Artigo
| Chemotype verboccidentafuran in Baccharis punctulata (Asteraceae): furanic sesquiterpenes correlation and molecular docking into the benzodiazepine receptor |
|
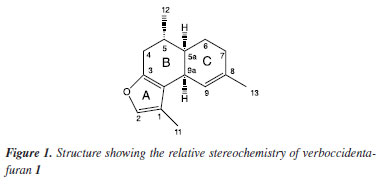
Jociani AscariI,*; Murilo S. de OliveiraI; André L. RüdigerI; Elaine ElisabetskyII; Domingos S. NunesIII; Jorge IulekIII; I. Coordenação de Licenciatura em Ciências Biológicas, Universidade Tecnológica Federal do Paraná, 85892-000 Santa Helena - PR, Brasil Received: 07/03/2024 *e-mail: jascari@utfpr.edu.br The compound verboccidentafuran (1) was identified from the essential oil of the leaves of Baccharis punctulata DC. (Asteraceae) specimens for the first time in Brazil. The variation of this compound found in male and female specimens may be associated to the existence of more than one type of chemotype, influenced by geographic variation, climatic conditions, stage of the vegetative cycle, genetic and ecological factors. The furanocadinan-type sesquiterpenes verboccidentafuran (1) and curzerene (2) have molecular descriptors with high similarities. Compound 2, found in Eugenia uniflora L. (Myrtaceae) and species of the genus Smyrnium L. (Apiaceae), is active to control seizures induced by pentylenetetrazole, what suggests a possible binding to the benzodiazepine-site of the GABAA (gamma-aminobutyric acid type A) receptor. Considering the occurrence and availability of1 in the species B. punctulata and its structural similarity to compound 2, docking calculations were performed for the interaction of both compounds with the human α1-β2-γ2 GABAA receptor. The results suggest that both compounds 1 and 2 must have affinity for the cavity at α1-γ2 interface and support the development of pharmacological research on the compound verboccidentafuran 1 as a drug candidate for modulating the GABA/benzodiazepine complex in the central nervous system. INTRODUCTION The furanosesquiterpene verboccidentafuran 1 was isolated and identified from the roots of Verbesina occidentalis (L.) Walter for the first time in 1978 by Bohlmann and Lonitz.1 The stereochemistry of this furanic derivative was better understood from the proposed synthesis for compound 1 using a route through an initial Diels-Alder reaction, thus being defined as (5α,5aα,9aα)-(±)-4,5α,5aα,6,7,9aα- hexahydro-1,5,8-trimethylnaphtho[2,1-b].2 Later, the compound 1 (Figure 1) was also found in species of the genus Baccharis,3-7 Chromolaena8 and Eupatorium.9
Among the species of the genus Baccharis, occurrence of 1 is also reported in Baccharis punctulata DC. This species is widely distributed in South America, particularly in Brazil, where it can be found in the Southeast and the South, in the Cerrado, Atlantic Forest and Pampa biomes.10 Schossler et al.11 observed the presence of 1 in the essential oil of B. punctulata leaves collected in the state of Rio Grande do Sul. González7 found compound 1 in the essential oil of leaves originating from Luján in the province of Buenos Aires, Argentina, from male and female specimens. However, other studies12,13 on the composition of essential oils from the leaves of this species obtained from different regions indicated the absence of this compound. Samples of essential oil from the leaves of B. punctulata collected in the West and Southwest regions of the state of Paraná, Brazil, between 2017 and 2019, were evaluated by gas chromatography coupled to mass spectrometry (GC-MS) by our group.14 Curzerene 2 (Figure 2) was isolated for the first time from the rhizomes of Curcuma zedoaria Roscoe15 and from the roots of Lindera strychnifolia.16 Ishii et al.16 observed that the isolated 2 presented itself as a racemic mixture, with the isomer in the smaller proportion attributed to the natural occurrence in the plant while the rest representing its racemate derived from the pyrolysis of furanodiene 3 (Figure 2).15,17-19
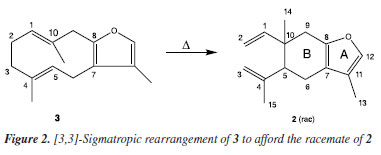
Eugenia uniflora is a Brazilian small tree whose essential oil from leaves contains furanodiene 3. Since the first chemical studies20 with this species, the same [3,3]-sigmatropic reaction producing curzerene 2 has been observed by heating during GC analysis. The occurrence of high proportions of curzerene 2 in essential oils from E. uniflora leaves has been published,21-25 yet GC techniques have been the only analytical method used. The effective determination of 2 concentrations in samples of essential oils from E. uniflora and Smyrnium spp. has been possible by using GC-MS combined with high-performance liquid chromatography (HPLC) analysis at room temperature.22,26-29 Both verboccidentafuran 1 and curzerene 2 are furanocadinan-type sesquiterpenes of rare occurrence in nature. 1 has a nonlinear furan structure with eight coplanar carbon atoms, while 2 has eight coplanar carbon atoms but with a linear structure. Our attention was drawn to the activity of curzerene 2 to control seizures induced by pentylenetetrazole, which suggests a possible binding to the benzodiazepine-site of the GABAA (gamma-aminobutyric acid type A) receptor.30 The molecular descriptors of both compounds show high similarities, such as identical MW (molecular weight), HBD (hydrogen bond donor), HBA (hydrogen bond acceptor), topological surfaces, zero rotatable bonds, and complexity indexes.31 The calculated XLogP3-AA values for 1 and 2 (3.8 and 4.6, respectively) are quite close and the data sets of molecular descriptors presented for both compounds indicate that they have the necessary prerequisites for oral absorption (TPSA (topological polar surface area) < 140 Å2)32 and for crossing the blood-brain barrier (values below 60 Å2).33 These data support our working hypotheses that both these sesquiterpenes might have comparable activities on the GABAergic mediated properties in the central nervous system. Considering the occurrence and identification of compound 1 from the essential oil of the leaves of B. punctulata specimens for the first time in Brazil and its structural similarity with compound 2, theoretical studies were developed on the interaction of both compounds with the benzodiazepine-site GABAA α1-β2-γ2. The structures were studied for conformers and optimized by quantum chemistry studies, and then used for receptor docking. The overall analysis might lead to a new molecular template for GABA receptors modulators.
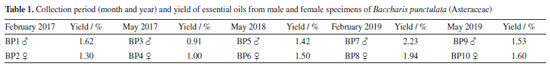
EXPERIMENTAL Plant material Aerial parts from female and male specimens of B. punctulata were collected in February and May of the years 2017, 2018 and 2019, in areas up to 1.5 km far from the point 26º00'42"S/52º47'40"W, Itapejara D'Oeste Town, in the southwest of the state of Paraná, Brazil. The collections were also carried out during the flowering period at 8:00 a.m. and with high incidence of sunlight. Exsiccates were taxonomically analyzed to confirm the species and deposited under the numbers ECT0003423 (female) and ECT0003426 (male) at the ECT Embrapa Clima Temperado Herbarium, Rio Grande do Sul, Brazil. Essential oil extraction and analysis Leaves from female and male specimens were separated and dried for 4 days at room temperature. Essential oils were obtained from 100 g samples by hydrodistillation in a Clevenger-type apparatus for 3 h.34 The yields of the essential oil samples were calculated considering the dry mass of each specimen. Identification of the composition of samples were carried out on a Shimadzu GCMS-QP2010 Plus at 70 eV using a non-polar column RTX-5MS (30 m × 0.25 mm × 0.25 µm). Analysis conditions were: 250 ºC for the injector, 250 ºC for the ion source and 280 ºC for the interface. Oven temperature programming was 60 ºC for the first 5 min, increasing at a rate of 3 ºC min-1 until the final temperature of 240 ºC. The split ratio used was 1/20. Chemical identification of the compounds was carried out by comparing the relative retention indices (RI) to a homologous series of n-alkanes (C8-C19) and mass spectra from the spectrometer database (NIST library), as published in the literature.35 The relative proportions of compounds present in the samples were determined quantitatively using a Shimadzu Gas 2010 Chromatograph with flame ionization detector (GC-FID) and an OV-5 column (30 m × 0.25 mm × 0.25 μm). Constant flow rate was 1 mL min-1 at 87.0 kPa. Helium was used as the carrier gas, split mode of 1/20, the injection volume was 1 μL of sample diluted in ethyl ether and temperature of 250 ºC for the injector, 250 ºC for the ion source and 280 ºC for the interface. Oven temperature programming was 60 ºC for the first 5 min, increasing at a rate of 3 ºC min-1 until reaching the final temperature of 240 ºC. Structural optimization of conformers To gain initial information about the relative stability of the conformers concerning specially the rings B and C for verboccidentafuran 1 and ring B for curzerene 2, initial 3D structures were obtained with ChemAxon program MarvinSketch36 for 1 and 2. The structures were then optimized with Gamess37 with the 6-31G(d) basis set and the B3LYP functional for subsequent docking calculations. We prepared the 1 molecule in four different conformations according to different half-chair conformations of rings B and C for subsequent calculations, and the 2 molecule to give two conformers considering its non-planar ring. The same protocol was also applied to flumazenil to prepare it for docking calculations (see "Molecular docking" sub-section), for which two conformers changing the seven member ring conformation were then prepared. Molecular docking The structures optimized by the GAMESS program37 (see "Structural optimization of conformers" sub-section) of the four conformers of 1 and of the two conformers of 2 were then submitted to the prepare_ligand4.py script of the AutoDockTools program.38 The human GABAA subtype α1-β2-γ2 receptor structure deposited at the Protein Data Bank (PDB)39 with code 6D6T40 was used for docking. This structure presents a flumazenil molecule inserted into a cavity between an α1 and the γ2 subunits (named chains D and E, respectively, in the PDB entry 6D6T). This receptor structure was prepared with AutoDockTools38 such that His102 of subunit α1, which is part of the interacting site (cavity), was considered at the N-δ or alternatively at the N-ε protonated state, implying two distinct docking runs in any of the subsequent combinations. Docking was performed with Vina41 in blind and site delimited (around the cavity occupied by flumazenil) approaches; in the former case, the search comprehended a large volume around the extra cellular domain (ECD), and in latter case, the search was focused on the flumazenil interaction site which had the protein side chain residues free to rotate (flexible docking), always using the Vina program maximum exhaustiveness. Statistical analysis The statistical evaluation was performed using Software R version 4.2.1, "Funny-Looking Kid".42 As graphical interface, the RStudio 2022.07.1+554 "Spotted Wakerobin" release for Windows43 was used. The principal components analysis (PCA), hierarchical group on principal components (HCPC) and description of categories, was applied by FactoMineR Package.44 For the multivariate analysis, the data were previously normalized using Z-scores. HCPC analysis was performed using the Ward method, and Euclidean distance was utilized. The plot of statistical analysis was performed using the factoextra Package 1.0.7.45 The data and imaging processing was performed in an x86_64-w64-mingw32/x64 (64-bit) platform, notebook Dell Inspiron 13-7348-B20, Intel(R) CoreTM i5-5200U processor, CPU @ 2.20GHz 2.20 GHz, and 8 Gb RAM DDR3L.
RESULTS AND DISCUSSION The essential oils from the leaves of B. punctulata specimens collected in the southwest region of Paraná showed yields in the range of 0.9-2.2% in relation to the initial dry mass of plant material (Table 1). All collections were carried out during the flowering period, at the same time and location, as well as sample preparation, extraction, and storage.
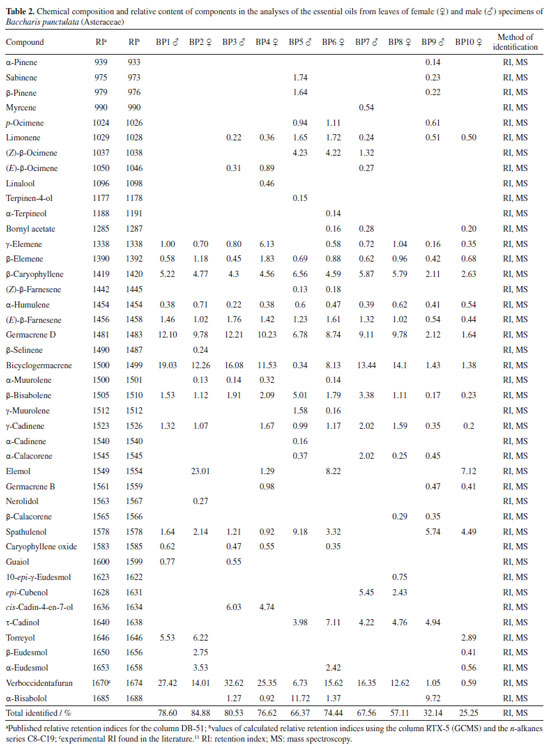
The chemical composition of the samples was analyzed using the GC-MS and GC-FID technique (Table 2). The major compounds found in the majority of female and male specimens in this study were: β-caryophyllene (2.11-6.56%), germacrene D (1.64-12.21%), bicyclogermacrene (1.38-19.03%) and verboccidentafuran (1) (0.59-32.62%). Compound 1 was the major one in specimens BP1 (27.42%), BP3 (32.62%), BP4 (25.35%), BP6 (15.62%), BP7 (16.35%) and BP8 (12.62%), observed for the first time in Brazil.
Ascari et al.14 analyzed the chemical composition of the essential oil from the leaves of B. punctulata specimens, collected in the western region of Paraná, Brazil, verifying that the majority were bicyclogermacrene (10.90-42.44%), germacrene D (11.29-21.18%), β-caryophyllene (5.59-14.06%) and δ-elemene (1.97-14.29%), and that compound 1 was absent. Like other studies7,11-13 that evaluated the chemical composition of the essential oil from leaves of the species B. punctulata, differences were observed in the chemical composition of the oils. Both differences observed, in yields (Table 1) and in chemical composition (Table 2), of the samples among the specimens and the collection period, can be associated with genetic factors46,47 as well as with the adaptation of each chemotype to edaphoclimatic factors,48-51 yet external stimuli can redirect the metabolic pathways. We then analyzed the data variability through PCA, shown in Figure 3a, the first two dimensions express 44.02% of the data variability. In this Figure, the samples (represented by black spots) are dispersed across all dimensions, while the direction and contribution of each variable to the construction of the PCA graph are depicted. In Dim.1, the right side of the graph is composed of individuals such as BP5, with strongly positive coordinates on the axis, characterized by high values for variables such as (Z)-β-farnesene, (Z)-β-ocimene, limonene, p-ocimene and γ-muurolene. In contrast, individuals on the left side of the graph, such as BP4 and BP3, are characterized by high values for variables such as verboccidentafuran, caryophyllene oxide, bicyclogermacrene and (E)-β-ocimene, along with low values for the variable α-humulene. Dim.2 distinguishes individuals such as BP5, BP4, and BP3 from individuals such as BP10, BP9 and BP2. In the positive coordinate on the Dim.2 axis, represented by individuals BP4 and BP3, there are high values for the variables verboccidentafuran, caryophyllene oxide, bicyclogermacrene and (E)-β-ocimene, along with low values for the variable α-humulene, as already elucidated by Dim.1. Conversely, in contrast to the positive coordinate, the negative coordinate on Dim.2, exemplified by individuals BP10, BP9, and BP2, entails low values for the variables (E)-β-farnesene and β-bisabolene, which they share.
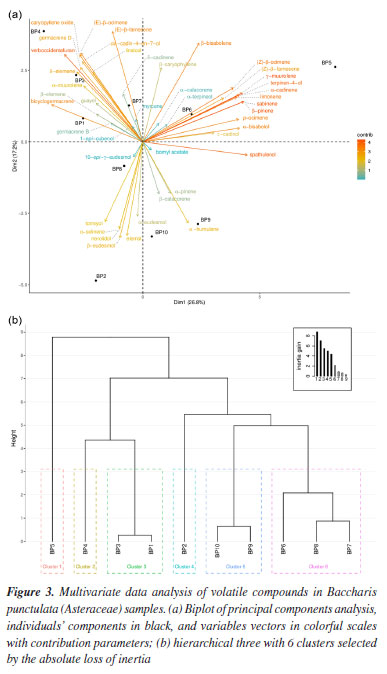
The individual BP5, although positioned on the positive side of the Dim.2 axis like BP3 and BP4, exhibits high values of distinct variables, thus confirming that Dim.1 is more crucial in discriminating for this individual than Dim.2. This underscores BP5's significant compositional variation relative to other specimens. The complete list of variables characterizing the dimensions can be found in the Supplementary Material, Tables S1 to S4. The HCPC analysis provided 6 different clusters (Figure 3b). The suggested partition is the one with the higher relative loss of inertia (i(cluster n + 1) / i(cluster n)) (absolute loss of inertia, (i(cluster n) - i(cluster n + 1)), was plotted with the tree, in Figure 3b). It is possible to reconnoiter the cluster combination for the same collections in the 2018 and 2019 years, however, for samples collected in 2017, it was not possible to distinguish an effective clustering as seen in hierarchical tree. Quantitative values for the quantitative variables with p ≤ 0.05, describing each cluster, can be found in Supplementary Material, Table S4. Cluster 3 is made of male individuals collected in February and May from 2017, BP1 and BP3, respectively. This cluster is characterized by high values for the variables guayol, verboccidentafuran and caryophyllene oxide. At comparing BP1 with the female specimen BP2 collected in the same period, February 2017, the same major concentration of the volatile compounds was not observed, and BP2 was grouped in cluster 4, that shows high values of norolidol, β-selinene, β-eudesmol, elemol, α-eudesmol and torreyol. This female specimen, collected in May 2017, shows a different highest compound content, as individuals like BP4, grouped in cluster 2. That show high levels of linalool, δ-elemene, (E)-β-ocimene, germacrene B, β-elemene and α-muurolene. Cluster 1 made BP5, a male individual collected in May 2018, which is characterized by the presence of α-pinene, a compound not observed in other collections. This cluster is also defined by low values for the variables germacrene D, β-cariophyllene, (E)-β-farnesene, verboccidentafuran, and bicyclogermacrene. Cluster 6 comprehends the male and female individuals BP6, BP7 and BP8, that share high values of the variables τ-cadinol, 1-epi-cubenol. Cluster 5 is made of individuals such as BP9 and BP10 and is characterized by low values for the variables germacrene D, β-caryophyllene, (E)-β-farnesene, verboccidentafuran and bicyclogermacrene. Verboccidentafuran is the primary compound in the initial collections. However, a decline in its relative concentration was observed across subsequent collections, as depicted in the boxplot (Figures 4a and 4b), indicating a significant decrease in its production in May 2019.
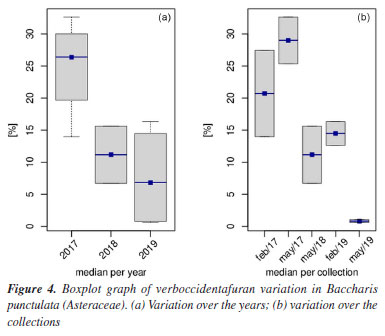
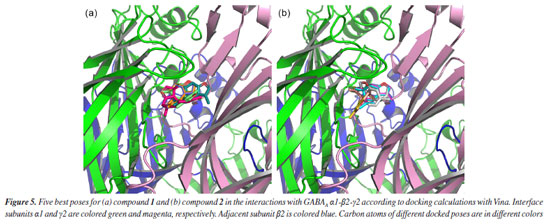
As previously noted for this species collected in the Western region of Paraná by Ascari et al.,14 where the presence of verboccidentafuran was not detected, the production of this compound may be associated with the existence of multiple chemotypes, influenced by geographic variation, climatic conditions, stage of the vegetative cycle, genetic, and ecological factors.34,36-38 Our study reports for the first time this compound as a major constituent in B. punctulata collected in Brazil. As expected, at a blind docking calculations, the majority of the best poses of compound 1 and compound 2 occupy the interface between subunits α1/γ2 (the one occupied by flumazenil in the experimental structure PDB code 6D6T), with interaction energies with this site estimated down to -8.7 kcal mol-1 for compound 1 and -7.0 kcal mol-1 for compound2, that compares well to energy estimates of down to -9.3 kcal mol-1 for flumazenil in the same docking run, energy that associated to an RMSD (root mean square deviation) of this pose to the experimental structure of only 0.781 Å for the rings and the proximal atoms (1.128 Å if one includes the flexible tail) confirm its preference for such site as well. Focusing on the docking study into this cavity, with flexible side chains for constituent residues, the five best compound1 poses presented favorable interaction energies running from -8.8 to -7.4 kcal mol-1, illustrated in Figure 5a. Yet, the five best compound 2 poses presented energies running from -8.5 to -8.0 kcal mol-1 and these are illustrated in Figure 5b. Interactions for the best poses for compounds 1 and 2 are highlighted in supplementary Figures 1SA and 1SB, respectively, as calculated by the PLIP server.52 Thus, our docking results suggest that both compounds 1 and 2 might also bind to this cavity; in fact, this observation for the latter correlates to the observed pharmacological results for its anticonvulsant activity.30 Other properties and potential activities for them were indicated by the swissADME53 and Passonline54 (limited to the 50 best predicted activity values) servers, whose output results are shown in Tables S5 and S6 (Supplementary Material), respectively; noteworthy are the drug likeliness rules not violated and the predicted permeability to the blood-brain barrier (BBB) for both compounds.
CONCLUSIONS This work has shown the presence of the compound verboccidentafuran 1 in the essential oil of the leaves of male and female specimens of B. punctulata, what is reported for the first time in Brazil. At the pursuit for a structural basis for verboccidentafuran 1 and curzerene 2 central nervous system activities, we carried out docking calculations in the human α1-β2-γ2 GABAA receptor, whose structure is available at the Protein Data Bank under code 6D6T. Our calculations indicated that both compounds must have affinity for the cavity at α1-γ2 interface that, in the cited Protein Data Bank structure, presents a flumazenil molecule. Therefore, we hypothesize that they might interact with this receptor at this cavity too and these interactions should lead to the anticonvulsant activity. These results and ADME predictions support the development of pharmacological research on the compound verboccidentafuran 1, as a drug candidate for modulating the GABA/benzodiazepine complex in the central nervous system.
SUPPLEMENTARY MATERIAL Statistics tables used in this work are available at http://quimicanova.sbq.org.br, in the form of a PDF file, with free access.
ACKNOWLEDGMENTS In memory of Mr. Vicanor Matuchaki Ascari for his help on Baccharis punctulata collections. Fuels and Chromatography Laboratory of the Fundação Universidade Regional de Blumenau are also acknowledged.
REFERENCES 1. Bohlmann, F.; Lonitz, M.; Phytochemistry 1978, 17, 453. [Crossref] 2. Bohlmann, F.; Trantow, T.; Liebigs Ann. Chem. 1983, 1983, 1689. [Crossref] 3. Zdero, C.; Bohlmann, F.; King, R. M.; Robinson, H.; Phytochemistry 1986, 25, 2841. [Crossref] 4. Jakupovic, J.; Schuster, A.; Ganzer, U.; Bohlmann, F.; Boldt, P. E.; Phytochemistry1990, 29, 2217. [Crossref] 5. Loayza, I.; Abujder, D.; Aranda, R.; Jakupovic, J.; Collin, G.; Deslauriers, H.; Jean, F.-I.; Phytochemistry1995, 38, 381. [Crossref] 6. Malizia, R. A.; Cardell, D. A.; Molli, J. S.; González, S.; Guerra, P. E.; Grau, R. J.; J. Essent. Oil Res. 2005, 17, 194. [Crossref] 7. González, M. D.; J. Essent. Oil Res. 2019, 31, 573. [Crossref] 8. Murakami, C.; Lago, J. H. G.; Perazzo, F. F.; Ferreira, K. S.; Lima, M. E. L.; Moreno, P. R. H.; Young, M. C. M.; Chem. Biodiversity 2013, 10, 621. [Crossref] 9. de Souza, T. J. T.; Bordignon, S. A. L.; Apel, M. A.; J. Nat. Prod. 2017, 80, 45. [Crossref] 10. Heiden, G.; Baumgratz, J. F. A.; Esteves, R. L.; Rodriguesia 2012, 63, 649. [Crossref] 11. Schossler, P.; Schneider, G. L.; Wunsch, D.; Soares, G. L. G.; Zini, C. A.; J.Braz. Chem. Soc. 2009, 20, 277. [Crossref] 12. Minteguiaga, M.; González, A.; Cassel, E.; Umpierrez, N.; Fariña, L.; Dellacassa, E.; Chem. Biodiversity 2018, 15, e1800017. [Crossref] 13. Budel, J. M.; Wang, M.; Raman, V.; Zhao, J.; Khan, S. I.; Rehman, J. U.; Techen, N.; Tekwani, B.; Monteiro, L. M.; Heiden, G.; Takeda, I. J. M.; Farago, P. V.; Khan, I. A.; Molecules 2018, 23, 2620. [Crossref] 14. Ascari, J.; de Oliveira, M. S.; Nunes, D. S.; Granato, D.; Scharf, D. R.; Simionatto, E.; Otuki, M.; Soley, B.; Heiden, G.; J. Ethnopharmacol. 2019, 234, 1. [Crossref] 15. Hikino, H.; Agatsuma, K.; Takemoto, T.; Tetrahedron Lett. 1968, 9, 2855. [Crossref] 16. Ishii, H.; Tozyo, T.; Nakamura, M.; Takeda, K.; Tetrahedron 1968, 24, 625. [Crossref] 17. Takeda, K.; Horibe, I.; Minato, H.; Chem. Commun. (London) 1968, 378. [Crossref] 18. Takeda, K.; Ishii, H.; Tozyo, T.; Minato, H.; J. Chem. Soc. C 1969, 1920. [Crossref] 19. Hikino, H.; Agatsuma, K.; Konno, C.; Takemoto, T.; Chem. Pharm. Bull. 1970, 18, 752. [Crossref] 20. Weyerstahl, P.; Marschall-Weyerstahl, H.; Christiansen, C.; Oguntimein, B. O.; Adeoye, A. O.; Planta Med. 1988, 54, 546. [Crossref] 21. Maia, J. G. S.; Andrade, E. H. A.; da Silva, M. H. L.; Zoghbi, M. G. B.; J. Essent. Oil Res. 1999, 11, 727. [Crossref] 22. Chang, R.; de Morais, S. A. L.; Napolitano, D. R.; Duarte, K. C.; Guzman, V. B.; do Nascimento, E. A.; Rev. Bras. Farmacogn. 2011, 21, 392. [Crossref] 23. Rodrigues, K. A. F.; Amorim, L. V.; de Oliveira, J. M. G.; Dias, C. N.; Moraes, D. F. C.; Andrade, E. H. A.; Maia, J. G. S.; Carneiro, S. M. P.; Carvalho, F. A. A.; Evidence-Based Complementary and Alternative Medicine 2013, 2013, 279726. [Crossref] 24. Figueiredo, P. L. B.; Pinto, L. C.; da Costa, J. S.; da Silva, A. R. C.; Mourão, R. H. V.; Montenegro, R. C.; da Silva, J. K. R.; Maia, J. G. S.; J. Ethnopharmacol. 2019, 232, 30. [Crossref] 25. Silva, J. S.; Damiani, C.; da Cunha, M. C.; Carvalho, E. E. N.; Boas, E. V. B. V.; Sci. Hortic. 2019, 250, 366. [Crossref] 26. Baldovani, N.; Tomi, F.; Casanova, J.; Phytochem. Anal. 2001, 12, 58. [Crossref] 27. Quassinti, L.; Maggi, F.; Barboni, L.; Ricciutelli, M.; Cortese, M.; Papa, F.; Garulli, C.; Kalogris, C.; Vittori, S.; Bramucci, M.; Fitoterapia 2014, 97, 133. [Crossref] 28. Santos, F. R.; Braz-Filho, R.; Castro, R. N.; Quim. Nova 2015, 38, 762. [Crossref] 29. Maggi, F.; Barboni, L.; Papa, F.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Vittori, S.; Food Chem. 2012, 135, 2852. [Crossref] 30. Abbasi, N.; Mohammadpour, S.; Karimi, E.; Aidy, A.; Karimi, P.; Azizi, M.; Asadollahi, K.; J. Biol. Regul. Homeostatic Agents 2017, 31, 683. [Crossref] 31. PubChem, https://pubchem.ncbi.nlm.nih.gov/, accessed in November 2024. 32. Ertl, P.; Rohde, B.; Selzer, P.; J. Med. Chem. 2000, 43, 3714. [Crossref] 33. Shityakov, S.; Neuhaus, W.; Dandekar, T.; Förster, C.; Int. J. Comput. Biol. Drug Des. 2013, 6, 146. [Crossref] 34. Stalh, E.; Schild, W.; Drogenanalyse II: Inhaltsstoffe und Isolierungen; Gustav Fischer Verlag, Stuttgart: New York, 1981. 35. Adams, R. P.; Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, Illinois, USA, 2009. 36. ChemAxon - Software Solutions and Services for Chemistry & Biology, https://chemaxon.com/, accessed in November 2024. 37. Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S.; Windus, T. L.; Dupuis, M.; Montgomery Jr, J. A.; J. Comput. Chem. 1993, 14, 1347. [Crossref] 38. Morris, G. M.; Huey, R.; Lindstrom, W.; Sanner, M. F.; Belew, R. K.; Goodsell, D.; Olson, A. J.; J. Comput. Chem. 2009, 30, 2785. [Crossref] 39. Berman, H. M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T. N.; Weissig, H.; Shindyalov, I. N.; Bourne, E. B.; Nucleic Acids Res. 2000, 28, 235. [Crossref] 40. Zhu, S.; Noviello, C. M.; Teng, J.; Walsh Jr., R. M.; Kim, J. J.; Hibbs, R. E.; Nature 2018, 559, 67. [Crossref] 41. Trott, O.; Olson, A. J.; J. Comput. Chem. 2010, 31, 455. [Crossref] 42. R Core Team; R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing, Vienna, Austria. 2022. 43. R Studio Team; RStudio: Integrated Development for R; RStudio, PBC, Boston, MA, 2020. 44. Lê, S.; Josse, J.; Husson, F.; Journal of Statistical Software 2008, 25, 1. [Crossref] 45. Kassambara, A.; Mundt, F.; factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Package, version 1.0.7; R Foundation for Statistical Computing, Vienna, Austria, 2020. [Crossref] 46. Agostini-Costa, T. S.; Gomes, I. S.; Fonseca, M. C. M.; Alonso, A. M.; Pereira, R. C. A.; Junior, I. M.; da Silva, J. P.; Pereira, A. M. S.; da Silva, D. B.; Vieira, R. F.; Vaz, A. P. A.; Planta Med. 2016, 82, 1431. [Crossref] 47. Talhaoui, N.; Gomez-Caravaca, A. M.; Roldán, C.; León, L.; de la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero A.; J. Agric. Food Chem. 2015, 63, 1722. [Crossref] 48. Besten, M. A.; Jasinski, V. C. G.; Costa, A. G. L. C.; Nunes, D. S.; Sens, S. L.; Wisniewski Jr., A.; Simionatto, E. L.; Riva, D.; Dalmarco, J. B.; Granato, D. J.; J. Braz. Chem. Soc. 2012, 23, 1041. [Crossref] 49. Polatoglu, K.; The Natural Products Journal2013, 3, 10. [Crossref] 50. Bueno, P. C. R.; Abarca, L. F. S.; Anhesine, N. B.; Giffoni, M. S.; Pereira, F. M. V.; Torres, R. B.; de Sousa, R. W. R.; Ferreira, P. M. P.; Pessoa, C.; Cavalheiro, A. J.; Planta Med. 2021, 87, 148. [Crossref] 51. Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J.; Trends Food. Sci. Technol. 2021, 110, 78. [Crossref] 52. Salentin, S.; Schreiber, S.; Haupt, V. J.; Adasme, M. F.; Schroeder, M.; Nucleic Acids Res. 2015, 43, 443. [Crossref] 53. Daina, A.; Michielin, O.; Zoete, V.; Sci. Rep. 2017, 7, 42717. [Crossref] 54. Filimonov, D. A.; Lagunin, A. A.; Gloriozova, T. A.; Rudik, A. V.; Druzhilovskii, D. S.; Pogodin, P. V.; Poroikov, V. V.; Chem. Heterocycl. Compd. 2014, 50, 444. [Crossref]
Editor handled this article: Nelson H. Morgon |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access