Artigo
| Renewable hydrocarbons obtained by catalytic pyrolysis of Jatropha curcas L. OIL |
|
Jesyka M. GuedesI,* I. Programa de Pós-Graduação em Ciências Naturais, Universidade do Estado do Rio Grande do Norte, 59610-210 Mossoró - RN, Brasil Received: 10/15/2024 *e-mail: jesyka.mg@gmail.com The growing global demand for energy and environmental concerns have driven the search for renewable fuel sources. This study investigated the production of renewable hydrocarbons through the catalytic pyrolysis of Jatropha curcas L. oil, using the mesoporous material KIT-6 impregnated with cobalt oxide (Co3O4) as the catalyst. The catalysts were characterized using techniques such as X-ray diffraction (XDR), X-ray fluorescence (XRF), scanning electron microscopy (SEM) with energy dispersive spectroscopy (EDS), and textural analysis. The catalytic cracking study was carried out through thermogravimetry and analytical pyrolysis. The mesoporous structure of KIT-6 was preserved after impregnation, although there was a reduction in surface area and total pore volume. The pyrolysis tests showed that Co/KIT-6 reduced the reaction temperature, favoring the formation of hydrocarbons in the C10-C18 range, suitable for biofuels such as biokerosene and green diesel. Compared to the thermal process, the use of the catalyst significantly increased the production of hydrocarbons while reducing the formation of undesirable oxygenated compounds. It is concluded that Co/KIT-6 is a promising catalyst for the sustainable production of biofuels from vegetable oils. INTRODUCTION The growing demand for fossil fuels, combined with environmental concerns, has driven the search for new energy sources, such as high-energy-density liquid biofuels.1-3 In this context, the production of biofuels from oilseeds that do not compete with the food market has garnered considerable interest.4-6 Various non-edible biological sources are being studied for biofuel production through catalytic pyrolysis, including agro-industrial residues, lignocellulosic biomass, forest residues, and microalgae.7-9 Recent studies show that agricultural residues have high potential for this application due to their abundance and low cost, resulting in bio-oils rich in oxygenated compounds and hydrocarbons.7 Similarly, lignocellulosic biomass derived from forest residues and urban solid waste has demonstrated efficiency in bio-oil production, particularly when mesoporous catalysts are used to promote the deoxygenation of compounds.8,9 Thus, using non-edible sources for pyrolysis helps meet energy demands without competing with food production, highlighting the relevance of the present study on Jatropha curcas L. oil. This oilseed stands out as a promising biomass due to its high oil yield, low acidity, and ease of cultivation.10,11 One of the most widely used methods for biofuel production is the catalytic cracking of vegetable oils. This process involves increasing the temperature in the presence of catalysts, favoring the formation of products similar to fossil fuels at lower temperatures.12-15 Although heterogeneous catalysts, such as metal oxides, are widely employed, they have limitations, such as low specific surface area and thermal stability, requiring the use of catalytic supports.16,17 In this scenario, porous materials have shown great catalytic potential in biofuel production due to their structural properties, which enable greater selectivity in product formation.15 Among these materials, KIT-6 stands out for its high average pore diameter, wall thickness of approximately 4-6 nm, three-dimensional cubic symmetric structure (Ia3d), high pore volume, large surface area, and bicontinuous network of interpenetrating channels.18 Cobalt oxide (Co3O4) also exhibits excellent performance in biofuel production due to its high catalytic activity in the deoxygenation of organic compounds.19,20 Previous studies21,22 have shown that cobalt supported on silica can achieve hydrocarbon yields exceeding 90% in the green diesel range. This study aimed to obtain renewable hydrocarbons from the thermocatalytic cracking of Jatropha oil using the mesoporous material KIT-6 impregnated with cobalt oxide as a catalyst.
EXPERIMENTAL Synthesis of the KIT-6 mesoporous material The synthesis of the mesoporous material (KIT-6) occurred under acidic conditions by the hydrothermal method and was performed according to the proposal by Kleitz et al.23 following the molar ratio: 1 TEOS: 0.017 P123: 1.83 HCl: 195 H2O: 1.31 BuOH. For the synthesis of 200 g of gel, 4.9 g of the non-ionic triblock copolymer surfactant Pluronic P123 (EO20PO70EO20, MW = 5800, Aldrich) was dissolved in 176.4 mL of distilled water (H2O) and 7.6 mL of concentrated hydrochloric acid (HCl, 37%). The mixture was stirred for 6 h at 303.15 K. Then, 6 mL of n-butanol (C4H9OH, Vetec, 99.4%) was added, and the stirring was continued for 1 h at the same temperature.Subsequently, 11.4 mL of TEOS (Sigma-Aldrich, 98%) based on silica were added to the solution. This mixture was stirred under the same conditions and then the resulting gel was aged in a Teflon autoclave at 373.15 K for another 24 h under static hydrothermal conditions. The precipitated product was cooled, filtered, washed with an alcoholic solution of HCl (2%), then dried overnight at 373.15 K, and finally calcined in a muffle furnace at 823.15 K with a heating ramp of 283.15 K min-1, in the air for 6 h. Synthesis of the Co/KIT-6 catalyst The deposition of cobalt oxide on the KIT-6 support was carried out using the wet impregnation method with excess solvent, employing cobalt nitrate hexahydrate (Co(NO3)2·6H2O, 99%) as the precursor, as described by Sanz et al.24 The oxide concentration was adjusted to 10% relative to the support mass, using 0.5 g of KIT-6. For the preparation, cobalt nitrate was dissolved in 40 mL of distilled water, after which the support was added to the solution. The mixture was kept under constant stirring on a hot plate at 343 K until a homogeneous gel was formed. The resulting gel was transferred to an oven and dried at 343 K for 12 h. After impregnation, the solid was subjected to a calcination process to decompose the nitrate ions and convert the metal salt into oxide. Calcination was performed with gradual heating at a rate of 10 K min-1 up to 673 K, a temperature that was maintained for 6 h to ensure the complete conversion of the compound. Catalysts characterization X-ray diffraction analysis (XRD) was performed on Rigaku - Mini Flex II equipment using a monochromatic CuKα beam as the radiation source and a nickel filter with a 30 kV tube voltage and 15 mA. The high angle method was used using a calibration slit, 0.02 step, and 1 s time for reading 2θ angle from 5º to 90º. The low-angle method used 0.005 steps and a time of 0.4 s to read the 2θ angle from 0.5º to 3º. The chemical composition of the catalysts was investigated by X-ray fluorescence spectrometry in a Bruker S2 Ranger apparatus (Billerica, Massachusetts, USA) using lead (Pd) anode radiation in vacuo, maximum power of 50 W, the maximum voltage of 50 kV, and 2 mA current with XFlash® silicon bypass detector. The results of the thermal analysis tests were obtained on Shimadzu TGA 50 equipment, using an alumina crucible as sample port, N2 as purge gas with a flow rate of 100 mL min-1, the tests were conducted from 298.15 to 1073.15 K with a heating rate of 283.15 min-1 using 10 mg of sample. To quantify changes in textural properties N2 adsorption/desorption isotherms were used. The area and the pore diameters of the samples were determined using the ASAP 2020 surface property analyzer from Micrometrics (Norcross, Georgia, USA), under a constant temperature of 77 K. For this procedure, the materials were previously degassed at a temperature of 573.15 K for 10 h. Before performing the analysis, the samples were degassed at 393.15 K for 6 h. The specific area was determined by the BET method (Brunauer, Emmett, and Teller), the total pore volume was obtained at a pressure of 0.98 p/p0, by the Gurvich method. To estimate the mean diameter and distribution of pores, the mathematical model BJH (Barrett, Joyner, Halenda) for the desorption isotherm was used. Scanning electron microscopy (SEM) analyses were performed on Shimadzu's MIRA3 FERG equipment. For analysis of energy dispersive spectroscopy (EDS) the equipment FEG Quanta 450 environmental with EDS/EBSD was used. Obtaining Jatropha oil and catalytic testing by thermogravimetry The Jatropha seeds were initially ground and then dried in an oven at 343 K for 48 h to remove all moisture. Subsequently, the oil was extracted using a Soxhlet system with hexane as the solvent for a period of 10 h. After extraction, the solvent was recovered. The catalytic cracking of the Jatropha oil was obtained by thermobalance on Shimadzu TGA 50 equipment at the atmospheric pressure, using an alumina crucible. The purge gas with a flow rate of 100 mL min-1 in N2 was used. The tests were conducted from 303.15 to 1073.15 K with a heating rate of 283.15 K min-1 using 10 mg of sample, 10% m/m of the catalyst in oil being used. Analytical pyrolysis The thermal and thermocatalytic pyrolysis of Jatropha curcas oil was carried out using a PY-2020iS Control pyrolyzer from Frontier LAB, coupled to a QP 2010 GC/MS (gas chromatography-mass spectrometry) system from Shimadzu. For pyrolysis, the samples were placed in a stainless steel crucible (EcocupSF) with a volume of 50 μL. In the case of catalytic pyrolysis, the catalyst was previously mixed with the oil at a mass ratio of 10% (0.1 g of catalyst per 1 g of oil), ensuring adequate contact during heating. The oil-catalyst mixture was carefully homogenized before being transferred to the crucible. The analyses were performed under a helium gas atmosphere with a flow rate of 3.0 mL min-1, a pyrolyzer temperature of 500 ºC, a split ratio of 200:0, and a pyrolyzer-GC interface temperature of 250 ºC. The fast pyrolysis products were separated using an RTX-1 PONA capillary chromatographic column (100% dimethylpolysiloxane) with a length of 30 m, a diameter of 0.25 mm, and a stationary phase thickness of 0.25 μm. The initial column pressure was 49.5 kPa, with a flow rate of 1.00 mL min-1 and a linear velocity of 36.1 cm s-1. The chromatographic oven was programmed as follows: temperature at 40 ºC for 5 min, followed by heating up to 280 ºC at a rate of 10 ºC min-1. The total oven programming time was 46 min. The GC/MS interface temperature was maintained at 250 ºC. The detection range in the mass spectrometer was set between 20 to 400 m/z, with a scan interval of 1 s. The quantity of each identified compound is expressed as a percentage by the software. The data tabulation was performed as percentages, and the division of the compounds generated during pyrolysis was classified into hydrocarbons, oxygenated compounds, and others. Based on the amounts of hydrocarbons obtained, the selectivity percentage for each carbon range was calculated.
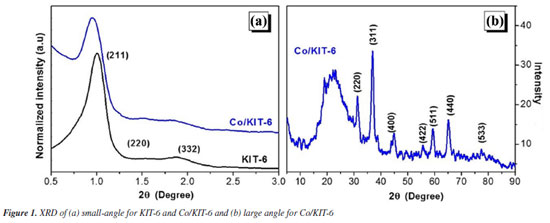
RESULTS AND DISCUSSION The low-angle X-ray diffractograms for KIT-6 and Co/KIT-6 (Figure 1) showed three diffraction peaks whose Miller indices are (211), (220) and (332). These peaks are characteristic of the three-dimensional mesoporous structure with space group Ia3d, typical of ordered cubic structures.25 Impregnation with cobalt oxide in KIT-6 did not significantly modify the structure of the mesoporous material, therefore the structural characteristics of KIT-6 were maintained. Co3O4 was identified as the only type of cobalt oxide formed with peaks referring to the reflection planes (220), (311), (400), (422), (511), (440) and (533) according to crystallographic reference (JCPDS: 65-3103). An increase in the interplanar distance of KIT-6 for Co/KIT-6 was observed, due to the insertion of the oxide in the mesoporous material.26
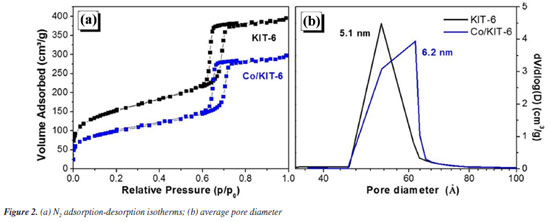
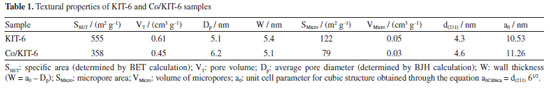
The N2 adsorption and desorption results (Figure 2) show that both samples presented type IV isotherms (Figure 2a), with a hysteresis loop H1 referring to mesoporous materials, and highly ordered as expected for KIT-6.27 The isotherm profile of the materials indicates that this material has an ordered and uniform pore distribution, which was maintained after the cobalt oxide impregnation. The textural properties of the materials are listed in Table 1. It is possible to observe that the specific areas (SBET) and the total pore volume (VT) decrease with the oxide impregnation, probably due to the oxide deposition in different places of the material, such as in the external area, in the micropores and inside the mesopores.
According to the TGA/DTG curves of the catalysts before calcination (Figure 3) and the mass loss values (Table 2), it is possible to identify each mass loss event. The first event refers to the output of physisorbed water from the catalysts. In KIT-6, this occurs due to the hygroscopicity of the mesoporous material, which can promote moisture retention. The second event is related to the loss of mass of the organic driver. From the data presented, it is evident that all organic drivers are removed at temperatures below 823.15 K in KIT-6. Thus, it is confirmed that the calcination temperature used was ideal for the total removal of the organic driver.
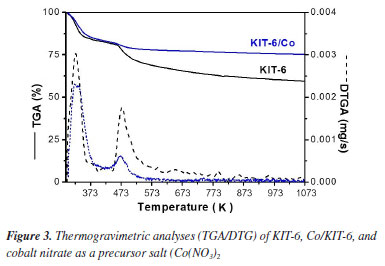
Co/KIT-6 features two weight loss events. The first, the output of physisorbed water, occurs at 417.15 K. The second, between 417.15 to 567.15 K, is associated with the reaction of nitrate ions, releasing NOx and forming cobalt oxide (Co3O4).28 The predefined calcination temperature guarantees the removal of nitrate from the used source and the formation of cobalt oxide. X-ray fluorescence (XRF) analysis of Co/KIT-6 resulted in 7.8% cobalt oxide, confirming its presence in the catalyst. The experimental proportion approached the theoretical value, indicating that the impregnation process was correctly conducted on the KIT-6 mesoporous material. The images obtained through scanning electron microscopy (SEM) of the KIT-6 and Co/KIT-6 are shown in Figure 4.
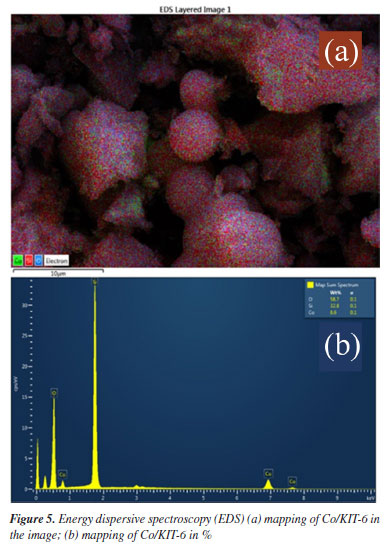
The SEM images (Figure 4) of the catalysts show a compacted morphology with irregular shapes and sizes, maintained after impregnation of cobalt oxide in the structure.29,30 The EDS (Figure 5) technique showed that the cobalt oxide is evenly distributed in the material and presents results close to the theoretical ones, corroborating the results of the XRF technique.
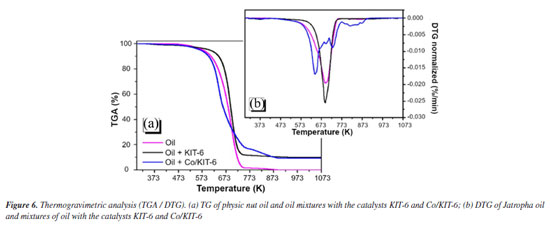
The TGA/DTG analysis (Figure 6) showed that the thermal cracking of Jatropha oil and the mixture with KIT-6 presents a single event, with mass loss up to 766.15 K, corresponding to the decomposition/volatilization of saturated oils and unsaturated. Similar results were found in a previous study.31 The presence of pure KIT-6 did not lead to further mass loss events in Jatropha oil, as expected. The remaining 10% is due to the mass of the catalyst.
During thermocatalytic cracking with Co/KIT-6, two mass loss events were observed, the quantification of mass loss values are shown in Table 3.
The first event, possibly related to chain breakage, occurs up to 673.15 K, and the volatilization/decomposition of the oil in the presence of the catalyst occurs with a maximum peak (DTG) at 641.15 K, confirming the efficiency of the catalyst at lower temperatures compared to the thermal process, with a difference of 325.15 K to any less. The second event, up to 712.15 K, corresponds to the volatilization of compounds with higher molecular mass and/or chemical structures capable of performing interactions that hinder their volatilization. The products obtained from the analytical pyrolysis of Jatropha oil were quantified and divided into the following chemical groups: hydrocarbons, oxygenated compounds, unidentified compounds (others), according to data in Table 4.
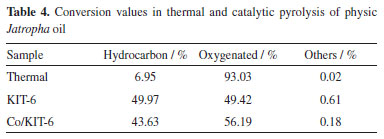
The results presented in Table 4 demonstrate that the presence of catalysts in the reaction promotes the greater formation of hydrocarbons, while in the process in the absence of catalyst it promoted the formation of oxygenated compounds. The linoleic and oleic fatty acids present in the oil composition are unstable at high temperatures, resulting in oxygenated compounds. Thermopyrolysis mainly formed lower molecular weight compounds, including cyclic compounds.32,33 Thus, it is possible to perceive that the compounds formed in thermopyrolysis are predominantly compounds of lower molecular mass, with a predominance of cyclic compounds, mainly referring to the gases formed in the process. The reaction with KIT-6 and Co/KIT-6 resulted in the formation of hydrocarbons, with greater formation of open-chain compounds. The porous structure of KIT-6 is capable of promoting the diffusion of compounds resulting from primary cracking.34 On the other hand, the presence of acidic sites in Co/KIT-6 promotes the formation of lower molecular mass hydrocarbons and cyclic compounds necessary for biofuel stability. The hydrocarbons obtained in thermal and thermocatalytic cracking were divided into carbon bands, C3 to C9 band 1 (similar to gasoline) and C10 to C18 band 2 (biokerosene and green diesel) and band 3 (similar to green diesel). These results are presented in Table 5.
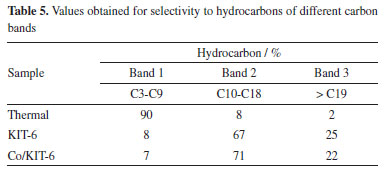
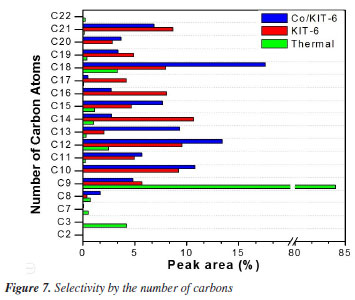
KIT-6, even with low acidity, for the pyrolysis process showed greater selectivity for hydrocarbons in the green diesel range (C10-C18). This result may be related to the diffusion of compounds in the porous structure of the material. Previous studies35,36 indicate that the diffusion process promotes the fragmentation of the carbon chains of these compounds, enabling the obtaining of hydrocarbons with a higher number of molecular masses. On the other hand, the presence of Co/KIT-6 in the process led to a greater formation of hydrocarbons corresponding to the range of biokerosene and green diesel. This behavior may be related to the deoxygenating properties of the catalyst, proving its efficiency. The catalytic cracking of fatty acids present in Jatropha oil, such as oleic acid (C18:1) and linoleic acid (C18:2), occurs due to the combined action of high temperature and the presence of catalysts like Co/KIT-6. In this process, thermal energy promotes the cleavage of carbon-carbon double bonds, generating free radicals. These radicals initiate chain reactions, such as beta-scission and deoxygenation, which result in the formation of saturated and unsaturated hydrocarbons with shorter chains, primarily in the C5 to C9 range. For example, in the case of oleic acid, the initial cleavage of the double bond generates alkyl radicals. These radicals can undergo dehydrogenation and rearrangements via beta-scission, leading to the formation of compounds such as nonane (C9H20). The presence of the Co/KIT-6 catalyst enhances these reactions by providing acidic and metallic sites that stabilize intermediates and promote deoxygenation, reducing the oxygen content in the final products and increasing the efficiency of hydrocarbon formation within the biofuel range. These mechanisms and chemical reactions are consistent with behaviors reported in the literature,37,38 demonstrating the efficiency of Co/KIT-6 in the catalytic cracking process. In the present study, a significant increase in the fraction of hydrocarbons in the C9 to C21 range was observed (Figure 7). These hydrocarbons are essential for producing biofuels with properties suitable for engine applications, including bio-kerosene and green diesel. Furthermore, the literature39 reports that cyclization reactions during cracking lead to the formation of aromatic and unsaturated hydrocarbons, which enhance the calorific content and improve the ignition properties of the biofuel. Thus, the results obtained confirm the effectiveness of the Co/KIT-6 catalyst in promoting deoxygenation reactions and optimizing the production of high-quality biofuels.
CONCLUSIONS The obtained catalysts exhibited good structural, chemical, textural, and morphological properties. Furthermore, tests conducted using thermogravimetric analysis with Jatropha oil demonstrated that all catalysts were efficient, with Co/KIT-6 standing out due to its higher catalytic activity, which significantly reduced the reaction temperature. Moreover, the reactions performed in the fast pyrolysis system using Co/KIT-6 resulted in a higher conversion of hydrocarbons. This performance highlights the catalyst's high selectivity in forming hydrocarbons, particularly within the bio-kerosene and green diesel ranges, while promoting a lower generation of oxygenated compounds. Therefore, these results reinforce the significant potential of Co/KIT-6 as a catalyst for producing renewable hydrocarbons, representing a promising alternative for the development of sustainable biofuels.
ACKNOWLEDGMENTS The authors would like to thank the Central Analítica-UFC (funded by Finep-CT-INFRA, CAPES-Pró-Equipamentos, and MCTI-CNPq-SisNano2.0) for the microscopy measurements, the Coordination for the Improvement of Higher Education Personnel, Brazil (CAPES, Finance Code 001), the National Council for Scientific and Technological Development, Brazil (CNPq, process No. 316044/2021-0), and the Ceará Foundation for the Support of Scientific and Technological Development (FUNCAP).
REFERENCES 1. Singh, A.; Rodríguez Jasso, R. M.; Gonzalez-Gloria, K. D.; Rosales, M.; Cerda, R. B.; Aguilar, C. N.; Singhania, R. R.; Ruiz, H. A.; Bioresour. Technol. Rep. 2019, 7, 100257. [Crossref] 2. Mona, S.; Kumar, S. S.; Kumar, V.; Parveen, K.; Saini, N.; Deepak, B.; Pugazhendhi, A.; Sci. Total Environ. 2020, 728, 138481. [Crossref] 3. Moonsrikaew, W.; Duangchan, A.; Mol. Catal. 2022, 523, 111712. [Crossref] 4. Li, G.; Zhang, C.; Wang, Z.; Huang, H.; Peng, H.; Li, X.; Appl. Catal., A 2018, 550, 67. [Crossref] 5. Kucharska, K.; Słupek, E.; Cieśliński, H.; Kamiński, M.; Chem. Pap. 2020, 74, 1199. [Crossref] 6. Ruatpuia, J. V. L.; Halder, G.; Vanlalchhandama, M.; Lalsangpuii, F.; Boddula, R.; Al-Qahtani, N.; Niju, S.; Mathimani, T.; Rokhum, S. L.; Fuel 2024, 370, 131829. [Crossref] 7. Yate, A. V.; Narváez, P. C.; Orjuela, A.; Hernández, A.; Acevedo, H.; Food Bioprod. Process. 2020, 122, 72. [Crossref] 8. Alherbawi, M.; McKay, G.; Mackey, H. R.; Al-Ansari, T.; Renewable Sustainable Energy Rev. 2021, 135, 110396. [Crossref] 9. Chiaramonti, D.; Prussi, M.; Buffi, M.; Tacconi, D.; Appl. Energy 2014, 136, 767. [Crossref] 10. Chen, R.-X.; Wang, W.-C.; Renewable Energy 2019, 135, 819. [Crossref] 11. Al-Layla, N. M. T.; Saleh, L. A.; Fadhil, A. B.; J. Anal. Appl. Pyrolysis 2021, 156, 105088. [Crossref] 12. Liu, J.; Wu, L.; Wang, R.; Xue, X.; Wang, D.; Liang, J.; Bioresour. Technol. 2024, 398, 130510. [Crossref] 13. da Costa, A. A. F.; Pires, L. H. O.; Padrón, D. R.; Balu, A. M.; da Rocha Filho, G. N.; Luque, R.; do Nascimento, L. A. S.; Mol. Catal. 2022, 518, 112052. [Crossref] 14. Bhattacharyya, M.; Shadangi, K. P.; Mahanta, P.; Mohanty, K.; Chem. Pap. 2023, 77, 4877. [Crossref] 15. Cao, X.; Li, L.; Shitao, Y.; Liu, S.; Hailong, Y.; Qiong, W.; Ragauskas, A. J.; J. Anal. Appl. Pyrolysis 2019, 138, 137. [Crossref] 16. Somo, T. R.; Mabokela, T. E.; Teffu, D. M.; Sekgobela, T. K.; Hato, M. J.; Modibane, K. D.; Chem. Pap. 2021, 75, 2237. [Crossref] 17. Ganesan, R.; Subramaniam, S.; Paramasivam, R.; Sabir, J. S. M.; Josephin, J. S. F.; Brindhadevi, K.; Pugazhendhi, A.; Sci. Total Environ. 2021, 757, 143781. [Crossref] 18. Konwar, R. J.; De, M.; J. Anal. Appl. Pyrolysis 2014, 107, 224. [Crossref] 19. Wen, J.-W.; Zhang, D.-W.; Zang, Y.; Sun, X.; Cheng, B.; Ding, C.-X.; Yu, Y.; Chen, C.-H.; Electrochim. Acta 2014, 132, 193. [Crossref] 20. Wang, A.; Shi, Y.; Yang, L.; Fan, G.; Li, F.; Catal. Commun. 2021, 153, 106302. [Crossref] 21. Ochoa-Hernández, C.; Yang, Y.; Pizarro, P.; de la Peña O'Shea, V. A.; Coronado, J. M.; Serrano, D. P.; Catal. Today 2013, 210, 81. [Crossref] 22. Shim, J.-O.; Jeon, K.-W.; Jang, W.-J.; Na, H.-S.; Cho, J.-W.; Kim, H.-M.; Lee, Y.-L.; Jeong, D.-W.; Roh, H.-S.; Ko, C. H.; Appl. Catal., B 2018, 239, 644. [Crossref] 23. Kleitz, F.; Choi, S. H.; Ryoo, R.; Chem. Commun. 2003, 2136. [Crossref] 24. Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E. S.; Microporous Mesoporous Mater. 2012, 158, 309. [Crossref] 25. Ramanathan, A.; Maheswari, R.; Barich, D. H.; Subramaniam, B.; Microporous Mesoporous Mater. 2014, 190, 240. [Crossref] 26. Toncón-Leal, C. F.; Múnera, J. F.; Arroyo-Gómez, J. J.; Sapag, K.; Catal. Today 2022, 394-396, 150. [Crossref] 27. Thommes, M.; Kaneko, K.; Neimark, A. V.; Olivier, J. P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. S. W.; Pure Appl. Chem. 2015, 87, 1051. [Crossref] 28. Tiernan, M. J.; Fesenko, E. A.; Barnes, P. A.; Parkes, G. M. B.; Ronane, M.; Thermochim. Acta 2001, 379, 163. [Crossref] 29. Dou, Y.-Q.; Zhai, Y.; Zeng, F.; Liu, X.-X.; Tu, B.; Zhao, D.; J. Colloid Interface Sci. 2010, 341, 353. [Crossref] 30. Boulaoued, A.; Fechete, I.; Donnio, B.; Bernard, M.; Turek, P.; Garin, F.; Microporous Mesoporous Mater. 2012, 155, 131. [Crossref] 31. Biswas, S.; Sharma, D. K.; J. Anal. Appl. Pyrolysis 2013, 99, 122. [Crossref] 32. Melo, A. C. R.; Araujo, A. S.; Silva, E. F. B.; Oliveira, R. M.; Fernandes Jr., V. J.; Luz Jr., G. E.; Souza, A. G.; Fuel Process. Technol. 2011, 92, 1340. [Crossref] 33. Vu, X. H.; Nguyen, S.; Dang, T. T.; Armbruster, U.; Journal of Vietnamese Environment 2014, 6, 270. [Crossref] 34. Yu, F.; Gao, L.; Wang, W.; Zhang, G.; Ji, J.; J. Anal. Appl. Pyrolysis 2013, 104, 325. [Crossref] 35. Benson, T. J.; Hernandez, R.; French, W. T.; Alley, E. G.; Holmes, W. E.; J. Mol. Catal. A: Chem. 2009, 303, 117. [Crossref] 36. Luz Jr., G. E.; Santos, A. G. D.; Melo, A. C. R.; Oliveira, R. M.; Araujo, A. S.; Fernandes Jr., V. J.; Fuel Process. Technol. 2011, 92, 2099. [Crossref] 37. Mascarenhas, A. J. S.; Oliveira, E. C.; Pastore, H. O.; Quim. Nova Esc. 2001, 25. [Link] accessed in February 2025 38. Negm, N. A.; Rabie, A. M.; Mohammed, E. A.; Appl. Catal., B 2018, 239, 36. [Crossref] 39. Karnjanakom, S.; Guan, G.; Asep, B.; Hao, X.; Kongparakul, S.; Samart, C.; Abudula, A.; J. Phys. Chem. C 2016, 120, 3396. [Crossref]
Associate Editor handled this article: Eduardo H. S. Sousa |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access