Artigo
| Ionic liquid-based dispersive liquid-liquid microextraction followed by HPLC for the determination of six effective composition of Silybum marianum |
|
Nan LiI,II; Peng SunI,II,* I. Heilongjiang Bayi Agricultural University, 163319 Daqing, Heilongjiang Province, P. R. China Received: 09/03/2024 *e-mail: byndsunpeng@sina.com A novel pretreatment method ionic liquid-based dispersive liquid-liquid microextraction followed by high-performance liquid chromatography (HPLC) was established and applied in the analysis of silychristin, silydianin, silybin, silybin B, isosilybin A, isosilybin B in Silybum marianum. The critical parameters affecting dispersive liquid-liquid micro-extraction (DLLME), including selection of dispersive solvent and extraction solvent, volume of dispersive solvent and extraction solvent, adjustment of pH, salt concentration, extraction time, were investigated by single factor study. Under optimum conditions, all of the target analytes presented good linearity (r > 0.9991) and satisfied recoveries (recoveries > 89.5%, relative standard deviation (RSD) < 4.6%). The limits of detection and quantification were 0.16 to 0.74 ng kg-1 and 0.42 to 2.52 ng kg-1, respectively. The developed method is sensitive, rapid, accurate and employable to simultaneously determine six target compounds in Silybum marianum. INTRODUCTION Silybum marianum ((L.) Gaertn.), a medicinal plant of Compositae, has a strong adaptability against drought and cold resistance.1 It is planted in Northeast China, Inner Mongolia, Hebei, Shanxi, and other places. As an economic crop for both medicine and food, its fruit is the main medicinal part, including silychristin (SCN), silydianin (SDN), silybin A (SBA), silybin B (SBB), isosilybin A (ISBA), and isosilybin B (ISBB).2 It has the functions of antioxidation,3 regulating immunity4 and protecting liver.5 A large number of studies have shown that silybin, as the highest content of Silybum marianum seed (SMS) lignin in the effective extract, can maintain the enzyme system in cells, enhance cell metabolism, protect cells and promote fat transfer.6-10 It has a significant effect on the treatment of liver diseases. It is commonly used in the treatment of simple or alcoholic fatty liver acute and chronic hepatitis, and other diseases with abnormal liver function.11-13 However, the standard methods for the determination of SCN, SDN, SBA, SBB, ISBA, and ISBB in SMS have not been established in China. Therefore, it is necessary to establish a rapid, accurate and efficient detection method for SMS compounds, which is conducive to the screening, processing and utilization of high-quality varieties of SMS. SCN, SDN, SBA, SBB, ISBA, ISBB in SMS are extracted mainly by normal temperature and ultra-high pressure extraction,14 ultrasonic assisted extraction,15 enzyme assisted extraction,16 supercritical CO2 extraction17 and microwave-assisted extraction.18 Special equipment is required for normal temperature and ultra-high pressure, supercritical CO2 extraction and microwave-assisted extraction, and corresponding enzymes need to be selected for enzyme assisted extraction. It is not easy to implement and popularize the method. It is important to establish a new method for sample pretreatment of SCN, SDN, SBA, SBB, ISBA, ISBB in order to obtain precise, accurate and reliable results, because it is difficult to extract multiple compounds from plant-derived sample. By optimizing the pretreatment conditions, we aim to enhance the method's selectivity, precision, sensitivity, and accuracy while improving analytical efficiency and reducing costs. There have been more and more ways to determine several components extracted from plant-derived sample simultaneously, such as solid-liquid phase extraction19 and dispersive solid-phase extraction.20 However, these detection methods have some shortcomings. Dispersive liquid-liquid microextraction (DLLME) was developed and applied by Rezaee et al.21 in 2006. DLLME has been widely applied to the analyses of various compounds in the environmental, food real samples and has attracted great attention due to its simplicity of operation, rapidity, low price, high recovery rate and environment friendly.22,23 Ionic liquids (ILs) are ionic media with unique physical and chemical properties. It is an environmentally friendly solvent and has attracted extensive attention in many fields. ILs is a potential solvent for DLLME to enrich typical organic compounds. In addition, these green solvents as sample preparation extractant have been used in such as supported liquid membrane extraction,24 liquid-liquid extraction,25,26 solid-phase microextraction,27,28 and so on. ILs as extractant is widely used in plant extraction.29-31 The above facts show that ILs, as a highly dispersed solvent, has great potential for the analysis of components from crop samples. Compared to traditional liquid-liquid extraction, ionic liquids dispersive liquid-liquid microextraction (ILs-DLLME) has the advantages of low sample consumption, high extraction efficiency, and easy operation. In the present work, we used ILs-DLLME combined with the high-performance liquid chromatography (HPLC) to simultaneously determine SCN, SDN, SBA, SBB, ISBA, ISBB in different SMS varieties. The key parameters affecting the efficiency of ILs-DLLME were researched.
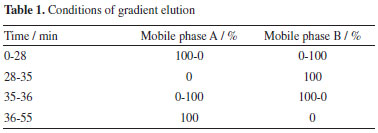
EXPERIMENTAL Materials SCN, SDN, SBA, SBB, ISBA and ISBB were purchased from the Alta Scientific Co., Ltd. (Tianjin, China). The stock solution was prepared by dissolving each substance in chromatographic grade methanol with a concentration of 1 mg mL-1 and storing it at 4 °C in the dark. Flat bottom glass insert (400 µL) was obtained from Agilent Technologies (5181-3377, USA). 1-Methyl-3-octyl imidazole hexafluorophosphate (99.5%, [OMIM]PF6), 1-hexyl-3-methylimidazole trifluoromethane sulfonate (99.5%, [HMIM]OTF) were purchased from the Macklin Reagent Co., Ltd. (Beijing, China). Methanol, ethanol and acetonitrile (chromatographic grade) were purchased from Merck (Shanghai, China). Acetone and ethanol chromatographic grade were purchased from Wuhan Huashen reagent Co., Ltd. (Wuhan, China). Phosphoric acid analytical grade was purchased from Kemio Chemical Reagent Co., Ltd. (Tianjing, China). Deionized water was used throughout the experiment. The other solvents were all analytical grade. Chromatographic analysis The chromatographic analysis was performed on an e2695 HPLC system (Waters Co., Ltd., USA) which was equipped with a diode-array detector system, an online degassing device, a thermostatic column compartment, and a two-element system. Chromatographic separation was carried out on a Waters carboxy-octadecyl silane bonded silica gel (250 mm × 4.6 mm, 5 µm) with an in-line filter in front of the column. The mobile phase flow rate was set at 1.0 mL min-1 and the mobile phase consisted of aqueous phosphoric acid-methanol-water (v/v/v, 0.5:35:65) (A) and phosphoric acid-methanol-water (0.5:50:50, v/v/v) (B). The chromatographic column temperature was set at 30 °C. The gradient elution procedure is shown in Table 1. The analytical injection was set at 10 µL. The method used a UV detector, with detection wavelength of target compounds at 288 nm.
Samples collection All of the SMS samples were sourced from a farm in Hulunbuir, China, in October 2021. After the homogenization, all of the SMS samples were kept in brown glass bottles with light-free at 4 °C. Extraction 0.5 g of the SMS were transferred into a 100 mL Teflon (PTFE) container. 50 mL 75% methanol solution was added and vortexed for 3 min. The extraction was carried out for 60 min at 60 °C under the water bath. After the extraction process, the sample was centrifuged at 8000 rpm for 10 min. The supernatant was obtained and then it was sieved through 0.45 µm membranes for further analysis ILs-DLLME Two mL of supernatant was rapidly injected into 100 µL [OMIM]PF6 to be mixed with 600 µL acetonitrile. A cloudy solution was formed in the polytetrafluoroethylene centrifugal tube and then mixed under ultrasonic for 2 min to improve the extraction rate. The mixture solution was centrifuged at 8000 rpm for 5 min; the fine particles dispersed in the extraction phase were deposited at the bottom of the polytetrafluoroethylene centrifugal. After removing the supernatant, the remaining sedimented phase was sucked by micro quantitative needle to be reconstituted into a mobile phase solution with 100 µL (phosphoric acid-methanol-water, v/v/v, 0.5:35:65) in flat bottom glass inserts with 400 µL. Then 10 µL of these treated samples were finally injected into HPLC for analyses.
RESULTS AND DISCUSSION Effect of extraction solvent and its volume The selection of extraction solvent is very important for extraction efficiency. Methanol and acetonitrile are the most commonly used as extraction solvents for target compounds from plant-derived sample.32 In this study, effect of three solvents including methanol and acetonitrile were evaluated for extraction efficiency, and the results showed that the average recoveries were higher for methanol as extraction solvent. Therefore, methanol was selected as the extraction solvent. SCN, SDN, SBA, SBB, ISBA, ISBB are flavonoids with high polarity. Therefore, methanol aqueous solution was investigated as extraction solvent to analyze the compounds, and the results are shown in Figure 1. Consequently, 75% methanol was selected as extraction solvent for the subsequent studies.
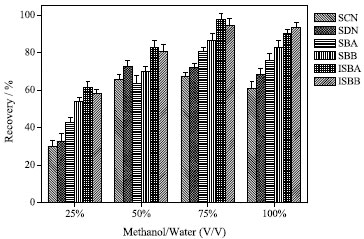 Figure 1. Effect of methanol aqueous solution (v/v) on extraction efficiency (methanol solvent, 50 mL; pH, 7.0; volume of extractant, 400 µL; dispersant, acetonitrile, 500 µL; extraction time, 5 min; no salt)
Comparing the extraction recoveries obtained by using different volumes of extraction solvents (i.e., 20, 30, 40, 50, 60, 70 and 80 mL), it showed that by using 50 mL of 75% methanol it was possible to obtain satisfactory extraction recoveries, and further increasing the solvent volume will not significantly improve the extraction recoveries, as shown in Figure 2. Thus, 50 mL was selected as the appropriate 75% methanol volume used in the following tests.
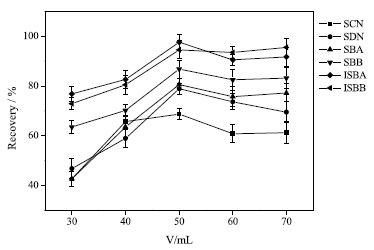 Figure 2. Effect of volume of extractant on extraction efficiency (75% methanol solvent, 50 mL; pH, 7.0; volume of extractant, 400 µL; dispersant, acetonitrile, 500 µL; extraction time, 5 min; no salt)
Optimization of ILs-DLLME conditions In order to obtain the optimal conditions of ILs-DDLME, a series of parameters affecting the extraction efficiency were studied, including extraction solvent, dispersive solvent, extraction time, pH value and ionic strength. The recovery was used as an indicator and 0.5 g samples (each analyte with 100 ng g-1 added to each sample) were used to optimize the extraction conditions. All experiments were made in triplicate, and the blank control sample was 0.5 g sample without any target compound. Effect of extraction solvent and its volume Appropriate extractant is the key to improve the analytical sensitivity. The effects of [OMIM]PF6 and [HMIM]OTF on the extraction efficiency of SCN, SDN, SBA, SBB, ISBA, ISBB were investigated. The results showed that [OMIM]PF6 had better extraction efficiency for SCN, SDN, SBA, SBB, ISBA, ISBB with less interference. Therefore, [OMIM]PF6 was selected as the extractant in this paper. The recoveries increased with the increase of volume of [OMIM]PF6. 20, 40, 60, 80, 100, 120 and 140 µL were investigated. When 100 µL of [OMIM]PF6 was added, the extraction recovery reached its maximum and stabilized, as shown in Figure 3. This is because the ILs-DLLME process reached equilibrium with 100 µL of [OMIM]PF6 as the extraction agent. Therefore, 100 µL [OMIM]PF6 was used as the optimal extraction solvent volume in subsequent experiments.
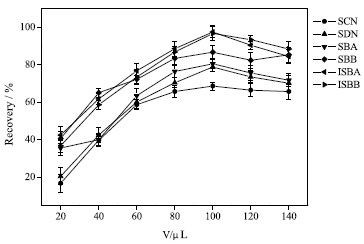 Figure 3. Effect of volume of ILs on extraction efficiency (75% methanol solvent, 50 mL; pH, 7.0; volume of extractant, 600 µL; dispersant, acetonitrile, 500 µL; extraction time, 5 min; no salt)
Effect of dispersant and its volume Dispersion solvent is considered to be another important factor affecting the extraction efficiency.33 The effects of methanol, ethanol, acetonitrile and acetone as dispersion solvents were studied. The results are shown in Figure 4. When acetonitrile was used, the recovery rate was the highest. Perhaps due to the high polarity of acetonitrile, the target compound comes into full contact with the extractant, thereby improving the extraction efficiency. Therefore, acetonitrile was selected as dispersant.
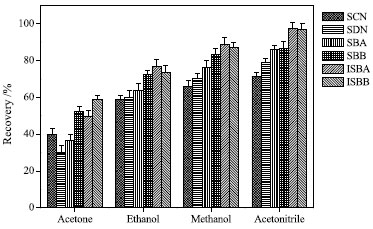 Figure 4. Effect of type of dispersant on extraction efficiency (75% methanol solvent, 50 mL; pH, 7.0; volume of extractant, 600 µL; dispersant, acetonitrile, 500 µL; extraction time, 5 min; no salt)
Because the volume of acetonitrile would affect the solubility of extraction solvent in water,34 the effect of the volume of dispersion solvent on the recovery was also investigated. In Figure 5, when 600 µL acetonitrile was used for extraction, the recovery of each analyte was the highest.
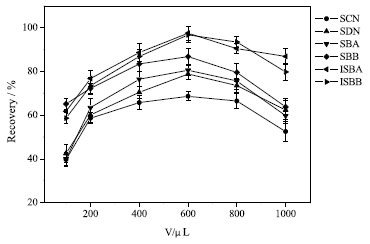 Figure 5. Effect of volume of dispersant on extraction efficiency (75% methanol solvent, 50 mL; pH, 7.0; volume of extractant, 600 µL; dispersant, acetonitrile, 600 µL; extraction time, 5 min; no salt)
Effect of sample pH value The pH value is another main factor affecting ILs-DLLME. The pH value of the sample solution can affect the stability and existing form of the tested compounds by deionizing analytes.35 The effect of sample pH value in the range of 5.0-8.0 on extraction efficiency was studied. Possibly because of analytes containing pKa between 6.4 and 7.4, the recovery of analytes was little affected by pH value. Therefore, the proposed experiment was carried out without pH factor. Effect of extraction time The establishment of extraction equilibrium takes some time. Ultrasound can accelerate the mass transfer between the two phases, shorten the extraction time and improve the extraction efficiency,36 so it is widely used to assist ILs-DLLME. The effect of ultrasonic time of 1-5 min on the extraction results was investigated. Figure 6 clearly shows that the optimal extraction time is 2 min. When the extraction time is more than 2 min, the recovery decreases slightly, mainly because some extraction solvents containing some target compounds enter the sample solution phase under ultrasonic conditions. Therefore, in the preprocessing process, the extraction time is set to 2 min.
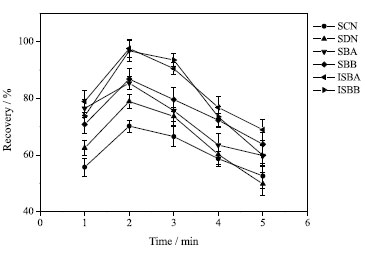 Figure 6. Effect of extraction time on extraction efficiency (75% methanol solvent, 50 mL; volume of extractant, 600 µL; dispersant, acetonitrile, 500 µL; ultrasound time, 2 min; no salt)
Effect of ionic strength The effect of ionic strength on extraction efficiency was studied by adding 0, 2, 4, 6 and 8% sodium chloride to the prepared samples. The results showed that the extraction efficiency increased with the increase of salt dosage. When the salt concentration was 4%, the extraction efficiency was the best (see Figure 7). The results showed that different salt concentrations had no significant effect on the recovery of six effective composition of Silybum marianum. Therefore, the ionic strength of the sample solution was set to 4% for further experiments.
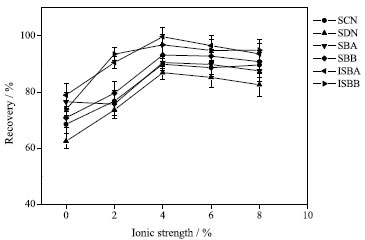 Figure 7. Effect of ionic strength on extraction efficiency (75% methanol solvent, 50 mL; volume of extractant, 600 µL; dispersant, acetonitrile, 500 µL; ultrasound time, 2 min; sodium chloride, 4%)
Analytical evaluation Linearity and limit of detection The proposed method was verified, including the linearity, precision, repeatability, limit of detection (LOD), limit of quantification (LOQ) and recovery of the six target compounds in SMS under the optimized conditions. The values obtained through validation test are shown in Tables 2 and 3.
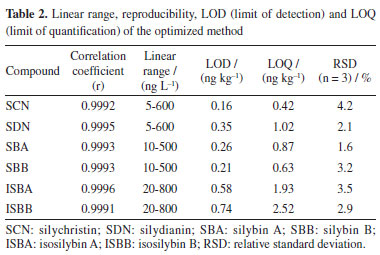
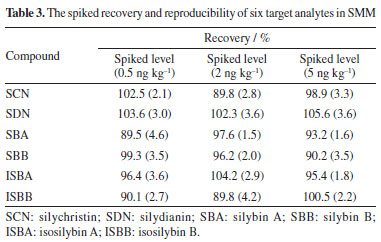
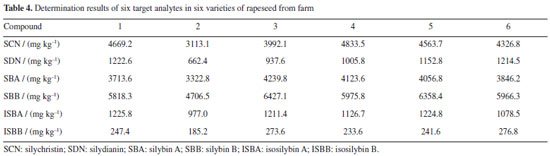
The linearity of the established method was evaluated by observing the target compounds at various concentrations. A standard curve was drawn based on the peak area (Y) and the concentration of spiked samples (X, ng kg-1), which demonstrated the linear relationship between peak area and the concentration of the six target compounds. For each level, three replicate extractions were carried out. Good linearity of response (r ≥ 0.9991) within the investigated concentration range was obtained for six targets in SMS. LOD and LOQ achieved on signal-to noise ratios of 3 and 10, for six target compounds ranged from 0.16 to 0.74 ng kg-1 and 0.42 to 2.52 ng kg-1, respectively. In order to determine the accuracy, three different concentration levels (0.5, 2.0 and 5.0 ng kg-1) were used for recovery experiments under the optimal conditions for analytes, respectively. The recovery values and relative standard deviations (RSD) are summarized in Table 3. The average recoveries obtained were in the range of 89.5 to 105.6% and RSDs were in the range of 1.5 to 4.6%, which indicates that the established method exhibited a high extraction efficiency for the six target compounds. Real samples analysis The above method was used to determine the concentration of the six target analytes in SMS, and the results are listed in Table 4. The high-performance liquid chromatograms of six target analytes are shown in Figure 8. SCN, SDN, SBA, SBB, ISBA, ISBB were detected in all SMS, which indicates that these six compounds are commonly in SMS, with SBB content being the highest, with the average reaching 37.6% among the detected compounds.
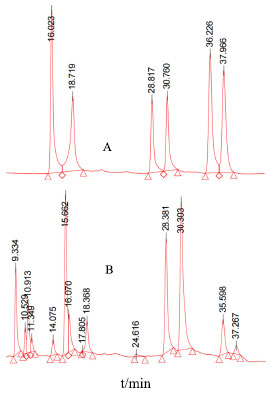 Figure 8. High-performance liquid chromatograms of six target analytes (A: 50 µg mL-1 standard solution of six target analytes; B: sample solution. SCN tR = 16.023 min, SDN tR = 18.719 min, SBA tR = 28.817 min, SBB tR = 30.760 min, ISBA tR = 36.226 min, ISBB tR = 37.966 min)
CONCLUSIONS In this work, ILs-DLLME followed by HPLC under ultrasound assisted extraction was established as an effective analytical technique for the determination of SDN, SBA, SBB, ISBA, ISBB in SMS. The developed method provides good linearity, good precision and high sensitivity, meets the requirements to determine SDN, SBA, SBB, ISBA, ISBB in SMS. The analytical technique has the advantages of simple operation and high enrichment factor. The analytical technique shows that the method has sufficient precision and accuracy, and can reliably analyze all targets in plant-derived samples.
ACKNOWLEDGMENTS This work was supported by the School Cultivation Subject of Heilongjiang Bayi Agricultural University (No. PTJH201905).
REFERENCES 1. Ha, E.-S.; Han, D.-G.; Seo, S,-W.; Kim, J.-M.; Lee, S.-K.; Sim, W.-Y.; Yoom, I.-S.; Kim, M.-S.; Molecules 2019, 24, 2180. [Crossref] 2. Surai, P. F.; Antioxidants 2015, 4, 204. [Crossref] 3. Luangchosiri, C.; Thakkinstian, A.; Chitphuk, S.; Stitchantrakul, W.; Petraksa, S.; Sobhonslidsuk, A.; BMC Complementary Altern. Med. 2015, 15, 334. [Crossref] 4. Jahanian, E.; Mahdavi, A. H.; Jahanian, R.; Livestock Science 2021, 249, 104529. [Crossref] 5. Mengesha, T.; Gnanasekaran, N.; Mehare, T.; BMC Complementary Med. Ther. 2021, 21, 104. [Crossref] 6. Wu, T.; Liu, W.; Guo, W.; Zhu, X.; Biomed. Pharmacother. 2016, 81, 460. [Crossref] 7. Aykanat, N. E. B.; Kacar, S.; Karakaya, S.; Sahinturk, V.; Pharmacol. Rep. 2020, 72, 199. [Crossref] 8. Shariati, M.; Shaygannejad, V.; Abbasirad, F.; Hosseininasab, F.; Kazemi, M.; Mirmosayyeb, O.; Esmaeil, N.; Inflammation 2019, 42, 1203. [Crossref] 9. Silva, C. M.; Ferrari, G. D.; Alberici, L. C.; Malaspina, O.; Moraes, K. C. M.; Mol. Cell. Biochem. 2020, 468, 129. [Crossref] 10. Ledur, P. C.; Santurio, J. M.; Toxicon 2020, 185, 97. [Crossref] 11. Gillessen, A.; Schmidt, H. H.-J.; Adv. Ther. 2020, 37, 1279. [Crossref] 12. Teksoy, O.; Sahinturk, V.; Cengiz, M.; İnal, B.; Ayhancı, A.; Appl. Biochem. Biotechnol. 2020, 191, 528. [Crossref] 13. Colletta, C.; Colletta, A.; Placentino, G.; J. Diabetes Metab. Disord. 2020, 19, 883. [Crossref] 14. Duan, L.; Carrier, D. J.; Clausen, E. C.; Appl. Biochem. Biotechnol. 2004, 114, 559. [Crossref] 15. Takase, M.; Feng, W.; Wang, W.; Gu, X.; Zhu, Y.; Li, T.; Yang, L.; Wu, X.; Fuel Process. Technol. 2014, 123, 19. [Crossref] 16. Wianowska, D.; Gil, M. In Water Extraction of Bioactive Compounds - From Plants to Drug Development; Dominguez González, H.; González Muñoz, M. J., eds.; Elsevier, 2017, p. 385. [Crossref] 17. Yang, G.; Zhao, Y.; Feng, N.; Zhang, Y.; Liu, Y.; Dang, B.; Asian J. Pharm. Sci. 2015, 10, 194. [Crossref] 18. Zheng, X.; Wang, X.; Lan, Y.; Shi, J.; Xue, S. J.; Liu, C.; Sep. Purif. Technol. 2009, 70, 34. [Crossref] 19. Wang, Q.; Feng, Q.; Hu, G.; Gao, G.; Zhu, X.; Epri, J. E.; Microchem. J. 2021, 175, 107098. [Crossref] 20. Sun, P.; Gao, Y.; Lian, Y.; Food Analytical Methods 2017, 10, 3217. [Crossref] 21. Rezaee, M.; Assadi, Y.; Hosseini, M.-R. M.; Aghaee, E.; Ahmadi, F.; Berijani, S.; J. Chromatogr. A 2006, 1116, l. [Crossref] 22. Wu, Q. H.; Li, Z.; Wang, C.; Wu, C. X.; Wang, W. N.; Wang, Z.; Food Analytical Methods 2011, 4, 559. [Crossref] 23. Junza, A.; Dorival-García, N.; Zafra-Gómez, A.; Barrón, D.; Ballesteros, O.; Barbosa, J.; Navalón, A.; J. Chromatogr. A 2014, 1356, 10. [Crossref] 24. Zeng, C. J.; Hu, Y.; Luo, J. W.; Microchim. Acta 2012, 177, 53. [Crossref] 25. Anvar, S. A.; Torbati, M.; Farajzadeh, M. A.; Mogaddam, M. R. A.; Food Analytical Methods 2020, 13, 1282. [Crossref] 26. Li, F.; Peng, C.; Yuan, K.; Xu, Q.; Song, H.; J. Mol. Liq. 2021, 339, 117048. [Crossref] 27. Tian, Y.; Feng, J.; Wang, X.; Luo, C.; Sun, M.; J. Chromatogr. A 2019, 1583, 48. [Crossref] 28. Yang, J.; Dong, X.; Hu, Y.-H.; Wang, Q.-Y.; Wang, S.-L.; Cao, J.; Zhang, H.-H.; J. Chromatogr. A 2019, 1602, 150. [Crossref] 29. Li, Y.; Fang, F.; Sun, M.; Zhao, Q.; Hu, Y.; Sui, Z.; Liang, Z.; Zhang, L.; Zhang, Y.; Talanta 2020, 213, 120848. [Crossref] 30. Si, R.; Han, Y.; Wu, D.; Qiao, F.; Bai, L.; Wang, Z.; Yan, H.; Talanta 2020, 207, 120247. [Crossref] 31. Fang, L.; Tian, M.; Yan, X.; Xiao, W.; Row, K. H.; J. Chromatogr. A 2019, 1592, 31. [Crossref] 32. Li, J.; Wu, F.; Zhang, Y.; Feng, J.; Wang, X.; Yang, Y.; Wang, Z.; Zhang, H.; Food Chem. 2023, 399, 133901. [Crossref] 33. Huang, P.; Zhao, P.; Dai, X.; Hou, X.; Zhao, L.; Liang, N.; J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2016, 1011, 136. [Crossref] 34. Jing, X.; Huang, X.; Zhang, Y.; Wang, M.; Xue, H.; Wang, X.; Jia, L.; Food Chem. 2022, 367, 130664. [Crossref] 35. Binjawhar, D. N.; Mohammedsaeed, W.; J. King Saud Univ., Sci. 2024, 36, 103303. [Crossref] 36. Elik, A.; Sarac, H.; Durukan, H.; Demirbas, A.; Altunay, A.; Microchem. J. 2022, 181, 107809. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access