Artigo
| Terpenes of Melipona quadrifasciata geopropolis |
|
Marta S. D. FreitasI I. Escola de Química e Alimentos, Universidade Federal do Rio Grande, 95500-000 Santo Antônio da Patrulha - RS, Brasil Received: 08/14/2024 *e-mail: luizufrr@gmail.com Melipona quadrifasciata is a stingless bee native to Brazil, known for producing a geopropolis with complex composition and diverse biological activity. The study aimed to identify the terpenes present in geopropolis collected from Melipona quadrifasciata colonies located in the state of Rio Grande do Sul, Brazil, to establish a geographic profile that enhances the understanding of the phytochemical composition of geopropolis from southern Brazil. The volatile fraction was analyzed by headspace-gas chromatography/mass spectrometry (HS-GC/MS) and identified fifty-six (56) mono- and sesquiterpenes, with particularly high concentrations of α- and β-pinene. These two compounds were present in all geopropolis samples at concentrations exceeding 50% and were identified as geographical markers of M. quadrifasciata geopropolis from the state of Rio Grande do Sul. Additionally, gas chromatography-mass spectrometry (GC-MS) analysis of the hexane fraction obtained via cold extraction under ultrasound revealed the presence of abietane diterpenes and pentacyclic triterpenes, particularly amyrins, with α-amyrin presented in all geopropolis samples. INTRODUCTION Native bees, also called "stingless bees" (SB), have their stingers atrophied so they do not use them for defense.1 SBs play a crucial ecological role, particularly in pollinating a significant portion of the flora.2 In nature, SBs species exhibit extensive diversity, exceeding 600 species, and are distributed across tropical regions.3 SBs produce honey, pollen, wax, and propolis/cerumen, which are natural products with considerable potential for bioprospecting.4 Certain SBs species produce a specific type of propolis, known as geopropolis, which is formed by blending plant resins with wax and varying amounts of soil.2 Geopropolis has a diverse chemical composition, comprising wax, resins, balsam, and secondary metabolites such as phenolic compounds, terpenes, and alkaloids.3 The variability in plant sources significantly influences these metabolites, thus impacting the aroma and biological activity of propolis.5 Given the importance of the biological potential of geopropolis, the study of a geographical marker becomes essential to explore and ensure its therapeutic and commercial properties fully. Geopropolis, rich in bioactive compounds such as flavonoids, terpenes, and phenolic acids, has demonstrated strong antimicrobial, antioxidant, and anti-inflammatory properties, making it a valuable resource in natural medicine and the pharmaceutical and cosmetic industries.2-5 Melipona quadrifasciata, a native SB species in Brazil, thrives in the Atlantic Forest along the Brazilian coast, from the state of Paraíba to Rio Grande do Sul.6 In Rio Grande do Sul, SBs inhabit two distinct biomes, the Atlantic Forest and Pampa, with the latter hosting a unique flora due to the occurrence of species exclusive to grassland environments.7 Propolis produced by SBs contains a diversity of bioactive compounds derived from plants in the bee's environment. Terpenes, a class of low-polarity compounds found in the propolis of various SB species, exhibit antimicrobial and antifungal activities that remain crucial for hive protection.8 The variety of aromas emitted by propolis is influenced by terpenoids present in the plant species visited by bees, particularly mono- and sesquiterpenes.9-11 Leonhardt and Blüthgen12 demonstrated that SBs from Borneo, an island in the Pacific Ocean, utilize olfactory cues to locate trees from which to collect resins. Specifically, they rely on mono- and sesquiterpenes to identify and recognize the source of the resins. The mono- and sesquiterpenes found in geopropolis, in addition to their aromatic properties, exhibit antioxidant, antimicrobial, analgesic, anxiolytic, and anticancer activities, making them promising candidates as potential therapeutic alternatives.13,14 In addition to the terpenoids responsible for the aroma, geopropolis contains diterpenes and triterpenes with potential biological activities. Torres et al.8 identified 26 diterpene skeletons in the geopropolis of two SB species, Melipona quadrifasciata and Tetragonisca angustula, demonstrating potential antibacterial activity. Triterpenes, such as α- and β-amyrins, have also been identified in SB geopropolis, showing potential cytotoxic activity against K562 erythroleukemia cells and skin cancer cells.14-16 The objective of this study is to identify the terpenoids present in the geopropolis of Melipona quadrifasciata, describing and providing chemically insights into the origin of the geopropolis produced in southern Brazil.
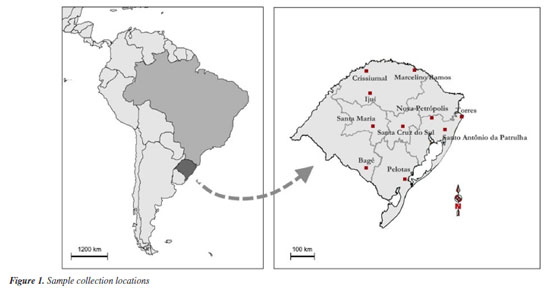
EXPERIMENTAL Collected samples The geopropolis samples of Melipona quadrifasciata were collected in sterile flasks from different cities of Rio Grande do Sul, Brazil, between August 2021 and November 2021, namely Santo Antônio da Patrulha (SAP), Marcelino Ramos (MAR), Santa Maria (SMR), Nova Petrópolis (NPP), Bagé (BAG), Pelotas (PEL), Santa Cruz do Sul (SCS), Ijuí (IJU), Crissiumal (CRI), and Torres (TOR) (Figure 1). Subsequently, the samples were crushed, packed, and refrigerated (1 to 6 ºC) for further processing and analysis.
Volatile compounds - headspace-gas chromatography/mass spectrometry (HS-GC/MS) The headspace extraction was conducted at 80 ºC for 15 min, utilizing 1.0 g of ground geopropolis in a 20.0 mL glass flask. Subsequently, one milliliter of the vapor phase from the headspace was injected in the GC-MS (Shimadzu, model GC-MS/MS TQ8050), equipped with an AOC 6000 autosampler. The injection was performed in splitless mode, employing helium as carrier gas at a flow rate of 1.02 mL min-1, while maintaining the injector temperature at 220 ºC. For compounds separation, a fused-silica capillary column model RTX-5MS (5% phenyl-95% polydimethylsiloxane, 30 m × 0.25 mm, 0.25 μm) was utilized. The oven temperature was programmed to increase from 60 to 246 ºC at a rate of 3 ºC min-1. Mass spectrometer analysis was led using electron ionization (70 eV), with a mass scan range from 41 to 415 Da. The temperatures of the ion source and the GC-MS/MS interface were maintained at 220 and 240 ºC, respectively. Arithmetical retention indices (RI) were calculated using a standard n-alkanes solution (C8 to C30). Compounds were identified by comparing the mass spectra from the substances present with those reported in the literature and hits on spectral libraries Wiley and NIST.17 Di- and triterpene extraction The geopropolis samples were first pulverized into a fine powder, and then 10 g of each was separately subjected to hexane extraction for 1 h under ultrasound and then allowed to rest for 24 h. The resulting extractor solution was filtered and then evaporated to dryness in a rotary evaporator under reduced pressure (60 rpm in a water bath at 40 ºC). Thirty milligrams aliquot of each sample of the hexane fraction was solubilized in 1.5 mL of double-distilled n-hexane and analyzed in a Shimadzu gas chromatograph (model GC2010 Plus) coupled to a mass detector (model GCMS-QP2110 Ultra) of the same manufacturer, operated at 70 eV, with a fused silica capillary column Rtx-5MS® (30 m × 0.25 mm × 0.25 μm). Helium was used as the carrier gas. One microliter of each sample was injected using a Shimadzu autoinjector (model AOC-5000 Plus). The analysis was conducted under the following conditions: injector temperature: 240 ºC; injection mode: 1:20 split ratio with 3.0 mL min-1 purge; gas flow control: linear velocity; carrier gas flow: 1.0 mL min-1; program: 50-290 ºC (4 ºC min-1); mass spectrometer interface temperature: 280 ºC, ion source temperature: 260 ºC. Compounds were identified by comparing the mass spectra from the substances present with those reported in the literature17 and hits on spectral libraries Wiley and NIST.17 Statistical analysis Principal component analysis (PCA) and cluster analysis, using the Euclidean distance and the UPGMA (unweighted pair-group average) method were applied to describe the relationship between the volatile compounds (n = 56) and the collection region (n = 10). The analyses were performed using the PAST software, version Past4.17.18
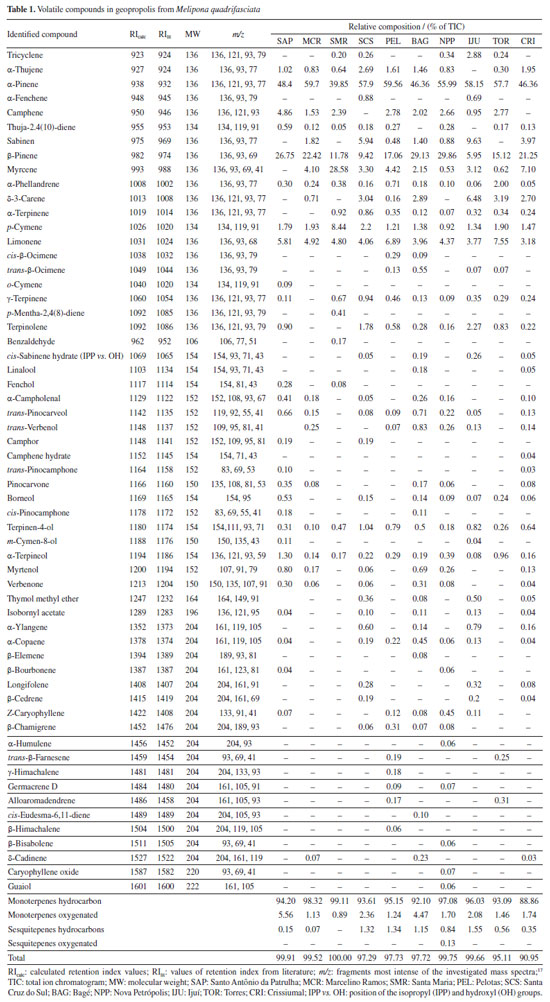
RESULTS AND DISCUSSION Terpenoids were identified in 10 samples of geopropolis obtained from Melipona quadrifasciata colonies from southern Brazil. The volatile fractions analyzed by HS-GC/MS revealed the presence of mono- and sesquiterpenes, while diterpenes and triterpenes were identified in the hexane fraction using GC-MS. In total, 37 monoterpenes, 19 sesquiterpenes, 4 diterpenes, and 3 triterpenes were identified across the samples. Mass spectra and fragmentations of the main compounds are in the Supplementary Material (Figures 1S-8S). Volatile compounds Table 1 presents fifty-six (56) mono- and sesquiterpenes identified in Melipona quadrifasciata geopropolis, which are responsible for the odor and aroma of the samples. The geopropolis from the SMR sample exhibited the lowest number of volatile terpenes (17 compounds), while the geopropolis from BAG had the highest number (36 compounds). This variability found in the chemical composition of propolis contributes to the distinct aromas of each sample collected in different regions.
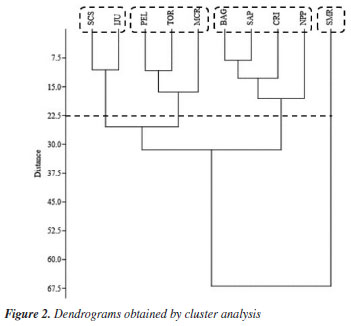
The compounds α-pinene, α-phellandrene, β-pinene, p-cymene, terpinen-4-ol, α-terpineol, and limonene were found in all geopropolis samples, with α-pinene being the predominant compound across samples. The β-pinene was the second most common compound, except in samples from SCS and IJU, where its concentration was notably lower (9.42 and 5.95%, respectively). The sample of SMR had the lowest concentration of α-pinene (39.85%). The α- and β-pinene terpenes accounted for over 67% of the compounds identified in Melipona quadrifasciata geopropolis, except in the SMR sample, where it constituted 52% of the compounds. Myrcene was the second most abundant compound in SMR geopropolis (28.58%). Terpenes are common compounds found in propolis and exhibit potential biological activity. The terpenes α-pinene, β-pinene, limonene, α-phellandrene and terpinen-4-ol have been identified in propolis from stinging bees in different regions of Brazil, as well as in other countries such as Mexico.19-22 Studies11,16,21-23 involving Meliponini species indicate that geopropolis from stingless bees is rich in terpenes, often at higher percentages than those found in Apis mellifera. The statistical analysis allowed a more detailed visualization of the presence of 56 volatile compounds in the 10 geopropolis samples. The dendrogram (Figure 2) demonstrates an ordering by cluster, where the relationship between the collection sites and the volatile compounds present can be verified, mainly in relation to the majority compounds, that is, β-pinene was decisive in the construction of the clusters. We observed that there are three groups formed with more than one sample and the fourth group with the isolated SMR sample. The first cluster formed by the SCS and IJU samples occurred due to the low concentration of β-pinene present in relation to the other samples. The second and third cluster were formed in relation to the concentrations of α-pinene and β-pinene among themselves, that is, the second group had the highest concentration of α-pinene and the third of β-pinene.
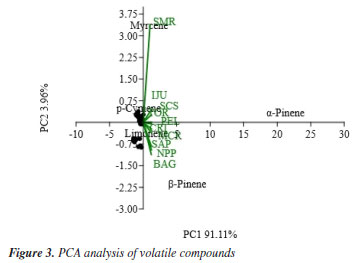
As indicated by the dendrogram, the major compounds were decisive in the formation of the clusters, and these same compounds were used for the PCA. Figure 3 shows the PCA of the samples alongside the volatile compounds with the highest concentration. Once again, the SMR sample is distinct from the others, mainly due to the presence of myrcene, as the second major compound. The quadrants for α-pinene and β-pinene can also be observed, along with their correlation to the samples. This analysis confirms the data already discussed in the dendrogram due to the difference in concentration between α- and β-pinene and the clusters formed. These results demonstrate that these two compounds serve as markers for the analyzed geopropolis samples from south of Brazil.
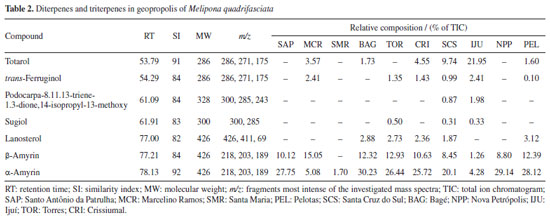
α-Pinene is originated from the resins of Pinus spp., a common conifer in the state of Rio Grande do Sul, southern Brazil. Notably, even in the Pampa biome (BAG sample), which lacks large trees, α-pinene was identified as the major component in the volatile compounds of geopropolis. Many studies20,24,25 have reported on the chemical composition of propolis and geopropolis from Brazil, highlighting a diversity of volatile compounds. However, the literature20,21,26,27 indicates that volatile compounds from propolis collected in southern Brazil contain α- and β-pinene in concentrations exceeding 50%, similar to the results found in our study. This finding suggests that geopropolis collected in southern Brazil, particularly in Rio Grande do Sul State, predominantly contains α-pinene as the major volatile compound, thus geographically characterizing geopropolis from this region. As previously noted by Leonhardt et al.,28 stingless bees use terpenes as resin identifiers, and in this context, they likely use resins rich in α-pinene from the flora of Rio Grande do Sul. Terpenes of the hexane fraction In the hexane fraction of the ten geopropolis samples, four abietane diterpenes and three triterpenes were identified (Table 2). Totarol and trans-ferruginol were found in almost all samples, while podocarpa-8,11,13-triene-1,3-dione,14-isopropyl-13-methoxy was found only in SCS and IJU samples. The abietane diterpene sugiol was found in three samples.
Pentacyclic triterpenes α- and β-amyrin were present in all samples, with α-amyrin ranging from 1.70 to 30.23% and β-amyrin ranging from 1.26 to 15.05%, except in the SMR sample where β-amyrin was absent. The variation in the percentage of α- and β-amyrin did not show uniformity in the geopropolis samples of Melipona quadrifasciata. This result is quite common, as the composition of α- and β-amyrin in geopropolis varies between bee species and geographic regions.14,29,30 Additionally, the pentacyclic triterpenoid lanosterol was identified in the samples BAG, TOR, CRI, SCS, and PEL of geopropolis obtained from Melipona quadrifasciata. The abietane diterpene totarol, found in higher percentages in geopropolis samples, has known antibacterial efficacy and has already been identified in geopropolis produced by Melipona quadrifasciata in another state in the southern region of Brazil.8,31 Abietane diterpenes are used as biomarkers for coniferous species of the families Araucariaceae, Podocarpaceae, and Pinaceae.32 Thus, it is plausible that the resin collected by SBs for geopropolis production also originates from the Podocarpus lambertii species, which is quite common in Rio Grande do Sul.33 According to Zhao et al.,34 lanosterol plays a key role in inhibiting the aggregation of lens proteins and reducing cataract formation, suggesting a new strategy for the prevention and treatment of cataracts. This compound found in geopropolis corroborates with medicinal usage by some indigenous peoples who used geopropolis as medicine in the treatment of ophthalmological infection.35
CONCLUSIONS In conclusion, the analysis of Melipona quadrifasciata geopropolis samples reveals a diverse terpenoid profile, with the monoterpene α-pinene as the predominant volatile compound, exceeding 50% concentration in some samples. Influenced primarily by regional flora such as Pinus and Podocarpus species, its presence, in high concentrations, geographically characterizes geopropolis as coming from southern Brazil. The samples showed abietane diterpenes and pentacyclic triterpenes, mainly α-amyrin, present in all samples of Melipona quadrifasciata geopropolis. Additionally, the detection of totarol, a bioactive abietane diterpene, further highlights the medicinal potential of geopropolis, particularly in terms of its antibacterial properties.
SUPPLEMENTARY MATERIAL Supplementary Material is available at http://quimicanova.sbq.org.br, in PDF format, with free access.
ACKNOWLEDGMENTS The authors thank the Fundação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
AUTHOR CONTRIBUTIONS Marta S. D Freitas: conceptualization, writing original draft preparation and editing; Matheus H. O. de Sousa: formal analysis and writing; Alexandre Piccinini: sample collection and identification; Adriana Flach: formal analysis; Neusa F. de Moura: conceptualization, writing, preparation, editing, supervision and funding acquisition.
REFERENCES 1. Popova, M.; Trusheva, B.; Bankova, V.; Phytomedicine 2021, 86, 153098. [Crossref] 2. Gabriel, M. B.; Carneiro, M. J.; de Camargo, R. C. R.; Sawaya, A. C. H. F.; Nat. Prod. Res. 2022, 36, 1626. [Crossref] 3. Zulhendri, F.; Perera, C. O.; Chandrasekaran, K.; Ghosh, A.; Tandean, S.; Abdulah, R.; Herman, H.; Lesmana, R.; J. Funct. Foods 2022, 88, 104902. [Crossref] 4. Campos, J. F.; dos Santos, H. F.; Bonamigo, T.; Domingues, N. L. C.; Souza, K. P.; dos Santos, E. L.; Oxid. Med. Cell. Longevity 2021, 2021, 2169017. [Crossref] 5. Ferreira, J. M.; Negri, G.; Salatino, M. L. F.; Message, D.; Salatino, A.; Quim. Nova 2022, 45, 1189. [Crossref] 6. dos Santos, L.; Hochheim, S.; Boeder, A. M.; Kroger, A.; Tomazzoli, M. M.; Dal Pai Neto, R.; Maraschin, M.; Guedes, A.; de Cordova, C. M. M.; J. Apic. Res. 2017, 56, 543. [Crossref] 7. Gonzatti, F.; Valduga, E.; Scur, L.; Wasum, R. A.; Rodriguésia 2021, 72, e03312018. [Crossref] 8. Torres, A. R.; Sandjo, L. P.; Friedemann, M. T.; Tomazzoli, M. M.; Maraschin, M.; Mello, C. F.; Santos, A. R. S.; Braz. J. Med. Biol. Res. 2018, 51, e7118. [Crossref] 9. Bankova, V.; Popova, M.; Trusheva, B.; Chem. Cent. J. 2014, 8, 28. [Crossref] 10. Teixeira, É. W.; Message, D.; Negri, G.; Salatino, A.; Stringheta, P. C.; J. Evidence-Based Complementary Altern. Med. 2010, 7, 307. [Crossref] 11. Torres-González, A.; López-Rivera, P.; Duarte-Lisci, G.; López-Ramírez, A.; Correa-Benítez, A.; Rivero-Cruz, J. F.; Nat. Prod. Res. 2016, 30, 237. [Crossref] 12. Leonhardt, S. D.; Blüthgen, N.; Biotropica 2009, 41, 730. [Crossref] 13. Souto, E. B.; Zielinska, A.; Souto, S. B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A. M.; Atanasov, A. G.; Marques, C.; Andrade, L. N.; Severino, P.; Int. J. Mol. Sci. 2020, 21, 1449. [Crossref] 14. Bonamigo, T.; Campos, J. F.; Alfredo, T. M.; Balestieri, J. B. P.; Cardoso, C. A. L.; Paredes-Gamero, E. J.; Souza, K. P.; dos Santos, E. L.; Oxid. Med. Cell. Longevity 2017, 2017, 1038153. [Crossref] 15. Cisilotto, J.; Sandjo, L. P.; Faqueti, L. G.; Fernandes, H.; Joppi, D.; Biavatti, M. W.; Creczynski-Pasa, T. B.; J. Pharm. Biomed. Anal. 2018, 149, 502. [Crossref] 16. Isidorov, V. A.; Maslowiecka, J.; Szoka, L.; Pellizzer, N.; Miranda, D.; Olchowik-Grabarek, E.; Zambrzycka, M.; Swiecicka, I.; Molecules 2022, 27, 7686. [Crossref] 17. Adams, R. P.; Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. 18. Hammer, Ø.; Harper, D. A. T.; Ryan, P. D.; PAST: Paleontological Statistics Software Package for Education and Data Analysis, version Past4.17; Palaeontologia Electronica, Oslo, Norway, 2001. 19. de Souza, E. C. A.; da Silva, E. J. G.; Cordeiro, H. K. C.; Lage Filho, N. M.; da Silva, F. M. A.; dos Reis, D. L. S.; Porto, C.; Pilau, E. J.; da Costa, L. A. M. A.; de Souza, A. D. L.; Menezes, C.; Flach, A.; Quim. Nova 2018, 41, 485. [Crossref] 20. Piccinini, A.; de Sousa, M. H. O.; Freitas, M. S. D.; Cesca, K.; de Moura, N. F.; Research, Society and Development 2022, 11, e193111234175. [Crossref] 21. Valcanaia, C. P.; Masote, J. B. B.; Sommer, H. F.; Schiquet, S.; Padilha, B.; Krepsky, L.; Paganelli, C. J.; Borges, P. P.; Danielli, L. J.; Apel, A. M. A.; Soares, K. D.; Althoff, S.; Alberton, M. D.; Botelho, T. K. R.; Guedes, A.; de Cordova, C. M. M.; Chem. Biodiversity 2022, 19, e2022003. [Crossref] 22. Pino, J. A.; Marbot, R.; Delgado, A.; Zumárraga, C.; Sauri, E.; J. Essent. Oil Res. 2006, 18, 53. [Crossref] 23. Portal, S. A.; Schiquet, S.; Amaral, B. P.; Krepsky, L. M.; Curbani, L.; Rebelo, R. A.; Rau, M.; Althoff, S. L.; Guedes, A.; de Cordova, C. M. M.; Chem. Biodiversity 2023, 20, e202300592. [Crossref] 24. de Lima, V. H. M.; Almeida, K. C. R.; Alves, C. C. F.; Rodrigues, M. L.; Crotti, A. E. M.; de Souza, J. M.; Ribeiro, A. B.; Squarisi, I. S.; Tavares, D. C.; Martins, C. H. G.; Miranda, M. L. D.; Rev. Bras. Farmacogn. 2020, 29, 807. [Crossref] 25. de Albuquerque, I. L.; Alves, L. A.; Lemos, T. L. G.; Dorneles, C. A.; de Morais, M. O.; J. Essent. Oil Res. 2008, 20, 414. [Crossref] 26. Simionatto, E.; Facco, J. T.; Morel, A. F.; Giacomelli, S. R.; Linares, C. E. B.; J. Chil. Chem. Soc. 2012, 57, 1240. [Crossref] 27. Ioshida, M. D. M.; Young, M. C. M.; Lago, J. H. G.; J. Essent. Oil Bear. Plants 2010, 13, 633. [Crossref] 28. Leonhardt, S. D.; Schmitt, T.; Blüthgen, N.; PLoS One 2011, 6, e23445. [Crossref] 29. Araújo, M. J. A. M.; Búfalo, M. C.; Conti, B. J.; Fernandes Jr., A.; Trusheva, B.; Bankova, V.; Sforcin, J. M.; Journal of Molecular Pathophysiology 2015, 4, 2. [Link] accessed in November 2024 30. dos Santos, D. C.; David, J. M.; Neto, O. C. S.; Lima, B. O.; Yatsuda, R.; Moreira, B. O.; Marques, L. M.; Frazão, R. F.; Quim. Nova 2024, 47, e-20230096. [Crossref] 31. Almuhayawi, M. S.; Saudi J. Biol. Sci. 2020, 27, 3079. [Crossref] 32. Otto, A.; Wilde, V.; The Botanical Review 2001, 67, 141. [Crossref] 33. Carvalho, P. E. R.; Espécies Arbóreas Brasileiras, 1a ed.; Embrapa: Colombo, PR, Brasil, 2003. 34. Zhao, L.; Chen, X. J.; Zhu, J.; Xi, Y. B.; Yang, X.; Hu, L. D.; Ouyang, H.; Patel, S. H.; Jin X.; Lin, D.; Wu, F.; Flagg, K.; Cai, H.; Li, G.; Cao, G.; Lin, Y.; Chen, D.; Wen, C.; Chung, C.; Wang, Y.; Qiu, A.; Yeh, E.; Wang, W.; Hu, X.; Grob, S.; Abagyan, R.; Su, Z.; Tjondro, H. C.; Zhao, X. J.; Luo, H.; Hou, R.; Jefferson, J.; Perry, P.; Gao, W.; Kozak, I.; Granet, D.; Li, Y.; Sun, X.; Wang, J.; Zhang, L.; Liu, Y.; Yan, Y. B.; Zhang. K.; Nature 2015, 523, 607. [Crossref] 35. Souza, R. C. S.; Yuyama, L. K. O.; Aguiar, J. P. L.; Oliveira, F. P. M.; Acta Amazonica 2004, 34, 333. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access