatty acids. Dipteryx lacunifera Ducke, known as "garampara" or "fava de morcego", occurs in Brazil's central-north region and has economic and energy value, along with antioxidant properties. For this study, ethyl acetate and ethylic ether soluble fraction of ethanol extract were subjected to chromatographic analysis leading to the isolation of two flavonoids, vitexin (1) and isoquercetin (6). In addition, analysis by electrospray ionization-ion trap mass spectrometry (ESI(-)-ITMS) and ultra-performance liquid chromatography coupled with electrospray ionization quadrupole-time-of-flight mass spectrometry (UPLC-ESI-QTOF-MS/MS) annotated twenty-one compounds (1-21), with seventeen of these being reported for the first time in D. lacunifera. Molecular network (GNPS) analysis grouped compounds (7-21) into flavonoids-O-glucoside, flavononol-O-glucoside, flavone-C-glucoside, proanthocyanidin, flavonol, and flavonoid-O-methoxylated classes. The FEtOAc fraction demonstrated potential antioxidant activity (half maximal effective concentration (EC50) 39.50 ± 6.51 mg mL-1), suggesting it as a promising source of antioxidants. However, neither fraction exhibited inhibition of tumor cell lines tested. These findings enhance our understanding of the chemical and biological potential of the D. lacunifera species. INTRODUCTION Brazil possesses one of the planet's richest floras, comprising approximately 20% of the world's plant biodiversity. Its diverse species exhibit therapeutic potential and serve as a source of new bioactive agents. It is noteworthy that numerous Brazilian plant species are chemically explored, placing Brazil in a privileged position for natural science, owing to its status as a mega-biodiverse country.1,2 In this context, Fabaceae is one of the largest angiosperms family, with about 650 genera and approximately 18,000 species currently distributed in three subfamilies: Faboideae (including the genus Dipteryx), Mimosoideae, and Caesalpinioideae.3 The family has a cosmopolitan distribution, mainly in regions such as tropical forests, deserts, plains, and alpine areas. In Brazil, approximately 200 genera and 1500 species are found, which are known for their economic and nutritional potential.4 The genus Dipteryx, belonging to the Faboideae subfamily and Dipterygeae tribe, comprises approximately 14 species found in the Amazon Rainforest, northeastern, and central regions of Brazil, as well as in Central America (Costa Rica and Panama) and Venezuela. Notably, its leaves, stems, and roots are used in popular medicine. Among the species in this genus, Dipteryxodorata is the most studied. Extracts from the seeds of this plant have yielded various chemical constituents, including flavonoids, which showed potential against breast cancer in rats. In addition to the seeds being used in the cosmetic, perfume, cigarette, condiment, and liqueur industries, it also exhibits therapeutic effects. The plants of this genus contain coumarins, flavonoids, terpenoids, fatty acids, and furanocassane diterpenoids in different parts of plants.5,6 Dipteryx lacunifera Ducke popularly known as "garampara", "fava de morcego", or "castanheira do gurguéia", is found in the mid-north region of Brazil (Maranhão and Piauí) and have high economic value and energy potential.7 Literature8 reports the presence of flavonoids 7,3',4'-trihydroxyflavone, (-)-butin, (-)-eriodictyol, luteolin, butein, and sulfuretin isolated from the fruit kernels of this species. The fruit shells are rich in essential oil, with their main chemical constituents β-farnesene, α-copaene, β-elemene, trans-caryophyllene, germacrene-D, bicyclogermacrene, γ-cadinene, spathulenol, and caryophyllene oxide and the chemical composition of the fixed oil from fruits includes fatty acids, which oleic acid being the major component.6 The species also presents an important nutritional and chemical variation, with several studies8-10 of the fruits showing compounds from the class of terpenoids (sesquiterpenes and diterpenes) and flavonoids. D. lacunifera stands out as a source of active substances with potential bioactivities, such as antioxidant, cytotoxic, and antimalarial action. Based on the potential antioxidant activity of the fruit kernels and compared to other studies, the bioactive compounds and biological potential of the leaves of D. lacunifera are still unreported. Therefore, this research focuses on chemical characterization and evaluates the antioxidant and cytotoxic activities of the polar fractions (ethyl acetate and ether) from D. lacunifera leaves.
EXPERIMENTAL Collection and identification of plant material The leaves of Dipterx lacunifera were collected from the Technical College of the Federal University of Piauí, Professor Cinobelina Elvas campus, located in the municipality of Bom Jesus-PI (-9.083239, -44.327733; altitude: 100 m), in February 2021 by Prof Dr Joxleide Mendes da Costa Pires Coutinho. A voucher specimen was deposited in the Graziela Barroso Herbarium (Federal University of Piauí, Minister Petrônio Portela campus) under the accession number TEPB 25571 (INCT - Virtual Herbarium of Flora and Fungi, 2021), SisGen No. AB29255. Preparation of extract and fractions The leaves of Dipteryx lacunifera were dried at room temperature and then powdered. The powdered plant material (1.2 kg) was subjected to extraction with ethanol (six times, 99.9%) at room temperature, with occasional agitation, followed by simple filtration. The solvent was removed in a rotary evaporator (Laborota 4000, Heidolph) under reduced pressure, yielding the ethanolic extract (EEtOH, 140.2 g, 11.2%). An aliquot of 80 g of the EEtOH extract was suspended in 750 mL of a MeOH/H2O mixture (1:2; v:v) and subjected to partition with hexane (1.60 L), yielding the hexane fraction (FHe, 28.3 g; 35.3%) and a precipitate (ppt, 10.0 g; 12.5%). Then, the soluble phase was subjected to partition with ethyl ether (1.60 L) and ethyl acetate (1.0 L), providing the FEt (3.9 g; 4.8%) and FEtOAc (7.6 g; 9.5%) fractions, respectively. Evaporation of hydro-methanolic solution afforded the FHM fraction (16.4 g; 20.5%). Isolation of constituents FEtOAc fraction (3.0 g) was subjected to column chromatography on Sephadex LH-20 (52 × 5 cm) and eluted with MeOH, providing 180 subfractions (S1-S180; 5 mL each). The subfractions were analyzed by thin-layer chromatography (TLC) on silica gel, and grouped according to the color and retention factor (Rf). Subfraction S122 (10.1 mg) yielded a yellow precipitate that was subjected to recrystallization with methanol, resulting in substance 1 (4.2 mg; yellow powder). Subfraction S145 (50.0 mg) was analyzed by TLC using as eluent CHCl3/MeOH (7:3) and ceric sulfate solution and subjected to column chromatography on Sephadex LH-20 (68 × 2 cm) using as eluent MeOH, providing 20 subfractions (SA1-SA20; 2 mL each). Subfraction SA10 (15.6 mg), after being analyzed by TLC using CHCl3/MeOH (7:3), was subjected to column chromatography on silica gel (68 × 2 cm, 60-200 μm) using CHCl3/MeOH in increasing order of polarity, resulting in 50 subfractions (SB1-SB50), which were grouped in according to the TLC analysis. Subfraction SB41 (10.4 mg) was subjected to column chromatography on silica gel (68 × 2 cm, 60-200 μm) using CHCl3/MeOH in increasing order of polarity, resulting in the isolation of substance 6 (3.0 mg; yellow powder). Analysis by electrospray ionization-ion trap mass spectrometry (ESI(-)-ITMSn) The subfractions (S122, S169, and SA41) obtained from FEtOAc fraction were dissolved in MeOH (50 ppm w:v) and filtered in a nylon membrane (47 mm diameter, 0.22 μm pore size, Chromafil® Xtra). Mass spectra were acquired by direct insertion into a mass spectrometer with an ion trap analyzer coupled to an electrospray ionization (ESI) source (amaZon SL Bruker Daltonics). The conditions used for the analyses were: ESI ionization source in negative ion mode, ESI(-), a mass range of m/z 100-1300 Da, a syringe flow rate of 3.0 μL min-1, capillary voltage of 4.5 kV, a drying gas flow rate (N2) of 5.0 L min-1, nebulization pressure of 8.0 psi, and a source temperature of 220 ºC. Data processing was performed using Compass 1.3 DataAnalysis software (version 4.0, Bruker Daltonics).11 Analysis by UPLC-ESI-QTOF-MS/MS and molecular networking The analyses of FEtOAc and FEt by high-resolution ultra-performance liquid chromatography coupled to electrospray ionization quadrupole-time-of-flight mass spectrometry (UPLC-ESI-QTOF-MS/MS) were performed on a Waters® Acquity H-Class Ultra Performance Liquid Chromatography system equipped with a quaternary solvent manager, a diode array detector (DAD), a column oven, and an autosampler (Sample Manager-FTN). A reverse-phase column (Waters Acquity UPLC HSS T3 100 × 2.1 mm, 1.8 µm) and a pre-column (Waters Acquity UPLC HSS T3 VanGuard 5 × 2.1 mm, 1.8 µm) were used for the analyses, with the mobile phase consisting of ultrapure H2O (A) and methanol (B). The gradient was applied at 0.5 mL min-1: 10 to 100% B over 8 min, followed by a hold at 100% B for 0.2 min. The analysis in mass spectrometry (MS2) mode was performed based on the application of a collision-induced dissociation energy gradient ranging from 10 to 30 eV, a mass range of m/z 100-1500 Da, and a scan time of 0.2 s. The Xevo G2-XS QToF mass spectrometer was used, equipped with an ESI source, a Q-ToF (quadrupole-time of flight) analyzer, and the MassLynx software (Waters®).12 The mass data in RAW format were converted to ".mzML" format using the Peak Picking filter in the MSConvert software (ProteoWizard).13 Subsequently, these files were uploaded to the Global Natural Products Social Molecular Networking (GNPS)14 platform using the WinSCP program.15 The parameters adopted for generating the molecular network were defined as follows: the precursor ion mass tolerance was set to 2.0 Da and the fragment ion tolerance was set to 0.9 Da. Ions with counts below 10 were removed from the MS/MS spectra. Molecular networks were generated using four matching peaks and a cosine score above 0.65. The data generated by the molecular network were imported and visualized in the Cytoscape version 3.7.0 software.16 The molecular networks obtained can be accessed on the GNPS website.14 The UPLC-MS/MS analyses were conducted at the Institute of Chemistry at São Paulo State University (UNESP), Araraquara campus. Nuclear magnetic resonance spectroscopy Nuclear magnetic resonance (NMR) spectra of 1H and 13C were obtained on a Bruker 400 spectrophotometer (400 MHz) using dimethyl sulfoxide (DMSO-d6) as the solvent and tetramethylsilane (TMS) as an internal reference for compound 1. For compound 6, a Bruker Avance III 600 spectrophotometer (600 MHz) was utilized, with methanol (CD3OD) as the solvent and tetramethylsilane (TMS) as an internal reference. The NMR analyses were conducted at the Institute of Chemistry at São Paulo State University (UNESP) and the University of São Paulo (USP) under the supervision of Professor Dr Mônica T. Pupo. Antioxidant activity The evaluation of the antioxidant activity (AA) of the fractions (FEtOAc and FEt) was performed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical method, using methodology adapted from the literature.17,18 A mass of 12.5 mg of each fraction was used in triplicate, and the solutions were prepared using MeOH at a concentration of 250 μg mL-1, and were diluted to 200, 150, 100, 50, and 25 μg mL-1. The synthetic compound BHT (2,6-di-tert-butyl-4-methylphenol) was used as a positive control. A stock solution of DPPH was prepared at a concentration of 40 μg mL-1 (MeOH) and protected from the light. The absorbance measurements of the reaction mixtures (2.7 mL of DPPH solution + 0.3 mL of the sample solution) were performed in triplicate after 30 min using a Lambda 25 spectrophotometer (PerkinElmer, Massachusetts, USA) at 516 nm. For the blank, 0.3 mL of the methanolic sample solution and 2.7 mL of MeOH were used. The equation of the analytical curve used to determine the concentration of DPPH was: A = 33.227c + 1.0607, with "c" as the equivalent of DPPH concentration, "A" the absorbance obtained in the maximum wavelength absorption (λmax) of 516 nm and the linear correlation coefficient R = 0.9997. To determine the remaining percentage (or residual) of DPPH (DPPHrem%), Equation 1 was used. 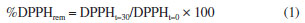 The results were presented in terms of half maximal effective concentration (EC50) with values in μg mL−1, determined from the first-order exponential curve of %DPPHrem versus the sample concentrations. The analysis was conducted at the Laboratory of Natural Products at the Federal University of Piauí, Teresina, Brazil. Cytotoxic activity The cytotoxic potential of the fractions (FEtOAc and FEt) was evaluated against tumor cell lines using methodologies adapted from the literature.19 The cell lines SNB-19 (glioblastoma), PC3 (prostate), and HCT 116 (colorectal adenocarcinoma), which were provided by the National Cancer Institute (USA), were cultured in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics. The cell lines were maintained in a CO2 incubator at 37 ºC with an atmosphere containing 5% CO2. The solid samples were weighed and diluted in DMSO to obtain a stock concentration of 50 mg mL-1. All samples were analyzed as a percentage (volume/volume) at a final concentration of 0.5%. For the tumor cell line assay, cells were plated at concentrations of 0.7 × 105 cells mL-1 (HCT-116), 0.1 × 106 cells mL-1 (SNB-19), 0.1 × 106 cells mL-1 (PC3) and then incubated with the samples at 37 ºC and 5% CO2 for 72 h. After this period, the cells were centrifuged and the supernatant was removed and replaced with 100 μL of 3-(4,5-dimethyl-2-thiazole)-2,5-diphenyl-2H-tetrazolium bromide (MTT). The plates were incubated for 3 h. Following the incubation, they were centrifuged again to remove the MTT solution. Absorbance was measured after the formazan precipitate was dissolved with 100 μL of analytical grade DMSO using a plate spectrophotometer at 595 nm. Cytotoxicity assays were conducted at the Experimental Oncology Laboratory, Federal University of Ceará (LOE-UFC) under the supervision of Professor Dr Cláudia Pessoa.
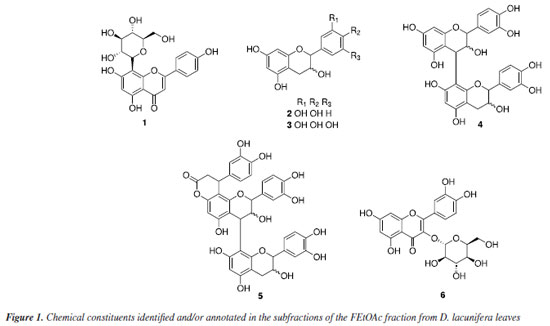
RESULTS AND DISCUSSION ESI(-)-ITMSn Through the analysis of the ESI(-)-ITMSn of the FEtOAc subfractions (S122, S169, and SA41) from Dipterx lacunifera leaves, four compounds (2-5) were annotated based on the precursor ions of the deprotonated molecules ([M − H]-) and fragment ions (Figure 1, Table 1, Supplementary Material: Figures 1S-6S). Compound 1 was identified through analysis of its mass spectra, as well as both 1H and 13C NMR spectroscopy (Figure 1, Table 1, Supplementary Material: Table 1S, Figures 17S-24S), corroborated by comparison with existing literature data.20 The mass spectrum displayed the deprotonated molecular ion at m/z 431 [M - H]-, consistent with the molecular formula C21H20O10. Additionally, presented fragment ions at m/z 341 [M - H - C3H6O3]-, 311 [M - H - C4H8O4]-, and 283 [M - H - C4H8O4 - CO]- corresponding to losses of 90 Da, 120 Da, and CO, respectively. These findings indicate that the compound is the flavonoid vitexin, marking the first report of this substance in the species. This flavone is found in various medicinal plants, such as hawthorn, millet, wheat leaves, mimosa, and bamboo, and exhibits various pharmacological activities such as anticancer, antiviral, antioxidant, and anti-inflammatory.21,22
The peak at m/z 289 [M - H]- is consistent with the molecular formula C15H14O6. The mass spectrum presented fragment ions at m/z 271 [M - H - H2O]-, indicative of the loss of one water molecule, and m/z 245 [M - H - CO2]- and m/z 227 [M - H - CO2 - H2O]- that correspond to the loss of carbon dioxide and water, respectively. Additionally, fragment ions at m/z 179 [M - H - C6H6O2]- (with a loss of 110 Da), m/z 137 [M - H - C8H8O3]-, and m/z 125 [M - H - C9H8O3]- are related to fragmentation of the type retro Diels-Alder (RDA) and cleavage of the C ring, respectively, suggesting to be the deprotonated form of (epi)catechin (2). This substance is widely present in the chemical composition of various plant-derived foods, such as green tea. It is responsible for anti-inflammatory action in lung diseases and bone repair, as well as in the prevention and treatment of diseases associated with obesity and diabetes.34-36 Compound 3 showed a peak of the deprotonated molecular ion at m/z 305 [M - H]-, corresponding to the molecular formula C15H14O7, as well as fragment ions at m/z 287 [M - H - H2O]- and m/z 261 [M - H - CO2]- corresponding to the loss of one molecule of water and carbon dioxide, respectively. Additional fragment ions at m/z 219 [M - H - CO2 - C2H2O]-, m/z 165 [M - H - C7H8O3]-, and m/z 137 [M - H - CO2 - C2H2O - C5H6O]- correspond to losses of 42, 140 and 82 Da, respectively. Furthermore, cleavage of the C ring resulted in the fragment ion at m/z 125 [M - H - C9H8O4]-, confirming the presence of a flavan-3-ol and indicating that it is likely to be (epi)gallocatechin. Compound 4 showed a peak of the deprotonated molecular ion at m/z 577 [M - H]-, which is consistent with the molecular formula C30H26O12, as annotated based on the fragmentation profile. In the MS/MS spectrum, the main fragmentation pathways showed heterocyclic ring fission (HRF) resulting in fragments at m/z 451 [M - H - C6H6O3]- and m/z 425 [M - H - C8H8O3]- corresponding to RDA fragmentation. The additional fragment ion in m/z 407 [M - H - C8H8O3 - H2O]- corresponds to the loss of water and through quinone-methide (QM) type cleavage with a fragment ion at m/z 289 [M - H - C15H12O6]-, indicating that it is a dimer of (epi)catechin, namely (epi)catechin-(epi)catechin. This compound exhibits several nutritional and pharmacological properties, such as vasoprotective, antioxidant, hepatoprotective, and anticancer effects.37 The mass spectrum of compound 5 showed a peak of the deprotonated molecular ion at m/z 739 [M - H]-, consistent with the molecular formula C24H20O9. Additional fragment ions were observed at m/z 629 [M - H - C6H6O2]- with a loss of 110 Da, and m/z 587 [M - H - C6H6O2 - C2H2O]- and m/z 569 [M - H - C6H6O2 - C2H2O - H2O]- that correspond to RDA fragmentation and the loss of one water molecule, respectively. Further fragmentations were observed at m/z 449 [M - H - C7H6O3]- with a loss of 138 Da and at m/z 435 [M - H - C16H16O6]- corresponding to RDA fragmentations. Additionally fragments ions at m/z 339 [M - H - C6H6O2 - C2H2O - C7H6O3 - C6H6O2]- correspond to an additional loss of 110 Da through QM cleavage, with a fragment ion at m/z 289 [M - H - C24H18O9]-, allowed annotated the compound as cinchonain. The mass spectrum of the isolated substance (6) showed a peak of the deprotonated molecular ion at m/z 463 [M - H]- corresponding to the molecular formula C15H14O7. There was a fragment ion at m/z 301 [M - H - Glu]- referring to the loss of one sugar unit, followed by the loss of 122 Da and carbon dioxide for the fragment ions at m/z 179 [M - H - Glu - C7H6O2]- and m/z 151 [M - H - Glu - C7H6O2 - CO]-, respectively. This suggests that it is isoquercetin (6), which was confirmed through 1H and 13C NMR spectra (Supplementary Material: Table 2S, Figures 25S-31S).38 The compound demonstrates a spectrum of beneficial properties, including antioxidant, anti-inflammatory, and anticoagulant. Moreover, it exhibits remarkable antiviral activity, notably reducing cellular infections caused by influenza, Zika virus, dengue virus, and other pathogens. Ongoing research is exploring its potential against COVID-19.39
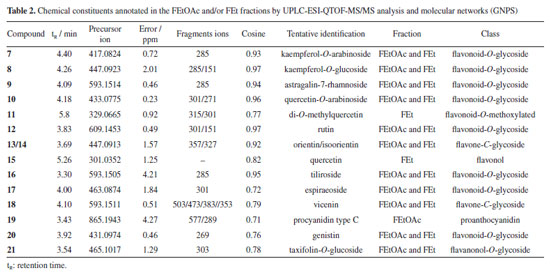
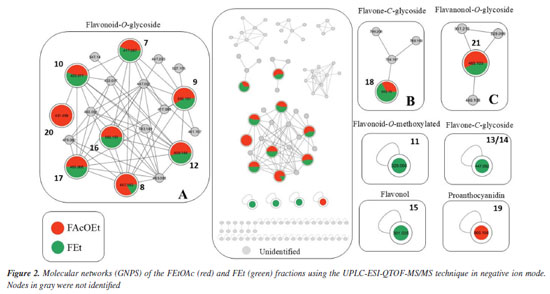
UPLC-ESI-QTOF-MS/MS The UPLC-MS/MS analysis of FEtOAc and FEt fractions, along with the data from the GNPS platform allowed for the construction of molecular networks. This analysis enabled the identification of flavonoids spanning various classes, such as flavonoids-O-glycoside, flavonol-O-glycoside, flavone-C-glucoside, proanthocyanidin, flavonol, isoflavone, and flavonoid-O-methoxylated, which were characterized based on high-resolution mass values (negative mode), retention times (tR), molecular formulae, errors (ppm), fragmentations, and comparison with literature data (Table 2). The molecular network in the negative mode presented three clusters and four individual nodes (Figure 2). Clusters A, B, and C presented nodes that were analyzed by GNPS as flavonoid-O-glycoside, flavone-C-glycoside, and flavanonol-O-glycoside, respectively. The isolated nodes were characterized as flavonoid-O-methoxylated (11), flavone-C-glycoside (13/14), flavonol (15), and proanthocyanidin (19). The nodes corresponding to substances 11, 13/14, and 15 indicate the presence of these substances only in FEt, while substances 19 and 20 are present only in FEtOAc. The substances 7, 8, 9, 10, 12, 16, 17, 18, and 21 are annotated in both fractions in different quantities according to the nodes (Figures 2 and 3, Table 2).
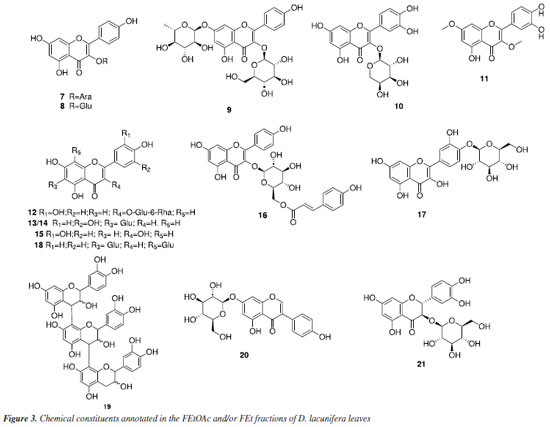
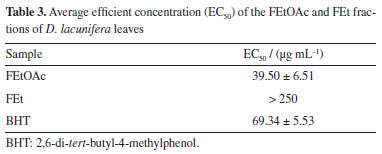
The peak with a tR of 4.40 min that is present in both fractions corresponds to the deprotonated molecular ion at m/z 417.0824 [M - H]-, which is consistent with the molecular formula C20H18O10, and generated a fragment ion at m/z 285 [M - H - Ara]- that refers to the loss of sugar unit, indicating that the compound is kaempferol-O-arabinoside (7). Compound 8, found in both fractions, exhibited a tR of 4.26 min and the mas spectrum showed deprotonated molecular ion at m/z 447.0923 [M - H]-, which is consistent with the molecular formula C21H20O11. Additionally, it generated a fragment ion at m/z 285 [M - H - Glu]-, attributed to the loss of a hexose and the subsequent type RDA fragmentation yielded an ion at m/z 151 [M - H - Glu - C8H6O2]-, indicating that it is likely kaempferol-O-glycoside. This substance was identified in the species Dipteryx alata.40 The mass spectrum of compound 9, present in both fractions, showed a peak of the deprotonated molecular ion at m/z 593.1514 [M - H]-, consistent with the molecular formula C27H30O15, with a tR of 4.09 min. Major fragment ion observed at m/z 285 [M - H - Glu - Rha]- corresponds to the loss of two sugar units, suggesting that this substance is astragalin-7-rhamnoside. The peak with a tR of 4.18 min, present in both fractions, corresponds to the ion with m/z 433.0775 [M - H]- and is compatible with the molecular formula C20H18O11. Fragment ions at m/z 301 [M - H - C5H8O4]-, which is related to the loss of a pentose molecule, and at m/z 271 [M - H - C5H8O4 - CH2O]-, which is caused by the additional loss of a methanal molecule, suggested this compound as quercetin-O-arabinoside (10). The identification of a quercetin derivative present in the FEt fraction at the peak tR of 5.88 min was based on the deprotonated molecular ion with m/z 329.0665 [M - H]-, which is compatible with the molecular formula C17H13O7, and fragment ions at m/z 315 [M - H - CH3]- and m/z 301 [M - H - 2CH3]- related to successive losses of methyl groups. This enabled the characterization of a flavonoid-O-methoxylated, suggesting that it is di-O-methylquercetin (11). The mass spectrum of compound 12 showed a peak of the deprotonated molecular ion at m/z 609.1453 [M - H]-, which is consistent with the molecular formula C27H30O16. Aglycone fragment ions were also observed at m/z 301 [M - H - Glu - Rha]- and type RDA fragmentation at m/z 151 [M - H - Glu - Rha - C8H6O3]-. This substance has already been reported in the genus Dipteryx, suggesting that it is the compound rutin.41 Compounds 13/14 are present in the FEt fraction with precursor ion at m/z 447.0913 [M - H]-, which is consistent with the molecular formula C21H20O11, along with RDA fragmentation. A fragment ion was generated at m/z 357 [M - H - C3H6O3]- followed by the loss of a methanal molecule, resulting in the fragment ion m/z 327 [M - H - C3H6O3 - CH2O]- that is consistent with a C-glycosylated flavone, annotated as orientin/isoorientin, which are substances with antioxidant, anti-inflammatory, antidiabetic, and antiobesity properties.42 The deprotonated molecular ion at m/z 301.0352 [M - H]- (tR = 5.26 min), compatible with the molecular formula C15H10O7, was detected only in the FEt fraction. This was attributed to the flavonoid quercetin (15), which is a substance that has a variety of pharmacological activities, such as antioxidant, anticancer, anti-aging, anti-inflammatory, and antiviral.43 This has already been reported in Dipteryx lacunifera.30 The peak with a tR of 3.30 min, present in both fractions, corresponds to compound 16, which presented the deprotonated molecular ion at m/z 593.1505 [M - H]-, consistent with molecular formula C30H26O13. This generated an ion fragment at m/z 285 [M - H - C15H16O7]- related to the loss of 308 Da, suggesting that the compound is tiliroside. This substance is found in several edible plants and is widely used to treat wounds, diarrhea, and diabetes. It also exerts antioxidant, anti-obesity, and anti-diabetic effects.44 The mass spectrum of compound 17, present in both fractions, indicated the presence of the deprotonated molecular ion at m/z 463.0874 [M - H]- (tR = 4.0 min), which is consistent with the molecular formula C21H20O12. A fragment ion at m/z 301 [M - H - Glu]- is related to the loss of a sugar unit, suggesting that it is spiraeoside. This substance has antiallergic properties, antitumor activities, and antioxidants. Additionally, it exhibits an inhibitory effect on the proliferation of colon cancer cells.45 The peak with a tR of 4.09 min and the deprotonated molecular ion at m/z 593.1511 [M - H]-, which is consistent with the molecular formula C27H30O15, was detected in both fractions. Fragment ions detected with losses of 90 and 120 Da were consistent with the fragment ions at m/z 503 [M - H - C3H6O3]- and m/z 383 [M - H - C3H6O3 - C4H8O4]-, respectively. Additional peaks at m/z 473 [M - H - C4H8O4]- and m/z 353 [M - H - 2C4H8O4]- are related to two successive losses of 120 Da, with characteristics that suggest that it was the flavonoid vicenin (18). This flavone-C-glycosylated has already been reported in Dipteryx lacunifera.46 The mass spectrum of compound 19, which is present in the FEtOAc fraction, is made up exclusively of (epi)catechin units. There was a peak of the deprotonated molecular ion at m/z 865.1943 [M - H]-, which is consistent with the molecular formula C45H38O18. Fragment ions arising from QM-type fission at m/z 577 [M - H - C15H12O6]- and m/z 289 [M - H - 2C15H12O6]- indicate that the substance is a type C procyanidin.47 The deprotonated molecular ion at m/z 431.0974 [M - H]-, which is consistent with the molecular formula C21H20O10, was detected in the FEtOAc fraction with a tR = 3.92 min. It showed a fragment ion at m/z 269 [M - H - Glu]- compatible with the loss of a sugar unit. This evidence suggests that it is the isoflavonoid genistin (20). This substance is found in a variety of dietary plants, such as soybeans and kudzu, and has anticancer, antioxidant, cardioprotective, neuroprotective, and antimicrobial properties.48 Compound 21 is present in both fractions, with a peak at m/z 465.1017 [M - H]- that is consistent with the molecular formula C21H22O12. A fragment ion at m/z 303 [M - H - Glu]-, referring to the loss of a hexose unit, suggests to be a taxifolin derivative. This substance has anti-inflammatory, antimicrobial, and other pharmacological activities, and was annotated as taxifolin-O-glucoside.49 Antioxidant and cytotoxic activities The fractions (FEtOAc and FEt) of Dipteryx lacunifera leaves were evaluated by scavenging the free radical DPPH. The antioxidant potential of FEtOAc fraction corroborates with the results obtained in mass spectrometry analyses, where it was possible to detect the presence of phenolic compounds, mainly from the flavonoids class. These are bioactive compounds with antioxidant properties.50 The FEtOAc fraction was the most active with EC50 = 39.50 ± 6.51 μg L-1, presenting better antioxidant potential than the positive control (Table 3). It was not possible to determine the EC50 for the FEt fraction, as the highest concentration tested (250 μg mL-1) did not result in a 50% reduction in the DPPH concentration.
The antiproliferative activity of the fractions was tested against three tumor cell lines: SNB-19, PC3, and HCT-116. The fractions did not reach 75% of tumor growth in tested cell lines, indicating that they are inactive against these tumor cell lines.
CONCLUSIONS The phytochemical composition of the fractions (FEtOAc and FEt) of the species Dipteryx lacunifera was investigated using ESI(-)-ITMSn and UPLC-ESI-QTOF-MS/MS analysis with molecular networks (GNPS). The fractions predominantly contained phenolic compounds, with twenty one compounds identified and/or annotated (1-21). The majority of the compounds belonged to the flavonoid class (flavonoids-O-glycoside, flavonol-O-glycoside, flavone-C-glucoside, proanthocyanidin, flavonol, isoflavone, and flavonoid-O-methoxylated). Among the compounds identified and/or annotated, seventeen (1-7; 9-11; 13/14; 16; 17;19-21) were reported for the first time in D. lacunifera. In the DPPH radical scavenging assay, the FEtOAc fraction showed better antioxidant activity, indicating the potential of this fraction as a source of antioxidant substances. This is the first study on D. lacunifera leaves, contributing to the understanding of their phytochemical composition and the remarkable bioactivity of the polar fractions of the leaf extract
SUPPLEMENTARY MATERIAL Spectrometric information (mass and NMR) and fragmentation proposals for the compounds annotated in the leaf fractions of Dipteryx lacunifera are provided in Figures 1S-31S, available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
ACKNOWLEDGMENTS We thank the Federal University of Piauí (UFPI) for the laboratory space; laboratory colleagues E. S. Monção Filho for assistance in the ESI-ITMS analysis and assistance in the interpretation of NMR and R. P. de Sousa for his partnership and assistance with chromatographic analysis; the National Council for Scientific and Technological Development (CNPq - 302197/2017-6; 402302/2021-4); and the National Institute of Science and Technology (INCT BioNat - 465637/2014-0).
REFERENCES 1. Berlinck, R. G.; Borges, W. S.; Scotti, M. T.; Vieira, P. C.; Quim. Nova 2017, 40, 706. [Crossref] 2. de Sousa, E. A. S.; Rosa, A. P. A.; dos Santos, R. R. L.; dos Santos, I. L.; de Sousa, V. C.; Carvalho, F. A. A.; Vieira Junior, G. M.; Chaves, M. H.; Quim. Nova 2019, 42, 192. [Crossref] 3. Espécies Arbóreas Brasileiras, Fabaceae, https://www.embrapa.br/agencia-de-informacao-tecnologica/tematicas/especies-arboreas-brasileiras/fabaceae, accessed in November 2024. 4. Silveira, F. S.; Miotto, S. T. S.; Revista Brasileira de Biociências 2013, 11, 93. [Link] accessed in November 2024 5. de Carvalho, C. S.; de Fraga, C. N.; Cardoso, D. B. O. S.; Lima, H. C.; Taxon 2020, 69, 582. [Crossref] 6. Vieira Júnior, G. M.; Silva, H. R.; Bittencourt, T. C.; Chaves, M. H.; de Simone, C. A.; Quim. Nova 2007, 30, 1658. [Crossref] 7. Ayres, M. C. C.; Brandão, M. S.; Vieira Junior, G. M.; Menor, J. C. A. S.; Silva, H. B.; Soares, M. J. S.; Chaves, M. H.; Rev. Bras. Farmacogn. 2008, 18, 90. [Crossref] 8. Vieira Júnior, G. M.; Sousa, C. M. M.; Cavalheiro, A. J.; Lago, J. H. G.; Chaves, M. H.; Helv. Chim. Acta 2008, 91, 2159. [Crossref] 9. Alexandre, L. S.; Oliveira, M. S.; Dittz, D.; Sousa, R. W. R.; Ferreira, P. M. P.; Pessoa, C.; Varotti, F. P.; Sanchez, B. A. M.; Banfi, F. F.; Chaves, M. H.; Vieira Junior, G. M.; Rev. Bras. Farmacogn. 2020, 30, 544. [Crossref] 10. Mendes, F. N. P.; Silveira E. R.; Phytochemistry 1994, 35, 1499. [Crossref] 11. DataAnalysis, version 4.0; Bruker Daltonics, Ettligen, Germany, 2010. 12. MassLynx, version 4.0; Waters® Corporation, Manchester, UK, 2003. 13. MSConvert, version 3.0; ProteoWizard, Palo Alto, CA, USA, 2017. 14. Global Natural Product Social Molecular Networking (GNPS), http://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=d475bd054e7049a6bda6c5b5887ab312, accessed in November 2024. 15. WinSCP, version 6.1.2; OpenSSL, Czech Republic, 2023. 16. Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N. S.; Wang, J. T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T.; Genome Res. 2003, 13, 2498. [Crossref] 17. Sousa, C. M. M.; Silva, H. R.; Vieira-Jr., G. M.; Ayres, M. C. C.; da Costa, C. L. S.; Araujo, D. S.; Cavalcante, L. C. D.; Barros, E. D. S.; Araujo, P. B. M.; Brandão, M. S.; Chaves, M. H.; Quim. Nova 2007, 30, 351. [Crossref] 18. Costa, D. A.; Chaves, M. H.; Silva, W. C. S.; Costa, C. L. S.; Acta Amazonica 2010, 40, 207. [Crossref] 19. Mosmann, T.; J. Immunol. Methods 1983, 65, 55. [Crossref] 20. Costa, E. C.; Menezes, P. M. N.; de Almeida, R. L.; Silva, F. S.; Ribeiro, L. A. A.; de Silva, J. A.; de Oliveira, A. P.; Araujo, E. C. C.; Rolim, L. A.; Nunes, X. P.; Heliyon 2020, 6, e05461. [Crossref] 21. He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W.; Fitoterapia 2016, 115, 74. [Crossref] 22. Costa, E. C.: Avaliação do Efeito Antitussígeno e Expectorante da Vitexina Isolada de Jatropha mutabilis e do Complexo de Inclusão Vitexina/β-Ciclodextrina; PhD Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2018. [Link] accessed in November 2024 23. Carvalho, G. F. S.; Marques, L. K.; Sousa, H. G.; Silva, L. R.; Ferreira, D. C. L.; do Amaral, F. P. M.; Maia Filho, A. L. M.; Figueredo-Silva, J.; Alves, W. S.; de Oliveira, M. D. A.; da Costa Junior, J. S.; Costa Junior, F. L. C.; Ramos, R. M.; Rai, M.; Uchôa, V. T.; J. Ethnopharmacol. 2020, 247, 112259. [Crossref] 24. Leal, S. S.; Gusmão, G. O. M.; Uchôa, V. T.; Silva, J. F.; Pinto, L. S. S.; Tim, C. R.; Assis, L.; Maia-Filho, A. L. M. M.; de Oliveira, R. A.; Lobo, A. O.; Pavinatto, A.; J. Funct. Biomater. 2023, 14, 438. [Crossref] 25. Costa, M. C. B.; Rodrigues, L. A. R. L.; Crisóstomo, J. M.; de Farias, L. M.; Lavôr, L. C. C.; de Sousa, R. R.; Frota, K. M. G.; Res., Soc. Dev. 2021, 10, e28510313185. [Crossref] 26. Jordão, A.; Millenium 2000, 19. [Link] accessed in November 2024 27. Islam, M.; Al-Amin, Md.; Siddiqi, M. M. A.; Akter, S.; Haque, M. M.; Sultana, N.; Chowdhury, A. M. S.; Dhaka Univ. J. Sci. 2012, 60, 11. [Crossref] 28. Mbikay, M.; Chrétien, M.; Front. Pharmacol. 2022, 13, 830205. [Crossref] 29. Dantas, C.; Abreu, L. S.; da Cunha, H. N.; Veloso, C. A. G.; Souto, A. L.; Agra, M. F.; Costa, V. C. O.; da Silva, M. S.; Tavares, J. F.; Phytochem. Anal. 2021, 32, 1011. [Crossref] 30. Arun, P. K.; Rajesh, S. S.; Sundaram, S. M.; Sivaramn, T.; Brindha, P.; Bangladesh J. Pharmacol. 2014, 9, 452. [Crossref] 31. Xu, Y.; Liang, P.-L.; Chen, X.-L.; Gong, M.-J.; Zhang, L.; Qiu, X.-H.; Zhang, J.; Huang, Z.-H.; Xu, W.; Front. Nutr. 2021, 8, 737539. [Crossref] 32. Zhang, M.; Vervoort, L.; Moalin, M.; Mommers, A.; Douny, C.; den Hartog, G. J. M.; Haenen, G. R. M. M.; Free Radical Biol. Med. 2018, 124, 31. [Crossref] 33. Desta, K. T.; Kim, G. S.; El-Aty, A. M. A.; Raha, S.; Kim, M.-B.; Jeong, J. H.; Warda, M.; Hacımüftüoğlu, A.; Shin, H.-C.; Shim, J.-H.; Shin, S. C.; J. Chromatogr. B 2017, 1053, 1. [Crossref] 34. Zheng, Y.; Zeng, X.; Chen, T.; Peng, W.; Su, W.; Nutrients 2020, 12, 224. [Crossref] 35. Yuzuak, S.; Ballington, J.; Xie, D.-Y.; Metabolites 2018, 8, 57. [Crossref] 36. Yan, T.; Hu, G.-S.; Wang, A.-H.; Hong, Y.; Jia, J.-M.; Nat. Prod. Res. 2014, 28, 1834. [Crossref] 37. Wu, J.; Fang, X.; Yuan, Y.; Dong, Y.; Liang, Y.; Xie, Q.; Ban, J.; Chen, Y.; Lv, Z.; Rev. Bras. Farmacogn. 2017, 27, 716. [Crossref] 38. Sendker, J.; Petereit, F.; Lautenschläger, M.; Hellenbrand, N.; Hensel, A.; Planta Med. 2013, 79, 45. [Crossref] 39. Bravo, L.; Goya, L.; Lecumberri, E.; Food Res. Int. 2007, 40, 393. [Crossref] 40. Alves-Santos, A. M.; Sampaio, K. B.; Lima, M. S.; Coelho, A. S. G.; de Souza, E. L.; Naves, M. M. V.; Food Res. Int. 2023, 164, 112366. [Crossref] 41. Sarri, D. R. A.: Análise Fitoquímica, Toxicidade e Avaliação das Atividades Antioxidante e Antimicrobiana das Folhas de Dipteryx alata (Baru) em Odontologia; MSc Dissertation, Universidade Federal do Tocantins, Palmas, Brazil, 2022. [Link] accessed in November 2024 42. Ziqubu, K.; Dludla, P. V.; Joubert, E.; Muller, C. J. F.; Louw, J.; Tiano, L.; Nkambule, B. B.; Kappo, A. P.; Mazibuko-Mbeje, S. E.; Pharmacol. Res. 2020, 158, 104867. [Crossref] 43. Okamoto, T.; Int. J. Mol. Med. 2005, 16, 275. [Crossref] 44. Grochowski, D. M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M.; Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395. [Crossref] 45. Liu, H.; Zhang, Z.; Zhang, L.; Yao, X.; Zhong, X.; Cheng, G.; Wang, L.; Wan, Q.; J. Biochem. Mol. Toxicol. 2020, 34, e22548. [Crossref] 46. Alves-Santos, A. M.; Fernandes, D. C.; Naves, M. M. V.; NFS J. 2021, 24, 26. [Crossref] 47. Enomoto, H.; Takahashi, S.; Takeda, S.; Hatta, H.; Molecules 2020, 25, 103. [Crossref] 48. Islam, A.; Islam, M. S.; Uddin, M. N.; Hasan, M. M. I.; Akanda, M. R.; Arch. Pharm. Res. 2020, 43, 395. [Crossref] 49. Liu, Y.; Shi, X.; Tian, Y.; Zhai, S.; Liu, Y.; Xiong, Z.; Chu, S.; Front. Pharmacol. 2023, 14, 1173855. [Crossref] 50. Almeida, J. R. G. S.; Araujo, C. S.; Pessoa, C. Ó.; da Costa, M. P.; Pacheco, A. G. M.; Rev. Bras. Frutic. 2014, 36, 258. [Crossref]
Editor handled this article: Jorge M. David |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access