Educação
| Laboratory electrochemistry "mystery box": from pre-service teachers' observations to inferences through predict-observe-explain strategy |
|
Carla MoraisI,* I. Science Teaching Unit, Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto, 4169-007 Porto, Portugal Received: 03/21/2024 *e-mail: cmorais@fc.up.pt Given the challenges associated with understanding electrochemistry content by engaging in prediction, observation, and explanation, laboratory activities can foster active participation and critical thinking, enabling individuals to proactively confront and revise their understanding. Since pre-service teachers should gain firsthand experience with predict-observe-based on a predict-observe-explain strategy, we propose a laboratory activity presented as a "mystery box" related to concentration cells coupled with an Arduino-based electronic data measurement system to identify how pre-service chemistry teachers move from observations to inferences in a qualitative research. Data was collected by written records and oral explanations during the practical interactions. Results suggest that the proposed laboratory activity allows pre-service chemistry teachers to access data and correct inferences related to electrochemistry content, promoting critical thinking. Data also showed a connection between the macroscopic and symbolic domains, and pre-service teachers recognized concepts associated with the activity, such as solution conductivity and the potential difference in chemical reactions, interpreting the system with prior knowledge, like electron flow direction and concentration cell components. However, additional strategies are needed for detailed and consistent observation and inference recording, enhancing the potential of such activities in favoring electrochemistry education in high schools. INTRODUCTION Different research on chemistry teaching and learning processes has shown that searching for methodologies and pedagogical approaches to simulate the scientific process in basic education is fundamental.1-3 Over the past two decades, a growing body of evidence has underlined the importance of science educators accessing the prior knowledge and misconceptions held by students regarding scientific concepts and phenomena.4,5 In parallel, more techniques for identifying and addressing students' beliefs and working with conceptual changes regarding learning have been developed.1,6,7 Conceptual change is understood here as conceptual profile change as elaborated by Mortimer,8 although many texts still reference the first term. In fact, the limitations of route learning have become apparent, demanding a shift towards more engaging and interactive methods aimed at promoting competency in scientific inquiry. Learning can be conceptualized as a transformative process that favors understanding natural phenomena, leading to conceptual profile change.9 Under the specificity of chemistry as an experimental and observational science, exploring different theoretical and practical perspectives in teaching and teacher education and training is therefore essential for creating broad possibilities to work with the complexity of chemical knowledge and scientific construction. Electrochemistry is a challenging subject for both students and teachers, often leading to misconceptions such as misunderstanding electron flow in electrolyte solutions and misattributing charges to electrodes, which prevent proper understanding and explanation of electrochemical phenomena. The concept of concentration cells, for example, suffers from similar educational hurdles. Predict-observe-explain (POE) is an effective pedagogical strategy to address these misconceptions by promoting active engagement, critical thinking, and conceptual profile change. Besides, collaborative laboratory activities that incorporate technological tools and encourage reflective discussions can enhance learning, communication skills, and metacognitive awareness, leading to a deeper understanding of electrochemistry concepts. Our work is situated within this perspective. We investigated how pre-service chemistry teachers report their observations and make inferences during a predict-observe-explain-based laboratory activity related to electrochemistry content, presenting an overview of this strategy and aspects related to electrochemistry teaching. Learning by the predict-observe-explain strategy Among pedagogical tools, educational strategies such as POE stand out for their capacity to promote active learning and deeper conceptual understanding, and are still considered important in education today.10,11 POE is a teaching strategy that strives to involve students actively in predicting the results of an action (an experiment, for example) before its execution, in observing or collecting data, and in creating hypotheses and explanations for the phenomenon.12-17 This strategy was elaborated considering various aspects related to students' pre-existing conceptions and knowledge to facilitate new understandings, enable the diagnosis of ideas to guide teaching proposals, and, beyond the acquisition of content knowledge, foster the development of scientific skills.18-20 Within chemistry education, engaging in POE-based activities is associated with overcoming misconceptions and effecting conceptual profile change via deeper understanding of the interplay between macroscopic, submicroscopic, and symbolic dimensions.21 By engaging students in predicting outcomes, observing phenomena, and subsequently explaining the observed results, POE generates a sense of inquiry and discovery in the classroom environment.5,10 Numerous researchers have proposed instructional modules employing the POE strategy to address chemistry concepts such as chemical equilibrium,22 while investigating the feasibility of implementing it. In other cases, researchers tackle misconceptions associated with electrochemistry23 or address its interplay with general chemistry knowledge13 while investigating the feasibility of their proposed activities. Although variations on the POE strategy, such as predict-observe-explain-explore (POEE), have been shown to improve student achievement and attitude toward Chemistry learning,7 integrating POE into the school curriculum remains an underexplored possibility with few studies examining how students perceive POE chemistry laboratory tasks.24-26 A unifying theme across these studies is their focus on both concept development and creating links between observational and inferential processes and their impact on learning. This exploration seeks to understand how these cognitive processes influence educational outcomes, underlining a holistic approach to understanding scientific concept acquisition. Central to this investigation is the pivotal role of observation, serving as the core of scientific inquiry and discovery.27 Its significance extends beyond mere data acquisition, permeating every facet of scientific inquiry and theory development. Heuristic scientific observation serves as both a catalyst and compass in the pursuit of knowledge.28,29 Building upon the significance of experimentation in science education, differentiating between observations and inferences assumes reinforced importance in generating a comprehensive understanding of scientific concepts, particularly in Chemistry. Another aspect concerns the misconceptions that often arise from a lack of verticalization between the macroscopic and submicroscopic levels of chemistry, increasing the challenge of relating observations to inferences.28 Issue is also discussed in other works21 outlining the relation between observation and inference processes in constructing meaning for chemical content at different representation levels, helping to overcome misconceptions. This underlines the need to establish coherent relations between empirical observations and theoretical constructs, especially when traditional laboratory classes aim to prove scientific truths and laws that may disrupt the continuum between empirical and theoretical chemical concepts.30,31 In the pedagogical landscape of chemistry teaching, laboratory-based practices serve as indispensable tools for improving experiential learning and conceptual understanding. By means of carefully designed experiments, students can engage with scientific phenomena firsthand thereby bridging the gap between theoretical knowledge and empirical observations. Moreover, integrating theoretical mediators such as concepts, representations, and symbolic models, aids the transition from macroscopic observations to submicroscopic interpretations.21,32 However, the effectiveness of laboratory-based methods, here understood as the capability to foster connections between different chemical knowledge domains, depends on the nuanced interplay between observation and inference, which is at the heart of scientific inquiry.2 As students grapple with experimental data and theoretical frameworks, they are asked to discern between empirical observations and the inferences drawn from them, a process that demands critical thinking and analytical reasoning. Fostering a holistic perspective of scientific thinking requires not only constructing knowledge from observations but also synthesizing disparate pieces of information into coherent conceptual frameworks.33,34 POE also incorporates principles from other strategies and approaches investigated in chemistry teaching such as inquiry-based science education, which emphasizes student-driven investigations, and active learning.35 Moreover, studies36-38 highlight the importance of developing discussion, negotiation, and critical thinking skills in science courses. Thus, pre-service teachers need to experience and engage firsthand with POE strategies and activities which can later be transferred to their professional contexts thus enhancing the development of these skills in their students. Collaborative efforts between educators, curriculum developers, and textbook writers are essential in integrating POE elements into the curriculum, thereby enriching science education and empowering students to become active participants in their learning process. Electrochemistry teaching and learning Given the conceptual development opportunities afforded by engaging in POE-centered activities, one should investigate them by incorporating topics and concepts that pose learning challenges. It this context, we endeavor to focus on the topic of electrochemistry in our educational pursuits. Electrochemistry poses significant challenges for both student learning and teacher training. regarding representations of contents related to electrochemistry concepts.39-42 The field is often perceived as one of the most daunting in chemistry, with students grappling to reconcile their intuitive beliefs with scientific principles.43 Common misconceptions such as the notion that electrons can flow through electrolyte solutions or that electrodes possess a positive or negative charge, thus explaining ion and electron flow, underline the need for targeted instructional strategies to address and rectify these erroneous notions.44,45 Few studies focus on the approach to concentration cells, despite teaching of this concept incurring in the same misconceptions and involving aspects related to the chemical balance of the system, the influence of ionic concentration on the voltage produced, electron flow and the Nernst equation.45,46 Misconceptions represent conceptual and propositional knowledge that diverges from the scientific consensus hindering students ability to understand and explain observable electrochemistry phenomena.47 Perceived misconceptions and learning apply not only to college and advanced chemistry students but also to future educators. Hence works that seek to identify misconceptions and create teaching models to overcome these difficulties, with the POE strategy being one possible perspective.48-50 We highlight this set of misconceptions and their occurrence in different educational contexts because our investigation sought to understand how some of these present themselves and how training action strategies can help mitigate them. In response to the pervasiveness of electrochemistry knowledge, a set of pedagogical strategies has been proposed for learning concepts through non-expository active strategies with greater student interaction.51-53 POE emerges as a pedagogical tool for fostering conceptual profile change and improving students' understanding of concepts and phenomena. Studies23,54 have demonstrated the potential of POE-based activities in mitigating misconceptions and enhancing students' learning abilities in electrochemistry. By engaging students in outcome prediction, observation of experimental results, and subsequent explanation of observed phenomena, POE-based activities encourage active engagement and critical thinking enabling students to confront and revise their assumptions. The use of diagnostic instruments such as pre- and post-tests allows educators to proactively identify and address students' misconceptions, laying the groundwork for meaningful learning experiences in electrochemistry.43,47 In light of this, we see POE as a valuable strategy for exploring electrochemistry concepts, particularly the laboratory construction of concentration cells. It underlines the significance of devising activities that foster observation, hypothesis formulation, and inferences for the application of scientific knowledge. Conceptualizing a laboratory activity encompasses a variety of experiments where observations are derived from visual, sensory, or data acquired by assorted sensors. The experimental framework can be enhanced by integrating technological tools like automated data collection systems. If the activity encourages information exchange and collaborative efforts, it can lead to further beneficial outcomes. By engaging in reflective discussions and sharing insights with peers, students further develop their conceptual understanding of electrochemistry phenomena while honing their communication and teamwork skills.55 Integrating reflective practices into group work activities fosters metacognitive awareness, empowering students to monitor their learning progress and adapt their strategies accordingly.56 Given the theoretical background and difficulties associated with electrochemistry learning, inviting participants to work in prediction, observation, and explanation activities can encourage active engagement and critical thinking, enabling them to confront and revise their knowledge proactively. Moreover, collaborative learning in laboratory classes fosters communication skills and enables dynamic interaction throughout the activity. To integrate these elements, we proposed a laboratory activity presented as a "mystery box," involving experimentation with concentration cells coupled to a low-cost electronic data measurement system developed with the Arduino system. Our objectives were:
Closely related to the objectives, our research asked: "How does a "mystery box" laboratory implemented through POE enable pre-service teachers to work with observations to make inferences associated with electrochemistry content?". The laboratory activity conducted is presented below, as well as the study's research design, data collection, and analysis procedures.
METHODOLOGY "Mystery box" laboratory activity The laboratory activity proposed was an investigation centered on a "mystery box" designed to involve participants in exploring electrochemical concepts. It tasked participants with discovering the contents of a sealed box containing a concentration cell (Figure 1), whose elements were completely concealed. The setup consisted of three beakers with solutions A, B, and C, a cardboard box from which two cables came out and were connected to an electrical circuit and Arduino, and an electrical plug that acted as a pair of electrodes for measuring electrical conductivity. Beaker A contained a potassium chloride solution to be used for calibrating the electrical conductivity measurement system. Beakers B and C contained solutions of the same ionic substance (copper sulfate, or zinc sulfate, or aluminum sulfate) at different concentrations. Inside the box was a concentration cell made up of two electrolytes equal to solutions B and C. The Arduino system was designed and assembled with three buttons that allowed for three operations: (1) calibration; (2) measurement of electrical conductivity; (3) measurement of electrical voltage. Electrical voltage measurement was collected through cables coming out of the "mystery box".
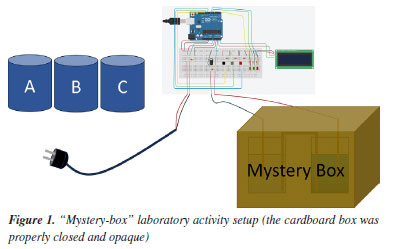
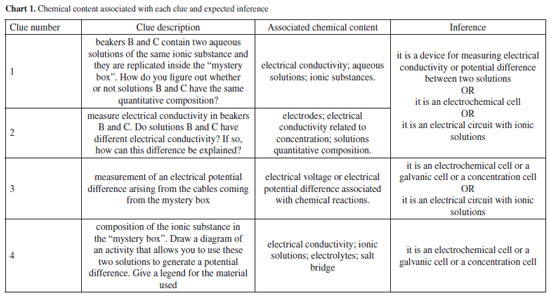
During the activity, the participants, working in groups of two or three, received information (e.g., measurements or data related to electrochemistry) or collected data along with questionings at each step - thereby referred to as clues - to propose inferences about the content of the "mystery box". In this context, the term "clues" refers to the information provided to participants or the data acquired through various measurements, which collectively form the empirical observations under consideration. During the activity, participants recorded their observations and inferences at three different moments: (i) at the end of clues 1 and 2, (ii) at the end of clue 3, and (iii) at the end of clue 4. Their content will be described in the "Data collection and analysis" sub-section. After recording the final observations and inference, participants gave an oral explanation describing the rationale behind the observations up to the final inference. Finally, the "mystery box" was opened and the participants were asked to reconstruct the concentration cell. Research design We conducted a qualitative research study to investigate how pre-service chemistry teachers report their observations and make inferences during a "mystery-box" laboratory activity. In this endeavor, we sought to delve deeper into the experiences and perspectives of pre-service chemistry teachers as they engaged in the activity. Through written records in the activity protocol and their oral rationale for the observations and inferences, we sought to uncover the intricate processes involved in their observational skills, inference formulation, and understanding of electrochemistry concepts. By exploring the qualitative aspects of their experiences, we intended to shed light on the underlying factors influencing their teaching practices and professional development. The study sample consisted of 20 pre-service chemistry teachers enrolled in the Master's program in Physics and Chemistry Teaching at the University of Porto, comprising 7 men and 13 women between 21 and 55 years old. Half of the participants were enrolled in the first year, and the other half in the second year of a two-year course. These participants are studying to become middle and high school physics and chemistry teachers. Before beginning, participants were informed of the research content and duration, its voluntary nature, and that they could withdraw from the study at any time. Additionally, they were assured that their identity would be kept confidential. Participants also voluntarily signed an informed consent form. Data collection and analysis Data were collected by means of written records in the activity protocol and an oral rationale of the observations and inferences produced by the participants. To maintain anonymity and avoid disclosing names, participants were identified by a code consisting of a letter followed by two digits. First-year participants were assigned the letter A, and second-year students were assigned the letter B. We followed a two-step analysis procedure. First, we organized the participants' responses based on the expected answer for each clue provided. One of the researchers coded the expected responses and associated the respective responses. This was then validated in regular meetings with all four researchers. Each clue was designed to allow some concepts to be listed by the participants, as illustrated in Chart 1. At each stage, participants were asked to record their observations and propose inferences about the contents of the "mystery box." For clues 1 and 2, a broader scope of inferences was deemed appropriate due to the insufficient information provided to deduce the precise contents of the box. After clues 3 and 4, the inferences deemed pertinent were exclusively those relating to electrochemical cells or concentration cells.
Subsequently, each participant was asked to give an oral explanation of their reasoning, describing an assembly schematic drawn by them according to their final hypothesis of the "mystery box" contents. After the "mystery box" was revealed, they were asked to compare it with the previous diagram and orally explain the differences, if applicable. Finally, the participants were asked to reconstruct the concentration cell using materials provided by the researcher. To assess the activity implementation and the participants' use of observations to make inferences based on electrochemistry knowledge, we analyzed the written records in the activity protocol related to observations and inferences, as well as the transcript of the oral rationale. Second, we categorized the audio transcripts of the oral responses according to procedures proposed in the literature, which involves selecting and preparing texts, reading and identifying relevant sections, and categorizing highlighted excerpts into thematic clusters with shared meanings.57 We established, as a priori categories, the use of concepts expected after each clue and the use of concepts not expected, but related to electrochemistry topics. The categories were defined as follows: (C1) concepts associated with clues 1 and 2; (C2) concepts associated with clue 3; (C3) concepts associated with clue 4; (C4) additional concepts were included by the participants but not directly anticipated, considering their indirect relation to the clues. Data discussion considers various aspects related to inquiry teaching, the POE strategy, and the challenges associated with teaching and learning electrochemistry.
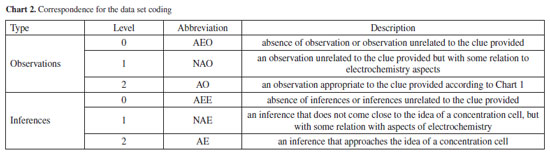
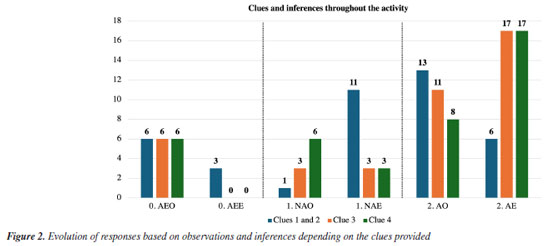
RESULTS AND DISCUSSION Given our objectives of evaluating the implementation of the proposed "mystery box" laboratory activity and identifying how the pre-service teachers work with observations to create inferences, we first analyzed their written records of observations and inferences in the activity protocol and then the oral explanations to verify the knowledge mobilized. Analysis of observations and inferences recorded by participants This section presents the results of the proposition of observations and inferences made by the participants. The table with the participants' individual evaluations is available in the Supplementary Material. The term "clue" used in this study refers to a measurement or data provided to participants at each activity step related to content on electrochemistry (Chart 1). We coded the data sets through three levels from 0 to 2 (Chart 2). Figure 2 provides a detailed summary of the number of participants who recorded observations and inferences in each activity step.
As observed, the absence of recordings remains constant throughout the process, showing that participants postponed recording their observations for each stage. Importantly, the process of observation recording, important in learning science, can often not receive due attention due to several factors. The quality of the observations tends to decrease, with 13 participants making appropriate observations after clues 1 and 2 and only 11 and 8 after clues 3 and 4, respectively. One possible explanation for this result is the increased complexity of the information being processed. Another hypothesis is that participants consider their formulated inferences appropriate and thus feel it unnecessary to indicate observations or repeat inferences. This data aligns with other findings which indicate that in inquiry-based activities participants may prioritize reaching the final answer over engaging with the intermediate steps.38 Given this possible limitation, as a way of not losing the connections between the clues and emerging knowledge, in the last part we asked participants to provide a complete description of their reasoning and inferences. However, developing strategies for students to keep written records throughout the process is a point of necessary improvement. Conversely, inferences increase in quantity and quality when moving from clues 1 and 2 to clues 3 and 4, with 17 participants adequately inferring that the contents of the "mystery box" were a concentration cell or something close. To confront the expected knowledge presented by the clues with the participants' knowledge, we have included some excerpts of their observations. The full list of observations and inferences is featured in the Supplementary Material. Clue 1 (beakers B and C contain two aqueous solutions of the same ionic substance and are replicated within the "mystery box") asked participants "How do you find out whether or not solutions B and C have the same quantitative composition?". With this, students are driven to create predictions and test possibilities, mobilizing knowledge about conductivity and concentration. For clue 2, participants measured the conductivity of the solutions to answer "Do solutions B and C have different electrical conductivity? If so, how can this difference be explained?". Upon interrogation regarding the observation rationale, participants were expected to attribute the variance to differences in concentration, premised on the pre-existing knowledge that the solutions had the same ionic compound. Within this framework, the "prediction" phase may require mobilization of pre-existing knowledge, "observation" refers to the juxtaposition of this knowledge against macro-level empirical data (conductivity measurements), and "explanation" implies the elucidation of the observed phenomenon. Despite the structured nature of the activity, few responses went beyond the anticipated inferences, with only six participants producing level 2 inferences:
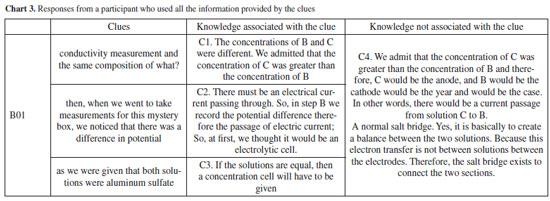
Participant B12 drew a diagram of an electrochemical cell without identifying what is connected between the two electrodes: whether a voltmeter, a voltage source, or another device. Despite insufficient data to definitively ascertain whether the study subject is a concentration cell or an electrochemical device, measuring conductivity differences linked to concentration variations enables the formulation of inferences grounded in electrochemical principles. Since the correlation between concentration and conductivity or potential differences in electrolytic systems is considered a challenging concept in electrochemistry teaching, this complexity might contribute to the limited frequency of accurate inferences.3,58,59 Hence, additional data may be needed during the observation and explanation processes to be correlated and influence conclusions. Participants may have initially doubted whether the "mystery box" contained a concentration cell or another electrochemical device. However, measuring conductivity differences associated with variations in concentration would have provided a crucial parameter to steer participants toward the correct inference. Of the eleven inferences classified as 1, six were justified by insufficient data to produce an inference, and the remaining three presented inferences not directly related to a concentration cell but with some electrochemical concepts: B09: Consisting of solutions B and C, it could be an instrument that allows you to carry out some measurements. B05: Two ionic solutions similar to B and C. B10: Is it a mixture of the two solutions? Considering our objectives, we can highlight that clues 1 and 2 may have enhanced the mobilization of knowledge expected from participants. Although more evidence may be necessary to establish greater clarity about the mobilization of domains, we can make the initial inference that some macroscopic (tangible electrical conductivity data) and submicro (quantity of charge carriers in solution) aspects were mobilized, and this aspect is important in evaluating the activity since the experiment involves a concentration cell. Even if the participants failed to express a complete inference, the association of macroscopic content with submicro content may have minimized the possibility of carrying misconceptions into the next steps,21 in this specific case, the concept that concentration and conductivity are related. Additionally, even if the inference is inadequate from the point of view of the "mystery box" content, it can generate a conceptual reformulation in the next step, which according to the POE strategy is a fundamental aspect of conceptual development.24 After clue 3, level 2 observations associated a potential difference measurement in a specific temperature, as expected. Answers classified as 1 failed to define this magnitude or, in the case of participant A10, stated that the "mystery box" voltage was low. We observe an increase in inferences that associate the contents of the "mystery box" with a concentration cell or a similar electrochemical system (electrochemical cell, galvanic cell, electrical circuit with ionic solutions, device for measuring the potential difference between two solutions). This was expected since a system containing solutions with different concentrations and registering a potential difference measurement can be associated with an energy source. Overall, after clue 4 a total of 17 participants correctly inferred that the "mystery box" contained a concentration cell or something related. Recordings of participants B03 and B05 illustrate this fact: B03: The potential difference between solutions B and C was measured, as the replicated solutions were inside the mystery box. Inside the box, there must be something dipped in each of the solutions connected to the crocodiles and there must be a salt bridge between the two solutions. There must be an electrode (anode and cathode) and then a salt bridge. B05: E0: standard reduction potential. One electrode is at B and the other at C. Since there is a d.d.p., there is an electric current passing. Interestingly, some participants cited other electrochemical elements and concepts, such as the "salt bridge between the two solutions", "electrode (anode and cathode)" or "standard reduction potential": B06: The potential difference between the two solutions, the same as solutions B and C, was measured. Inside the mystery box, there is a salt bridge between the two solutions, and an anode and a cathode are immersed in each solution and connected to each other. An Arduino wire is connected to each electrode (anode and cathode) to read and record the values, converting them into a potential value and the Arduino automatically calculates the potential difference → 32.21 mV. Based on their inferences to predict the hidden assembly, participants were tasked with sketching a diagram representing a setup that would allow them to use the solutions in beakers B and C to generate a potential difference. Additionally, they were prompted to name or otherwise identify their diagram. Subsequently, participants progressed to the final step and were confronted with the contents of the "mystery box". Upon observing the materials of the concentration cell setup, participants attempted to list the materials, describe the electrochemical assembly, and assess whether there were any differences compared to their proposed prediction following clue 4. Eight participants introduced other concepts about electrochemistry (A02, A04, A05, B03, B06, B09, B11, B12) such as redox reactions, cathode and anode: B03: There are 2 solutions, C being (+) concentrated and solution B being (-) concentrated. In solution C there is the anode electrode and in solution B there is the cathode. There is a salt bridge between the solutions and the Arduino that reads the potential difference, which is connected to the electrodes immersed in the solutions. The e- (electrons) pass from the anode to the cathode. A02, A04, and A05: How did the reduction in d.d.p occur? It may indicate that there was oxidation, allowing electrons to be released to prove the reduction in the other beaker. The latter had already correctly inferred that the assembly was a concentration cell. What these excerpts show is that participants included a new observation ("d.d.p. decreases over time. Because the d.d.p. was measured again after 10 minutes") which corroborated the previous prediction. Other six participants did not write observations, all of whom had already inferred that it was a concentration cell or a similar system, maintaining their answers after clue 4. Among them, three proposed inferences at the end of clue 4 with more elements than expected, detailing the structure of the cell and its different components: B06: Solution B and C in separate beakers, connected by a salt bridge. In solution B there is a cathode where reduction occurs and in solution C there is an anode where oxidation occurs. At the anode, the ions lose electrons, and these electrons pass through a connecting wire towards the cathode, creating an electric current. The Arduino is connected to each electrode (anode and cathode). The salt bridge [XXX - somewhat illegible] the ions, establishing a balance. B09: Concentration cell that functions as a voltage source. With copper sulfate solutions and copper electrodes. B11: A concentration cell containing solutions B and C as electrolytes and copper electrodes. When considering all clues, some interpretations and relations can be established with theoretical aspects. As already mentioned, the decrease in observation records is an aspect to be considered. Frequent exposure to more traditional teaching formats is one of its causes combined with the search for the final answer, consequently prioritizing the result rather than the process. When considering other possible causes, even though experimental practices are common in chemistry teacher education, research aspects or even practices with a higher investigative level are still little explored.60 Even though some observations were not explicitly recorded in the written records, from the perspective of the POE strategy, the participants' anticipated knowledge and understanding of electrochemistry concepts were mobilized. As a result, most participants were able to formulate their inferences, establishing connections between the data and their prior knowledge. This finding mirrors what has been observed in other studies.2 Moreover, participants not only mobilized the expected knowledge but also reported additional knowledge on electrochemistry. Initial inadequate inferences were reconstructed, demonstrating the meaningful role that the predict-observe-explain cycle plays in this process. Based on the results presented so far, the activity can be considered adequate in terms of the data provided and the inferences generated. However, strategies to ensure more detailed and constant recording by the participants are necessary. Analysis of audio records To explore the activity implementation and the relationship between levels of knowledge, specifically regarding electrochemistry, we highlight some excerpts from the transcriptions of the participants' statements in the last step. Recording of the explanation was conducted after clue 4, to discern whether they were able to correlate the various predictions, observations, and explanations, thereby expanding the written records. This instrument acknowledges that such records may not have been comprehensively documented. All transcriptions of the oral responses provided by the participants, when asked to explain what was in the "mystery box" and how they reached that conclusion, are in the Supplementary Material. Transcriptions were classified as level 1 or 2 if the ideas were presented in a non-articulated or articulated way, respectively, using the same criteria defined in Chart 1. Transcriptions were coded according to the use of clues and the incorporation of electrochemistry elements and concepts associated or not with the clue. Data showed that 14 of the participants managed to create a response with an adequate chain of ideas, of which 11 used more than one clue in the description and three used only one. Six participants mentioned concepts not directly linked to the clues and produced a confusing chain of ideas. In Charts 3 and 4, respectively, we present the responses from a participant who used all information provided by the clues, articulating them, and the responses provided by a participant who failed to highlight the information explicitly resulting in a poorly articulated response.
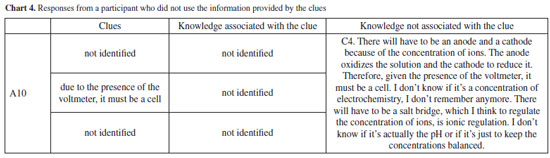
These excerpts show how participants were or were not able to relate the data obtained and provided (macro level knowledge), its interpretation, and their knowledge on electrochemistry. Importantly, the objective of the activity was not to teach electrochemistry concepts, since the participants already knew these contents, but rather to evaluate whether the activity allowed them to mobilize such knowledge. Participant B01 illustrates a step-by-step systematization of ideas which allows to construct an argument based on the knowledge provided and mobilized, following a structure similar to that found in other works.61 B01's case is similar to that of most participants, as shown in the Supplementary Material, and the macroscopic knowledge provided by the clues could be related to microscopic elements. However, we must consider responses such as those of participant A10, who despite correctly inferring that it was an electrochemical system, did not use the clues appropriately when constructing his argument. This data allows us to reflect on strategies to improve the activity. But it can also be used by educators to discuss aspects of an inquiry-based activity, Science, Technology, Engineering and Maths (STEM) proposals and to recognize possible difficulties regarding the content itself.36 This last aspect appears in other answers, mainly regarding the function of the salt bridge and the direction of electrons in the system: A04: This may indicate that there was an oxidation reduction reaction. We don't know the meaning yet. A10: There will have to be a salt bridge, maybe to regulate the concentration of ions, it's ionic regulation. I don't know if it's actually the pH, if it's just to keep the concentrations balanced. A11: The function would be to supply electrons to the solution. As reported in the literature,24,25,47 these two concepts - function of the salt bridge and current flow in the electrolyte solutions - can pose difficulties to electrochemistry learning and can be targets for misconceptions. Other challenges were less frequently observed, such as the difficulty in predicting electron flow direction, associated with the understanding of concentration's influence and, consequently, in identifying the cathode and anode, as with participants A4, A11, and B01. Thus, although there is an understanding of the electrochemical system, there are associated difficulties that can be identified and addressed.45,46
CONCLUSIONS Revisiting our research questions and objectives, this final section elucidates how the intervention data shed light on these inquiries and suggests future research directions. In chemistry education, experimental activities play a vital role in conceptual understanding, particularly those designed to emphasize observation and inferences formulation based on research results and findings. During the activity phases, we observed an enhanced quality in the inferences made. Despite initial inaccurate observations and inferences regarding the "mystery box" content, participants ultimately succeeded in identifying it. This success underlines the importance of the predict-observation-explanation and inference cycle, which could facilitate establishing connections between previous concepts and the data observed and revising initial ideas based on new evidence. Regarding our research question about how a "mystery box" laboratory implemented through a POE strategy could enable pre-service teachers to make inferences from observations associated with electrochemistry content, results suggest that participants engage with conceptual domains by data interaction which enable them to review and expand their knowledge. We can thus infer that participants progressively mobilized more concepts in response to reflection on new data throughout the activity. Initially, following clues 1 and 2 and the prompt to predict the box's contents, the teachers mobilized few concepts and the inferences differed from the proposed content. Upon accessing additional data through clue 3, however, a significant number of participants identified the box's contents more accurately, integrating electrochemistry concepts related to electrodes, oxidation, and reduction reactions. By clue 4, participants orally presented a synthesis with almost all elements of a concentration cell. Evidently, we should reflect on and discuss potential improvements to the activity regarding its application in diverse educational settings. In terms of activity evaluation, a point of improvement is how participants record information. Strategies should be developed for more rigorous participation, overcoming the belief that the end result (inference and explanation) is more valuable than the evidence provided. Not only the final oral explanation must be systematized, but all stages of the process in which the connection between knowledge domains takes place. Rather than focusing on teaching a specific chemistry topic, this investigation intended to assess knowledge mobilization using a POE strategy. Future research could explore how pre-service teachers see the integration of this methodology into educational environments and the necessary adjustments for its implementation in different school contexts. Moreover, the POE strategy is a comprehensive teaching strategy that encompasses both scientific content and the methods of chemistry, potentially aligning with discussions on the nature of science itself. Our study suggests that POE favors knowledge engagement in electrochemistry among pre-service teachers, offering a foundation for further research in this field.
SUPPLEMENTARY MATERIAL Transcripts of the research participants' audio responses and the table with the participants' individual evaluations are available in the Supplementary Material at http://quimicanova.sbq.org.br, as PDF file, with free access. The Arduino full code are available in: https://docs.google.com/document/d/1l2oYDYI2IabE5mj6MNqhpm-_eaF-f_OACNtVfNgG8as/edit?usp=sharing.
ACKNOWLEDGMENTS We would like to thank all the pre-service chemistry teachers who participated in the study, their willingness to contribute and share insights was greatly appreciated; the CIQUP, Faculty of Science, University of Porto (Project UIDB/00081/2020) and IMS (Institute of Molecular Sciences, Project LA/P/0056/2020), and the São Paulo Research Foundation (FAPESP, Process No. 2023/04544-7), and to Espaço Escrita from State University of Campinas.
REFERENCES 1. Treagust, D.; Chittleborough, G.; Mamiala, T.; Int. J. Sci. Educ. 2003, 25, 1353. [Crossref] 2. Adúriz-Bravo, A.; Science & Education 2013, 22, 1593. [Crossref] 3. Acar, B.; Tarhan, L.; International Journal of Science and Mathematics Education 2007, 5, 349. [Crossref] 4. Palmer, D.; Research in Science Education 1995, 25, 323. [Crossref] 5. Kibirige, I.; Osodo, J.; Tlala, K. M.; Mediterranean Journal of Social Sciences 2014, 5, 300. [Crossref] 6. Ausubel, D. P.; The Journal of General Psychology 1962, 66, 213. [Crossref] 7. Nadelson, L. S.; Heddy, B. C.; Jones, S.; Taasoobshirazi, G.; Johnson, M.; International Journal of Educational Psychology 2018, 7, 151. [Crossref] 8. Mortimer, E. F.; Science & Education 1995, 4, 267. [Crossref] 9. Letina, A.; Educ. Sci. 2020, 10, 325. [Crossref] 10. Gunstone, R. F. In Practical Science, vol. 1; Woolnough, ed.; Milton Keynes, Open University Press: London, 1990, p. 67-77. 11. Champagne, A. B.; Klopfer, L. In Annual Meeting of the American Educational Research Association; Pittsburg University Learning Research and Development Center: Pittsburg, 1979, p. 1-57. 12. Uriyah, N.; Supardi, Z. A. I.; Suryanti; Studies in Philosophy of Science and Education 2023, 4, 66, [Crossref] 13. Hilario, J. S.; International Journal of Education and Research 2015, 3, 37. [Link] accessed in November 2024 14. Chen, J.-C.; Interactive Learning Environments 2022, 30, 1252. [Crossref] 15. DeSmet, A.; Aelterman, N.; Bastiaensens, S.; Van Cleemput, K.; Poels, K.; Vandebosch, H.; Cardon, G.; De Bourdeaudhuij, I.; Computers & Education 2015, 88, 192. [Crossref] 16. Orozco, M.; Boon, M.; Arce, A. S.; European Journal of Engineering Education 2023, 48, 180. [Crossref] 17. Fitriani, A.; Zubaidah, S.; Susilo, H.; Al Muhdhar, M. H. I.; Eurasian J. Educ. Res. 2020, 85, 45. [Link] accessed in November 2024 18. Furqani, D.; Feranie, S.; Winarno, N.; Journal of Science Learning 2018, 2, 1. [Crossref] 19. Hong, J.-C.; Hwang, M.-Y.; Liu, M.-C.; Ho, H.-Y.; Chen, Y.-L.; Computers & Education 2014, 72, 110. [Crossref] 20. Banawi, A.; Sopandi, W.; Kadarohman, A.; Solehuddin, M.; International Journal of Instruction 2019, 12, 359. [Crossref] 21. Sarıtaş, D.; Özcan, H.; Adúriz-Bravo, A.; Science & Education 2021, 30, 1289. [Crossref] 22. Sahara, R.; Subarkah, C. Z.; Rahmatullah, S.; J. Phys.: Conf. Ser. 2019, 1402, 055035. [Crossref] 23. Karamustafaoğlu, S.; Mamlok-Naaman, R.; Eurasia Journal of Mathematics, Science and Technology Education 2015, 11, 923. [Crossref] 24. Tuysuz, A.; Özdemİr, Ö. F.; Research in Science & Technological Education 2024. [Crossref] 25. Ayvacı, H. Ş.; Journal of Baltic Science Education 2013, 12, 548. [Crossref] 26. Bariş, Ç. Ç.; J. Biol. Educ. 2024, 58, 271. [Crossref] 27. Rothchild, I.; Induction, Deduction, and the Scientific Method - An Eclectic Overview of the Practice of Science; The Society for the Study of Reproduction, Madison, Wisconsin, 2006. [Link] accessed in November 2024 28. Saritaş, D.; Özcan, H.; Adúriz-Bravo, A.; III Congreso Internacional de Tendencias en Innovación Educativa 2020; Universidad Continental, Perú, 2020, p. 67-76. [Link] accessed in November 2024 29. Norris, S. P.; J. Res. Sci. Teach. 1985, 22, 817. [Crossref] 30. Johnstone, A. H.; Journal of Computer Assisted Learning 1991, 7, 75. [Crossref] 31. Atkinson, A. B.; Journal of Economic Theory 1970, 2, 244. [Crossref] 32. Taber, K. S.; Chem. Educ. Res. Pract. 2013, 14, 156. [Crossref] 33. e Trindade, J. F.; Revista Thélos 2014, 9, 6. [Link] accessed in November 2024 34. Lawson, A. E.; Sci. Educ. 2010, 94, 336. [Crossref] 35. Crawford, B.; In Handbook of Research on Science Education, vol. II; Lederman, N. G.; Abell, S. K., eds.; Routledge: New York, 2014, ch. 26. [Crossref] 36. Herranen, J.; Kousa, P.; Fooladi, E.; Aksela, M.; International Journal of Science Education 2019, 41, 1977. [Crossref] 37. Medina, E. D.; Baraquia, L. G.; Polaris Global Journal of Scholarly Research and Trends 2023, 2, 116. [Crossref] 38. Strat, T. T. S.; Jegstad, K. M.; Journal of Science Teacher Education 2023, 34, 624. [Crossref] 39. Goes, L. F.; Fernandez, C.; Educ. Sci. 2023, 13, 1159. [Crossref] 40. Nogueira, K. S. C.; de Goes, L. F.; Fernandez, C.; Revista Electrónica de Enseñanza de las Ciencias 2017, 16, 410. [Link] accessed in November 2024 41. Goes, L. F.; Fernandez, C.; Eilks, I.; Educ. Sci. 2020, 10, 170. [Crossref] 42. Goes, L. F.; Chen, X.; Nogueira, K. S. C.; Fernandez, C.; Eilks, I.; Science Education International 2020, 31, 313. [Crossref] 43. Loh, A. S. L.; Subramaniam, R.; Tan, K. C. D.; Research in Science & Technological Education 2014, 32, 229. [Crossref] 44. Dorsah, P.; Yaayin, B.; International Journal of Innovative Research and Development 2019, 8, 33. [Crossref] 45. Sanger, M. J.; Greenbowe, T. J.; J. Chem. Educ. 1997, 74, 819. [Crossref] 46. Tsaparlis, G.; Isr. J. Chem. 2019, 59, 478. [Crossref] 47. Özkaya, A. R.; J. Chem. Educ. 2002, 79, 735. [Crossref] 48. Rahayu, S.; Treagust, D. F.; Chandrasegaran, A. L.; International Journal of Science and Mathematics Education 2022, 20, 1859. [Crossref] 49. Rogers, F.; Huddle, P. A.; White, M. D.; J. Chem. Educ. 2000, 77, 104. [Crossref] 50. Sanger, M. J.; Greenbowe, T. J.; J. Res. Sci. Teach. 1997, 74, 819. [Crossref] 51. Santos, T. N. P.; Batista, C. H.; de Oliveira, A. P. C.; Cruz, M. C. P.; Quim. Nova Escola 2018, 40, 258. [Crossref] 52. Silveira, M. M. S.; Lima, R. H.; Bernardes, G. S.; Alves, V. A.; da Silva, L. A.; Quim. Nova 2020, 44, 118. [Crossref] 53. Andrade, L. V.; Zimmer, C. G.; Quim. Nova Escola 2021, 43, 298. [Crossref] 54. Seyhan, H. G.; Türk, G. E.; International Journal of Research in Teacher Education 2023, 14, 54. [Crossref] 55. Thompson, J.; Soyibo, K.; Research in Science & Technological Education 2002, 20, 25. [Crossref] 56. Leopold, H.; Smith, A.; Educ. Sci. 2020, 10, 7. [Crossref] 57. Creswell, J. W.; Educational Research Planning, Conducting, and Evaluating Quantitative and Qualitative Research, vol. 1, 4th ed.; Smith, P. A.; Robb, C.; Buchholtz, M.; Mason, K., eds.; Pearson Education: Boston, 2012, ch. 8. 58. Schmidt, H.-J.; Marohn, A.; Harrison, A. G.; J. Res. Sci. Teach. 2007, 44, 258. [Crossref] 59. Lin, C.-Y.; Wu, H.-K.; Chem. Educ. Res. Pract. 2021, 22, 786. [Crossref] 60. Guidotti, C.; Heckler, V.; Revista Thema 2017, 14, 191. [Crossref] 61. El-Hani, C. N.; Pietrocola, M.; Mortimer, E. F.; Otero, M. R.; Science Education Research in Latin America; BRILL: Leiden, The Netherlands, 2020. [Crossref]
Associate Editor handled this article: Nyuara A. S. Mesquita |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access