Artigo
| Evaluation of thermal and mechanical properties of ZnSO4-B2O3-P2O5-Ho2O3 glass-ceramics |
|
Areej S. AlqarniI,* I. Department of Physics, College of Science, Princess Nourah Bint Abdulrahman University, P.O. Box 84428, 11671 Riyadh, Saudi Arabia Received: 07/01/2024 *e-mail: arsalqarni@pnu.edu.sa Associate In this study, a novel glass-ceramic (GC) obtained from ZnSO4-B2O3-P2O5-Ho2O3composition, coded as H0.0, H0.5 and H0.8, was synthesized via molten-solidified approach. The effect of varying content of Ho2O3 from 0.5 to 0.8 mol% on thermal and mechanical properties of the prepared GC were experimentally and theoretically scrutinized. The attained results showed that inclusion of Ho2O3 content amplified crystallite size from 42.3 to 50.5 mm. Besides, the values of elastic moduli (Youngs's, bulk, shear and longitudinal) computed via Makishima-Mackenzie's (MM) model were slightly augmented. Finally, the decent value of thermal stability (> 150ºC) and high fracture toughness of > 20000MPa mm1/2 achieved for the newly fabricated GC may be applicable in protective covers of portable electronics. INTRODUCTION Glass-ceramics (GC) with high hardness, reasonable Young's modulus, boosted fracture toughness and coupled with a suitable thermal stability are of great significance in modern optical displays systems.1-3 Categorically, these materials by virtue of their strong resistance to scratching and sharp contact damage find manifold applications in the protective layer of various portable electronics like smartphones, laptops, tablets, wearable devices, and radiation shielding applications.4,5 Although, glass-ceramics depend on multiple factors namely, chemical composition, crystallinity, thermal history, kinetics, phase composition, and additives.6 The mechanical evolution of brittle materials depends on internal micromechanical stresses, which occur upon cooling due to the elastic and thermal mismatch between the constituent phases.7 Over the past decades, several competing glass-ceramics have been developed and reported by renounced scholars for diverse practical usage wherein lithium disilicates (LDs) remains the topmost and commercially available glass-ceramics for dental implants and borne regeneration.8,9 Besides, synthesis and characterization of iron phosphate-based glass-ceramic for potential utilization in nuclear waste immobilization was performed and testified by Wang et al.10 Thereafter, microstructure and phase composition dependent microwave dielectric properties of borophosphate GC has been examined by Jiang et al.11 These GC's were commendable for low temperature co-fired ceramics (LTCC) application. However, the status of crystalline phases, thermal, magnetic, and density of ZnO-SrO-B2O3-Fe2O3-P2O5 glass-ceramics due to SrO and ZnO substitutions was analyzed.12 In 2021, the structural analysis of multicomponent borophosphate glass ceramic was accomplished by Haily et al.12 Further, Węgrzyk et al.13 had achieved and publicized high mechanical strength ZABS (ZnO-Al2O3-B2O3-SiO2) glass ceramics system routed with transition metal ions. Besides, a comprehensive analysis on structural, mechanical, gamma, and neutron attenuation characteristics of B2O3-PbO-TeO2-CeO2-WO3 glasses was conducted by Solak et al.14 Very recently, Acikgoz et al.15 examined the influence of praseodymium oxide on the structural, mechanical and photon, charged particles, and neutron shielding properties of alumina borate glass. Above and beyond, it has been well established that the thermo-mechanical evolution of GC materials can be promoted by introducing rare earth ions (REI's) impurities. This assertion is attributed to the strengthening and toughening potential of REI's as well as the purifying effect on the interface between grains and the effective solution.16 Ho2O3, among the 17 rare earth ions, are famous for their light emitting characteristic owing to its several high excited energy levels over the visible spectral region responsible for the intense visible emissions.17 On this claim, Ho was chosen in this study, aiming at achieving cheap, tiny, efficient and safe glass-ceramic host. As reported in Gupta et al.,18 Ho2O3 is commonly used as additive and supporting mediator in structural glass-ceramics. Specifically, it is more significant that the ions has profound impact on the dielectric, optical, thermal and mechanical properties of glass ceramics. In fact, small amount of these ions can significantly affect the microstructural morphology as well as thermo-mechanical properties of glass-ceramics. Moreover, incorporation of Ho2O3 greatly enriched durability, glass transformation temperatures (Tg), low electrical conductivity and thermal expansion coefficients in borophosphate glass ceramics.19 Encouraged by the aforementioned facts, the aim of the present work is to synthesize Ho3+-doped novel P2O5-B2O3-ZnSO4-based glass-ceramic by molten and solidified process. Borate (B2O3) and phosphate (P2O5) are chosen as exciting hosts because borate possess precious thermal strength, lower melting point, massive RE3+ solubility, and excellent transparency. Meanwhile, high gain, low dispersion, and comparatively higher refractive indices are the added advantages from phosphate which enables it to decolorize the borate flint units.20 Besides, the existence of ZnSO4 in P2O5-B2O3 glass-ceramic network enhance it physical, structural and optical performance.21 With this insight, herein, the effects of substituting P2O5 by Ho2O3 on the thermo-mechanical properties of the P2O5B2O3ZnSO4-based glass-ceramic was investigated. The overall outcomes of the present investigation established high values of microhardness, Young's modulus, fracture toughness and good thermal stability. From the aforementioned achievement, the conceptual design and customization of present glass ceramics in developing protective covers for electronic displays on smartphones and wearable devices can be extended.
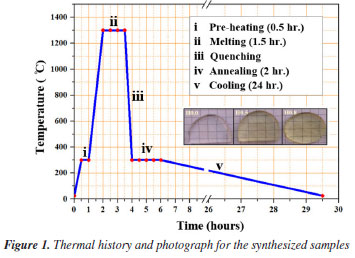
EXPERIMENTAL Synthesis of glass-ceramic samples The raw materials of high purity (99.999%) analytical grade reagents, ZnSO4, B2O3, H3PO4, and pure Ho2O3 (99.999%) were used in the fabrication of new glass ceramics (GC) with a well-defined stoichiometric compositions of (40-x)P2O5-30B2O3-30ZnSO4-xHo2O3 (with x = 0.0, 0.5, and 0.8 mol%) coded as H0.0, H0.5 and H0.8. These GC with constituent's batch of 20 g each were made via melting-solidified approach The resultant mixture of the compositions was gradually preheated at low temperature (300 ºC) for 30 min before being transferred to another furnace for complete melting at 1300 ºC for 90 min. Succeeding, the molten was poured on a warmed stainless-steel plate and solidified at 300 ºC for 2 h. To conclude, the obtained samples were kept in furnace and logically cool down to room temperature. Finally, samples were stored in a moisture free environment at room temperature. Some portions of the as-quenched samples were crushed into fine powder for structural and thermal analyses. Table 1 shows the sample compositions alongside the sample's codes. Additionally, the thermal history and photograph for the synthesized samples (with ~2 cm diameter) is presented in Figure 1.
Characterisation of the synthesized glass-ceramics By employing X-ray diffractometer (SmartLab Rigaku) equipped with Cu-Kα (wavelength ≈1.5406 Å) radiation, the microstructure status of our fabricated GC was exposed. This was conducted by logging the data in the angular range (2θ) from 10º to 80º with a step size of 0.02º. Additionally, the integrated EDX (energy-dispersive X-ray spectroscopy) in field-emission scanning electron microscope (FE-SEM) was used for microstructure analysis. In addition, the thermal stability potency of GC was verified by differential thermogravimetry analysis (DTA). This was piloted by using thermal analyzer (HITACHI STA7200 model) at a heating rate of 10º min-1 within nitrogen atmosphere. Last but not the least, the microhardness measurement was carried out by using digital microhardness tester of Shimadzu HVM-2 model at room temperature. Particularly, 100 g load was applied for a peak-load time of 10 s, and the diagonals of indentation were measured with an accuracy of ± 0.1l m. Afterwards, the Vickers microhardness values was deduced as an average of 10 readings collected from different position of the sample surface.
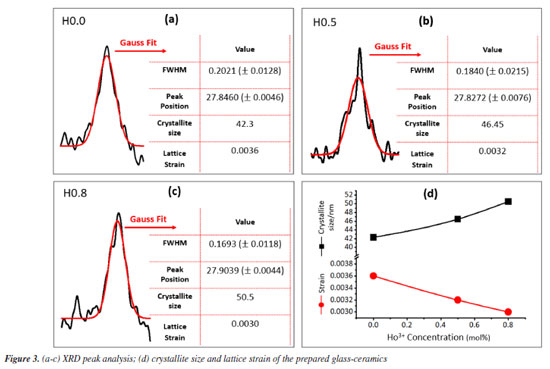
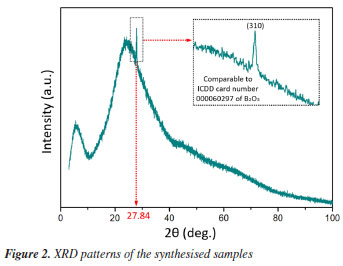
RESULTS AND DISCUSSION Structural properties The XRD (X-ray diffraction) pattern of the as-produced H0.8 sample is epitomized in Figure 2. The obvious wide diffraction halo around 20-40º indorse the amorphous nature of the present samples. In addition, a little diffraction peak noted at 27.84º was due to the reflection from the (310) lattice plane of B2O3 (based on ICDD card number 000060297), confirming the formation of the glass-ceramic phase. This peak was further analyzed and well fitted by using Gaussian function for all samples. The consequential fits are presented in Figures 3a, 3b and 3c, respectively. Evident from the said Figures together with Figure 3d affirmed that the crystallite size amplified from 40.30 to 50.00 nm and congruently strain decreased from 0.036 to 0.030 with increasing content of Ho3+ ions. The augmentation in the FWHM (full width at half maximum) and crystallinity stature of our GC originated from the quantum size effect. This remark is indeed consistent with the similar findings reported in Alqarni et al.4 and Gao et al.22 The enhanced crystallite size revealed an enhancement in the arrangement of atoms in the GC matrix with the replacement of phosphorus by holmium. This also indicated the formation of more bridging oxygens in the GC network
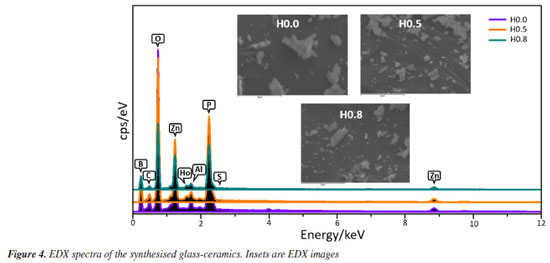
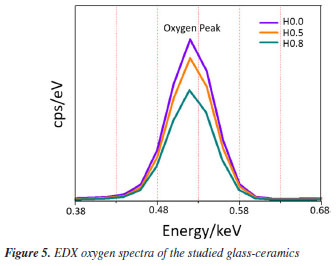
To validate the structural and elemental analysis of our produced glass-ceramics, the typical EDX plot of all samples is shown in Figure 4. An inset unveiled the corresponding EDX images. The EDX scrutiny attests that the elemental constituents harmonized with the initial stoichiometry during the fabrication process. Figure 5 shows the EDX oxygen spectra for H0.0, H0.5 and H0.8 samples. The intensity of the oxygen spectra decreased with the increase of Ho3+ which implied lack of non-bridging oxygen participation due to the formation of more bridging oxygen. This finding is in good agreement with the enhanced crystallite size revealed by XRD.
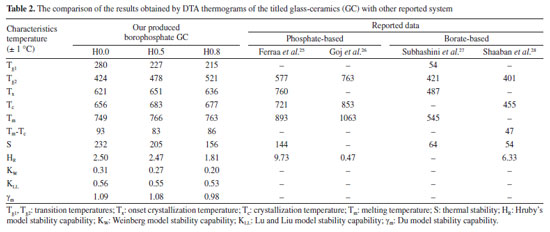
Thermal scrutiny of as-synthesized GC Typically, DTA or DSC results exposed both melting and recrystallization peaks of materials.23 Therefore, the thermal entities of present glass-ceramics were acquired by DTA thermograms and the attained results are presented in Figure 6. Evidently, the Figure 6 revealed relatively dip curve above 600 ºC terming crystallization temperature (Tc). The onset crystallization temperature is denoted by Tx and transition temperature is marked as Tg (Tg1 and Tg2). The melting temperatures (Tm) of the glass-ceramics was also ascertained from the DTA plot. Instantaneously, these parameters (Tg1, Tg2, Tx, Tm) together with thermal stability (S) evaluated by using the given the following expressions previously employed elsewhere24 and the attained data are tabulated in Table 2. Standard deviation was ± 1 ºC for all data. 
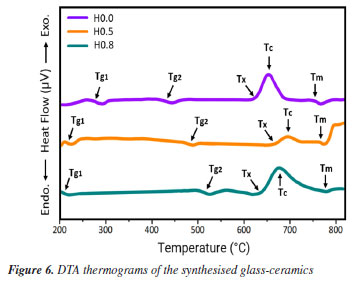
The stability capability of the present glass-ceramics was further computed by employing the following thermal models:29 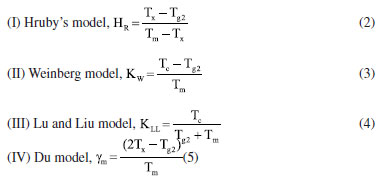 An inclusive result attained herein were compiled in Table 2 and presented in Figure 7, and also validated through a suitable comparison with those obtained for other phosphate and borate-based glasses. The occurrence of two Tg's in this study implied that the samples have two structural phases.30 Assertively, Tg2 value was in the range of the Tg for many phosphate glass systems,31-33 while Tg1 corresponds to the other phase present in the sample. It was found that the value of Tg2 increased with the increase of Ho3+ in good agreement with other analysis in the manuscript. However, the Tg1 values were relatively very low and reduced with the increase of Ho3+. This revelation is common in many plastics, polymers (network structure like ceramic-glass) which are partially crystalline: they, therefore, have a glass transition temperature below which the amorphous phase freezes (somewhat lower due to partial crystallinity). Indeed, it is also an indication of enhancing the crystallinity of the crystalline areas inside the glass-ceramic network. The behavior of Tg1, and also hardness Hv result from a structural changes of the glass-ceramic network with probably the presence of small crystallites B2O3.34
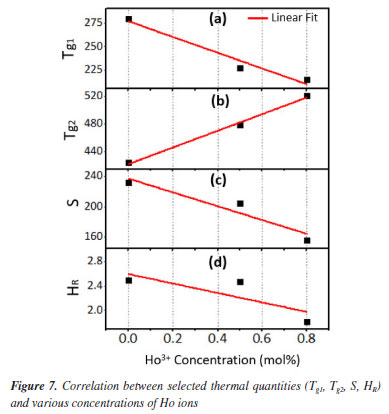
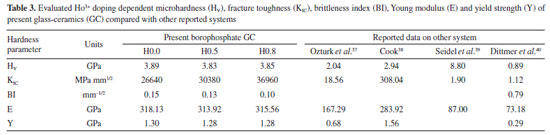
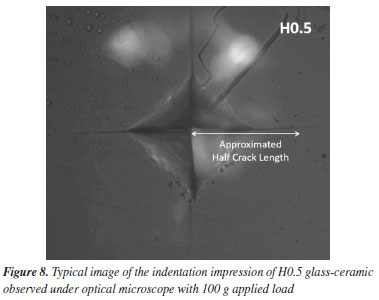
In this study, a decline in Tg1 value was observed with the consecutive addition of Ho3+ content. This may be attributed to the disparity in bond enthalpy values. Replacement of P-O having high bond enthalpy value with HO-O molecules possessing low bond enthalpy value reduced Tg1 value. Meanwhile, both Tg2, Tc, Tm and Tx values increase simultaneously. However, according to the golden rule of calorimetry, the value of the thermal stability must be greater or equal to 100 ºC.35 Undeniably, our produced glass ceramics divulged better values of S ranging from 236 to 156 ºC which is obviously > 100 ºC. Besides, holmium ions free sample coded H0.0 disclosed higher value of S than the H0.5 and H0.8 samples. This implied that H0.0 glass ceramic is more stable than its counterpart. The present findings harmonized with existing calorimetry parameters on other glass and glass-ceramics systems presented in Table 2. Apart from the S feature, the glass ceramic stability potential was further verified through Hruby's, Weinber, Liu and Liu as well as Du models and achieved data are listed in Table 2. Apparently, the thermal stability values evaluated via the aforementioned models are found to be less 3 which further assert good thermal stability of our investigated glass ceramics. Mechanical properties The microhardness of as-produced glass-ceramics was ascertained by using micro indentation hardness testing systems. Following the standard procedure earlier described in the Experimental section together with an in-depth protocol reported in the literature,36,37 the hardness value was deduced by employing the following classic equation of Vickers geometry:  where HV is the Vickers hardness number in kg mm-2, P is the normal load in kg, and d is the average diagonal length of the indentation in mm. Furthermore, other impressive mechanical derivatives including Young modulus (E), yield strength (Y), fracture toughness (KIC) and brittleness index (BI) were also calculated as follows: 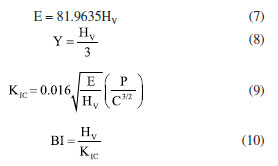 where E denotes elastic modulus, P is the load applied, and c is the crack length measured from the center of the indent. Considering the above formulations, the microhardness (HV), elastic modulus (E), yield strength (Y), indentation fracture toughness (KIC) and brittleness index (BI) of the present glass-ceramics have been evaluated and are enlisted in Table 3. However, the typical image of the indentation impression of H0.5 glass-ceramic sample observed under optical microscope is presented in Figure 8. Evident from Table 3 unveiled that the value of microhardness decreases with Ho doping regime. This declaration is attributed to the impurity phases and irregular grain orientation distribution within the structure of present GC. Besides, E, Y, KIC and BI values decrease meaningfully with increasing Ho doping. The reason behind this is because the aforementioned parameters are highly dependent on the hardness values.36 The matching result reported herein with those attained in similar systems; Bi1.75Pb0.25Sr2Ca2Cu3−xSnxO10+y superconductor ceramics,37 undoped and Ru doped BSCCO glass ceramic38 and MgO/Al2O3/SiO2/ZrO2 glass-ceramics39 is compiled in Table 3.
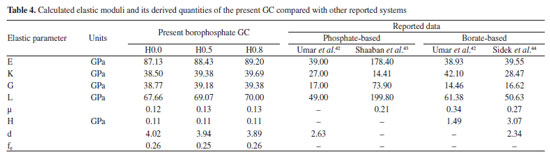
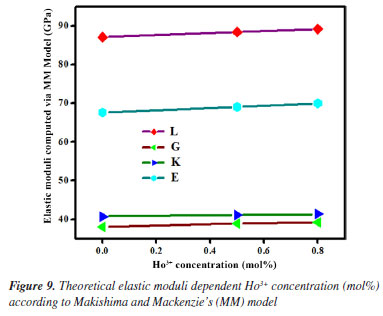
Theoretical evaluation of elastic moduli by using Makishima and Mackenzie's model By invoking the ionic the values of packing density (PD) and dissociation energy Gt, the elastic moduli namely, Young (E), bulk (K), shear (G) and longitudinal (L) modulus as well as its derived parameters such as micro-hardness (H), Poisson ratio (μ), fractal bond connectivity (d) and Fugacity (fe) of the current GC were theoretically computed by adopting Makishima and Mackenzie's (MM) model as follows:41 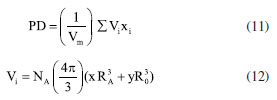 where RA and R0 are the ionic radius of metal and oxygen, respectively. 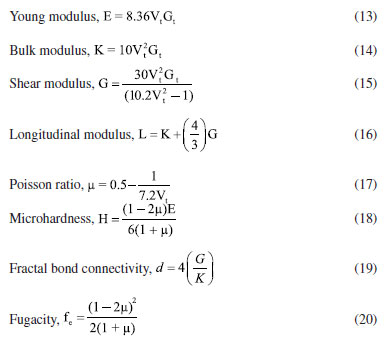 By using the above-described formulations, the elastic moduli and its consequential entities were evaluated and enlisted in Table 4. Figure 9 typified the correlation between elastic moduli and Ho3+ contents in the present GC. Furthermore, a comparison between our findings with existing data on other borate and phosphate-based glass system has been established. Observably, the elastic moduli including E, G, K and L slightly increase with the increase in the H2O3 content. The noted upsurge in these parameters is attributed to the growth in the median bonds per unit volume together with cross-link density.45 Similarly, increment in Ho3+ content may augment the Coulomb factor of the lattice energy in the glass-ceramic system. However, large discrepancy between L and G moduli was observed which may be accredited to the volume effects. Rationally, resistance to penetration or indentation of any depends on it micro-hardness.46 The unchanged behavior observed in micro-hardness values signified a constant rigidity and network connectivity in the present glass ceramic.
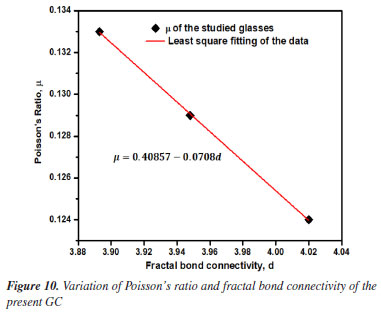
Basically, the variation of the Poisson's ratio (μ) in glass-ceramics reveal the dimensionality changes in its network. It has been established that the glass-ceramic network structure with 0.1 to 0.2 value of μ implied high cross-link density in the network whereas those within 0.3 to 0.5 range of μ specified low cross-link density in their network. Evidently, our attained value of μ ranging from 0.12 to 0.13 affirmed high cross-link density in the studied glasses.47 Besides, the evaluation of the effective dimension of certain materials for any disorderly mixture of fluid and solid backbone near the percolation limit as proposed by Bergman and Kantor48 by using fractal bond connectivity (d) analysis classified network dimension as follows: for a 2-dimensional network, d value falls around 2.0 meanwhile the 1-dimensional glass network structure is symbolized by d value around 1.0 and for a 3-dimensional network, the d value is around 3.0 to 4.0. In the studied glasses, d-value for all glasses found to be ~4.0 which certifies their 3D network. However, careful assessment of μ and d trend (see Table 4) testified that the network dimensionality (d) of the present borophosphate based glass-ceramic network is decreasing while the Poisson's ratio is almost constant with increasing concentration of Ho3+. This disclosure coincides with the proposed semi-empirical formulae μ = A - zd,49 which is well fitted to the curve epitomized in Figure 10 and yielded μ = 0.40857 - 0.0708d. The achieved values for the two constants A and z further validate the earlier claim with σ = 0.45 - 0.083D in oxyfluoro-zinc-tellurite.50 Another vital quantity herein examined is the fugacity defined as the fluctuation of free volume of a system.51 This unit play a very crucial role molecular dynamics of liquids. It detects the degree of rigidity of the glass network. A mathematical model by great scientist called Williams-Landel-Ferry (WLF) was proposed to describe the viscosity of inorganic glasses and their structural properties.52 Therefore, careful examination of Table 4 revealed that the values of fugacity remain almost constant. This indeed reinforced the outcome from micro-hardness and the Poisson ratio.
CONCLUSIONS In this study, some new kind glass-ceramic (GC) with stoichiometric composition of ZnSO4-B2O3-P2O5-Ho2O3 were produced via melt-solidified method to ascertain the impact of Ho2O3 contents on thermal variables and mechanical properties. The overall findings unveiled high values of thermal stability (> 150ºC) and fracture toughness (> 20000MPa mm1/2). Furthermore, by sensibly replacing P2O5 with Ho2O3, both crystallite size and elastic moduli values were remarkably enhanced. Thus, the obtained results suggest the possibility of using the current GC in protective covers of portable electronics.
ACKNOWLEDGMENTS Authors appreciate Princess Nourah Bint Abdulrahman University Researchers Supporting Project Number (PNURSP2025R479), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
REFERENCES 1. Guo, Y.; Li, J.; Zhang, Y.; Feng, S.; Sun, H.; iScience 2021,24, 102735. [Crossref] 2. Acikgoz, A.; Ceyhan, G.; Aktas, B.; Yalcin, S.; Demircan, G.; J. Non-Cryst. Solids 2021, 572, 121104. [Crossref] 3. Aktas, B.; Yalcin, S.; Albaskara, M.; Aytar, E.; Ceyhan, G.; Turhan, Z. Ş.; J. Non-Cryst. Solids 2022, 584, 121516. [Crossref] 4. Alqarni, A. S.; Hussin, R.; Alamri, S. N.; Ghoshal, S. K.; Ceram. Int. 2020, 46, 3282. [Crossref] 5. Aktas, B.; Acikgoz, A.; Yilmaz, D.; Yalcin, S.; Dogru, K.; Yorulmaz, N.; J. Nucl. Mater. 2022, 563, 153619. [Crossref] 6. Zhao, Q.; Li, Z.; Zhu, Y.; Inorganics 2023, 11, 187. [Crossref] 7. Hallmann, L.; Ulmer, P.; Kern, M.; J. Mech. Behav. Biomed. Mater. 2018, 82, 355. [Crossref] 8. Zhang, H.; Liu, J.; Shi, F.; Zhang, H.; Yuan, X.; Li, Y.; Li, T.; Zhao, X.; Wang, M.; Ceram. Int. 2023, 49, 216. [Crossref] 9. Pereira, R. M.; Ribas, R. G.; Montanheiro, T. L. D. A.; Schatkoski, V. M.; Rodrigues, K. F.; Kito, L. T.; Kobo, L. K.; Campos, T. M. B.; Bonfante, E. A.; Gierthmuehlen, P. C; Spitznagel, F. A.; Thim, G. P.; J. Appl. Oral Sci. 2023, 31, e20220421. [Crossref] 10. Wang, F.; Liu, J.; Wang, Y.; Liao, Q.; Zhu, H.; Li, L.; Zhu, Y.; J. Nucl. Mater. 2020, 531, 151988. [Crossref] 11. Jiang, D.; Chen, J.; Lu, B.; Xi, J.; Chen, G.; J. Mater. Sci: Mater. Electron. 2018, 29, 18426. [Crossref] 12. Haily, E.; Bih, L.; Jerroudi, M.; Azrour, M.; El bouari, A.; Manoun, B.; Mater. Today: Proc. 2021, 37, 3798. [Crossref] 13. Węgrzyk, S.; Herman, D.; Pancielejko, M.; J. Non-Cryst. Solids 2022, 582, 121443. [Crossref] 14. Solak, B. B.; Aktas, B.; Yilmaz, D.; Kalecik, S.; Yalcin, S.; Acikgoz, A.; Demircan, G.; Mater. Chem. Phys. 2024, 312, 128672. [Crossref] 15. Acikgoz, A.; Izguden, I.; Tasgin, Y.; Yilmaz, D.; Demircan, G.; Kalecik, S.; Aktas, B.; Ceram. Int. 2024, 50, 34573. [Crossref] 16. Alqarni, A. S.; Hussin, R.; Alamri, S. N.; Ghoshal, S. K.; Results Phys. 2020, 17, 103102. [Crossref] 17. Venkateswarlu, M.; Mahamuda, S.; Swapna, K.; Prasad, M. V. V. K. S.; Rao, A. S.; Shakya, S.; Babu, A. M; Prakash, G. V.; J. Lumin. 2015, 163, 64. [Crossref] 18. Gupta, G.; Bysakh, S.; Balaji, S.; Khan, S.; Biswas, K.; Allu, A. R.; Annapurna, K.; Adv. Eng. Mater. 2020, 22, 1901357. [Crossref] 19. Reddy, A. S. S.; Purnachand, N.; Kostrzewa, M.; Brik, M. G.; Venkatramaiah, N.; Kumar, V. R.; Veeraiah, N.; J. Non-Cryst. Solids 2022, 576, 121240. [Crossref] 20. Alqarni, A. S.; Hussin, R.; Alamri, S. N; Ghoshal, S. K.; J. Lumin. 2020, 223, 117218. [Crossref] 21. Naseer, K. A.; Marimuthu, K.; Vacuum 2021, 183, 109788. [Crossref] 22. Gao, Y.; Chen, J.; Yang, Y.; Zhou, D.; Qiu, J.; J. Eur. Ceram. Soc. 2023, 43, 3591. [Crossref] 23. Amrouch, S.; Velázquez, M.; Chalal, M.; Guyot, Y.; Belhoucif, R.; Lamrous, O.; Mater. Today Commun. 2022, 32, 104077. [Crossref] 24. Lu, J.; Wang, H.; Li, Y.; Zhou, Y.; Jiang, W.; Ceram. Int. 2023, 49, 7737. [Crossref] 25. Ferraa, S.; Barebita, H.; Moutataouia, M.; Nimour, A.; Elbadaoui, A.; Baach, B.; Guedira, T.; Chem. Phys. Lett. 2021, 765, 138304. [Crossref] 26. Goj, P.; Ciecińska, M.; Szumera, M.; Stoch, P.; J. Therm. Anal. Calorim. 2020, 142, 203. [Crossref] 27. Subhashini; Shashikala, H. D.; Udayashankar, N. K.; J. Alloys Compd. 2016, 658, 996. [Crossref] 28. Shaaban, K. S.; Saddeek, Y. B.; Sayed, M. A.; Yahia, I. S.; Silicon 2018, 10, 1973. [Crossref] 29. Monisha, M.; Murari, M. S.; Sayyed, M. I.; Al-Ghamdi, H.; Almuqrin, A. H.; Lakshminarayana, G.; Kamath, S. D.; Mater. Chem. Phys. 2021, 270, 124787. [Crossref] 30. Mohamed, E. A.; Ratep, A.; Abdel-Khalek, E. K.; Kashif, I.; Appl. Phys. A: Mater. Sci. Process. 2017, 123, 479. [Crossref] 31. Intawin, P.; Panyata, S.; Kraipok, A.; Tunkasiri, T.; Eitssayeam, S.; Pengpat, K.; Thermochim. Acta 2020, 690, 178699. [Crossref] 32. Li, X.; Yang, H.; Song, X.; Wu, Y.; J. Non-Cryst. Solids 2013, 379, 208. [Crossref] 33. Mandlule, A.; Döhler, F.; van Wüllen, L.; Kasuga, T.; Brauer, D. S.; J. Non-Cryst. Solids 2014, 392-393, 31. [Crossref] 34. Ducel, J. F.; Videau, J. J.; Mater. Lett. 1993, 18, 69. [Crossref] 35. Hirdesh; Khanna, A.; J. Lumin. 2018, 204, 319. [Crossref] 36. Koralay, H.; Arslan, A.; Cavdar, S.; Ozturk, O.; Asikuzun, E.; Gunen, A.; Tasci, A. T.; J. Mater. Sci.: Mater. Electron. 2013, 24, 4270. [Crossref] 37. Ozturk, O.; Asikuzun, E.; Tasci, A. T.; Gokcen, T.; Ada, H.; Koralay, H.; Cavdar, S.; J. Mater. Sci.: Mater. Electron. 2018, 29, 3957. [Crossref] 38. Cook, R. F.; J. Am. Ceram. Soc. 2020, 103, 2278. [Crossref] 39. Seidel, S.; Dittmer, M.; Höland, W.; Rüssel, C.; J. Eur. Ceram. Soc. 2017, 37, 2685. [Crossref] 40. Dittmer, M.; Yamamoto, C. F.; Bocker, C.; Rüssel, C.; Solid State Sci. 2011, 13, 2146. [Crossref] 41. Abd El-Moneim, A.; El-Mallawany, R.; Saddeek, Y. B.; J. Non-Cryst. Solids 2022, 575, 121229. [Crossref] 42. Umar, S. A.; Halimah, M. K.; Azlan, M. N.; Grema, L. U.; Ibrahim, G. G.; Ahmad, A. F.; Hamza, A. M.; Dihom, M. M.; SN Appl. Sci. 2020, 2, 291. [Crossref] 43. Shaaban, K. S.; Wahab, E. A. A.; Shaaban, E. R.; Yousef, E. S.; Mahmoud, S. A.; J. Electron. Mater. 2020, 49, 2040. [Crossref] 44. Sidek, H. A. A.; El-Mallawany, R.; Matori, K. A.; Halimah, M. K.; Results Phys. 2016, 6, 449. [Crossref] 45. Issa, S. A. M.; Saddeek, Y. B.; Tekin, H. O.; Sayyed, M. I.; Shaaban, K. s.; Curr. Appl. Phys. 2018, 18, 717. [Crossref] 46. Laopaiboon, R.; Bootjomchai, C.; Glass Phys. Chem. 2015, 41, 352. [Crossref] 47. wshah, A. A. A.; Halimah, M. K.; Alazoumi, S. H.; Umar, S. A.; Ibrahim, G. G.; Mater. Chem. Phys. 2021, 260, 124195. [Crossref] 48. Bergman, D. J.; Kantor, Y.; Phys. Rev. Lett. 1984, 53, 511. [Crossref] 49. Abd El-Moneim, A.; El-Mallawany, R.; J. Non-Cryst. Solids 2019, 522, 119580. [Crossref] 50. Abd El-Moneim, A.; J. Fluorine Chem. 2019, 217, 97. [Crossref] 51. Tafida, R. A.; Halimah, M. K.; Muhammad, F. D.; Chan, K. T.; Onimisi, M. Y.; Usman, A.; Hamza, A. M.; Umar, S. A.; Mater. Chem. Phys. 2020, 246, 122801. [Crossref] 52. Ojovan, M. I.; Molecules 2020, 25, 4029. [Crossref]
Editor handled this article: Eduardo H. S. Sousa |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access