Artigo
| Investigations on the effect of sodium trimetaphosphate and sodium sulfate concentrations on the properties of cross-linked cassava starch films |
|
Marisa Ferreira KarowI; Karine Laste MacagnanII I. Departamento de Ciência e Tecnologia de Alimentos, Faculdade de Agronomia Eliseu Maciel, Universidade Federal de Pelotas, 96160-000 Pelotas - RS, Brasil Received: 04/30/2024 *e-mail: angelitadasilveiramoreira@gmail.com Films based on pure native starch have limited applications, are easily breakable, very hygroscopic, and permeable to water vapor. One method for improving these properties is to modify starch by cross-linking. The aim was to evaluate the effect of the addition of sodium trimetaphosphate (STMP), sodium sulfate (SS) and native cassava starch (S) on the properties of modified cassava starch films using a 23 factorial design comparing the concentration of the crosslinkers (5.0, 12.5 and 20% STMP), SS (3.0, 9.0 and 15%) and native cassava starch (S) (5.0, 7.5 and 10%) m/v. All cross-linked cassava starch films had better easiness of handling, continuity, and brightness than the control film. Generally, cross-linking reduced the water solubility and tensile strength and increased thickness, elongation and the films' yellowish color and opacity. Reagents' concentration affected most of the dependent variables. The SMTP*SS interaction positively affected water vapor permeability (WVP), while negatively affected solubility. Tensile strength was positively affected by SMTP. As for elongation, the S*SS interaction was positive, while the effect of STMP*SS was negative. Treatment 1, using the lowest concentrations of reagents, presented positive results, such as excellent macro and microscopic appearance. These findings also qualify the crosslinked starch for use in coatings formulations. INTRODUCTION Biodegradable films were developed to minimize environmental damage caused by traditional non-biodegradable plastic materials, which are widely used in packaging including for foods.1 Biodegradable or edible films and coatings are among the new environmentally-friendly methods for post-harvest conservation of fresh fruits, minimally processed or processed by dehydration.2,3 To be suitable for food packaging, biodegradable films must be non-toxic and have certain essential characteristics, such as low hygroscopicity, selective permeability to gases and water vapor, as well as good mechanical properties such as elongation and tensile strength. And, preferably, they must have low cost.4 The coatings are applied or formed directly on the surfaces of fruits, forming thin membranes that are normally imperceptible to the naked eye and having various structural characteristics, which are dependent on the formulation of the film-forming solution.5 The coating should prevent excessive water loss or gain, reduce microbial proliferation and provide selective gas permeability (CO2 and O2).6 As these coatings become part of the consumed food, the materials used must be GRAS (generally recognized as safe), that is, they must be non-polluting, non-toxic, safe for use in foods, and inexpensive.7,8 Starches are among the most abundant organic materials in nature, and these polysaccharides have numerous industrial applications.9 They are widely used as powders for providing viscosifying, gelling and stabilizing agents. They have also been employed for making films and coatings of variable purposes,10 but some main features of the native forms limit their applicability. Cassava starch is extracted from the Manihot esculenta Crantz plant. It is also known as starch or manioc powder; it is a fine, white, odorless and tasteless powder. Cassava is known for its rapid growth, especially in tropical countries. However, its characteristics vary significantly depending on geographical location, cultivated variety, and plant age.11,12 Cassava starch typically contains 17% amylose and 83% amylopectin, this ratio being crucial for its physicochemical properties.12 Previously used as a filler material for producing eco-friendly and cost-effective plastic materials, starch now plays a crucial role as a raw material in various industries such as plastics, paper, textiles, and food packaging.13,14 Despite its benefits in terms of its biodegradability, biocompatibility, toxicity and cost-benefit ratio, starch does not fully meet the properties required for protective food coatings, particularly with regard to its poor mechanical performance.15,16 Native starch films and coatings are also typically very soluble and provide poor vapor barriers. To improve or adapt these properties, starch can be subjected to modifications that increase its applications and value.10,17-19 Modified starches have been used to develop biodegradable coatings and films for food packaging, as they represent improvements in the physical, chemical, morphological and mechanical properties compared to those made from native starch.18,20 Chemical modifications involve reactions including etherification, esterification, crosslinking, grafting or decomposition21 for addition of functional groups to starch. Cross-linking is one of the most important chemical modifications applied to polymers, including starch. The cross-linking of polymers is a process that occurs when linear or branched polymer chains are interconnected. Connections between the linear molecules produce high molecular weight three-dimensional polymers.22,23 Crosslinking agents are low-molecular mass molecules, preferably having at least two reactive functional groups enabling forming a bridge between polymer chains.24 For polysaccharide crosslinking, various agents are used, including boric acid,25 ferulic acid,26 and glutaraldehyde.27 One useful agent is sodium trimetaphosphate (SMTP), widely employed for its low toxicity, low cost and high reactivity. It reacts by replacing a few hydroxyl groups from starch, imparting anionic features to it - phosphate groups; it renders possible to obtain materials having properties improved both relative to those of natural starch and of enlarged application.28 Sodium sulfate (SS) is an alkalizing agent which inhibits starch gelatinization as well as promotes its crosslinking,29 allowing the crosslinking agent to deeply penetrate within the granule.29,30 As in any chemical reaction, the substrate concentration and amount of reagents influences the products yield and quality. Thus, the amount of starch in the reaction is also important.31 The objective of this study was to evaluate the effects of the concentrations of the cross-linking agent STMP, the alkalizing agent SS and the cassava starch on the physical, chemical and mechanical properties of films based on cross-linked cassava starch.
EXPERIMENTAL Cross-linking reactions For the chemical modification of native cassava starch, commercial (YOKI®, Brazil) native cassava starch with 12% (w/w) humidity - determined according to the AOAC 44-15A method32 - was purchased from a local store in the city of Pelotas, RS, and chemically modified by cross-linking. Sodium trimetaphosphate (STMP) p.a. (Synth®, Brazil), anhydrous sodium sulfate (SS) p.a. (Synth®, Brazil), glycerol p.a. (Synth®, Brazil), alcohol 96% (v/v), and distilled water were also used in the experiments. To evaluate the effects of SMTP cross-linking agent (5.0, 12.5 and 20% w/w), alkalizing agent SS (3.0, 9.0 and 15.0% w/w) and cassava starch (5.0, 7.5 and 10% w/w) a complete factorial design 23 (Table 1) was performed.
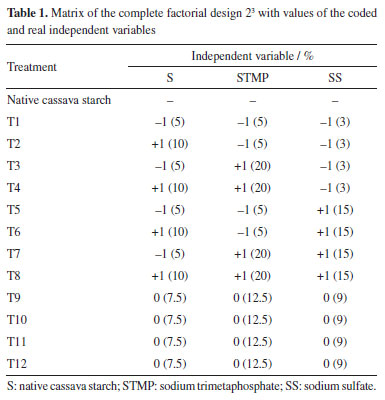
Cassava starch cross-linking was performed according to Soares et al.,21 with modifications in reaction time and reagent concentrations. The dispersions were performed in distilled water and at room temperature. These mixtures were placed in a water bath with mixing at 65 ºC for 15 min (BMA, RC Labor®, Brazil). The systems were then transferred to another water bath at 45 ºC and the pH was adjusted to 9.5 with a 1 M NaOH solution. Afterwards, SMTP at 5.0, 12.5 and 20.0% (w/w) and SS in proportions of 3.0, 9.0 and 15.0% relative to the mass of starch were added. When needed, the pH was again adjusted to 9.5 (K39-1420A, Kasvi®, Brazil). These suspensions were mixed for 120 min. After the reaction time, the pH was adjusted to 6.0 with a 1 M HCl solution. The samples were washed with ethanol (96%), filtered, dried in an oven at 45 ºC until constant weight, then crushed and sieved at a 100-mesh sieve. To control the safety of the modified starches obtained, the powders residual phosphorus content was determined using a commercial colorimetric kit (K020, Bioclin®, Brazil). The values of residual phosphorus in all treatments agreed with the Food and Drug Administration (FDA)7 and Food and Agricultural Administration (FAO)33 standards concerning starches modified with sodium trimetaphosphate, that is, less than 0.04%. Characterization of native and crosslinked starch Scanning electron microscopy (SEM) The morphology of native and modified starch was analyzed in a scanning electronic microscope (JSM-6610LV, Jeol®, USA). Samples, previously dried in an oven at 40 ºC, were manually dispersed on a carbon adhesive tape, contained in an aluminum sample holder and recovered with gold, with recovery thickness of 20 nm. The energy of the beam was 1 pA and the beam power, 10 kV. Infrared spectroscopy (IR) Infrared spectra were assessed by pelletizing 2 mg of the crushed and dried samples (100 mesh) in 200 mg potassium bromide spectroscopic grade; the analyses were conducted in an IR Prestige 21 spectrophotometer (Shimadzu®, Japan), at the wavenumber range of 4000 to 400 cm-1, in the transmittance mode, with 60 scans and resolution of 4 cm-1.34 Rheometric analysis Aqueous solutions of native and crosslinked cassava starches at 3% (m/v) were prepared with different concentrations of sodium trimetaphosphate crosslinking agent and sodium sulfate alkalizing agent. The tests were of the rotational kind, in a Rheostress 600-RS150 rheometer (Haake®, Germany) with a temperature controller (Peltier, ± 0.1 ºC). Viscosity was determined with the aid of shear stress vs. strain rate curves at 25 ºC, utilizing cone and plate geometry (sensor C35/1º; 0.052 mm gap) and shear rate of 0.1-400 s-1, during 400 s. The parameters consistency index k (kPa s-1) and flow index ɳ (dimensionless), of the Ostwald-de-Waele rheological model, were obtained from the viscosity curves (mPa s) versus strain rate (s-1). Preparation and characterization of films Based on the native cassava starch and the modified starches (T1 to T12) obtained by the crosslinking process described above according to a 23 factorial design, 13 films were prepared by the casting method. To the films were assigned the labels CF (control film) and F1 to F12, respectively. The film-forming solutions were prepared using a starch/glycerol/distilled water 3.0:0.9:97.1 (w/w/w). The mixtures were kept at 85 ºC for 30 min in a mechanical agitator (RW20.n, Ika Labortechnik®, Brazil) and 20 mL aliquots were distributed in 9 cm diameter Teflon® plates, followed by drying in an oven at 56 ºC for 24 h. The samples were then stored at 25 ± 3 ºC in an environment with a relative humidity (RH) of 55 ± 3%, which was obtained with the aid of a saturated solution of magnesium nitrate. Macroscopic and microscopic properties The films were macroscopically evaluated for their general appearance, following the parameters described by Gontard et al.,35 including the following: homogeneity (absence of insoluble particles and bubbles, uniform coloring), continuity (lack of ruptures or easily broken zones) and easiness of handling (easiness of removing films from the support, low or absent tackiness). SEM analysis of the films was performed using a digital scanning electron microscope (model 440, Leo®, United Kingdom) using a small fragment of each film previously dried in an oven at 40 ºC. The films were manually dispersed on adhesive carbon tape on an aluminum sample holder and coated with a 20 nm thick gold coating. The beam current used was 1 pA, and the beam power was 10 kV. Water vapor permeability (WVP) and solubility The permeability was determined according to Gontard et al.,35 the films were placed in cells containing silica gel (RH = 0%, 0 kPa vapor pressure), constituting a membrane. The cells were then placed inside a desiccator containing distilled water (RH = 100%, 3.17 kPa vapor pressure) in a room at 22 ± 3 ºC. After six days the cells were weighed on a semi-analytical scale. The permeability was calculated by means of Equation 1. 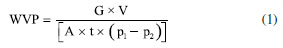 where: WVP = water vapor permeability (g mm m-2 day kPa); G = weight gained by the cell during 24 h (g); V = mean film thickness (mm); A = film permeation surface (m2); t = time (days); and p1 - p2 = vapor pressure gradient between the film surfaces (3.17 kPa). Water solubility of the films was determined according to the methodology described by Zamudio-Flores et al.,36 with modifications in relation to stirring time. Circular film samples having diameters of 2 cm were cut, and the initial percentage of dry matter in each sample was determined after drying in an oven at 105 ºC until constant weight. After weighing, the samples were placed in Erlenmeyer flasks with 50 mL of distilled water, followed by mixing at 200 rpm for 24 h at 25 ºC. After this period, the samples were dried in an oven at 105 ºC until constant weight. The percentage of total soluble material was calculated as follows (Equation 2): 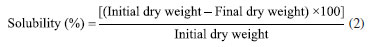 Thickness and mechanical properties The films thickness was evaluated according to the ASTM F2251-13 method37 using the arithmetic average of ten random measurements on the surface made using a digital micrometer (model IP-54, Insize®, Brazil). The results were expressed in mm. The tensile strength and percent elongation of the starch films were evaluated on a texture analyzer (Texture Analyzer, TA.XT Plus®, United Kingdom) according to the ASTM 882-12 method.38 Three samples from each treatment, having 80 mm length and 25 mm width, were evaluated at an initial clamp separation of 40 mm and a test velocity of 0.8 mm s-1. The tensile strength was calculated by dividing the maximum force at rupture of the films by the cross-sectional area; the results were expressed in MPa. Elongation was determined by dividing the final distance achieved at rupture of the films by the initial distance (40 mm) multiplied by 100; the results were expressed in percentage.39 Color and opacity The color and opacity of the films were determined by the average of five evaluations with one center point and four perimeter points using a colorimeter (CR 300, Minolta®, Japan) and the CIELAB color measurement system. The color space parameters obtained were L* (black/white) as well as the chromaticity coordinates a* (green/red) and b* (blue/yellow). The film opacity was analyzed with the relationship between the film opacity superimposed on the black standard (Sblack) and white standard (Swhite).40 Statistical analysis The analyses were performed in triplicate and the comparisons were made using an analysis of variance (ANOVA) test using the Statistica 7.0 program (StatSoft®, Germany, 2008), for the analysis of water vapor permeability, water solubility, thickness, tensile strength, elongation, color and opacity. A value of p < 0.05 was considered significant. The mathematical models were developed based on the regression coefficients.
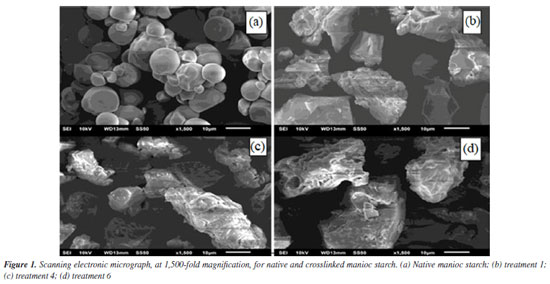
RESULTS AND DISCUSSION Characterization of native and crosslinked starch Scanning electronic microscopy (SEM) Figure 1 illustrates the morphology of native manioc starch and of some crosslinked manioc starches (T1, T4 and T6). SEM analysis revealed the characteristic structure of native manioc starch granules (Figure 1a), of truncated or oval shape, with sizes in agreement with those reported in the literature - 7 to 14 μm.41 As for crosslinking-modified starches, the breakage of characteristic starch granules and formation of irregular, bigger solid structures could be observed (Figures 1b to 1d). Treatment T4 (Figure 1c) resulted in apparently more compact granules; while in the treatments T1 and T6 samples (Figures 1b and 1d, respectively), which share lower amount of STMP crosslinking agent, orifices of different diameters in the fragments can be observed.
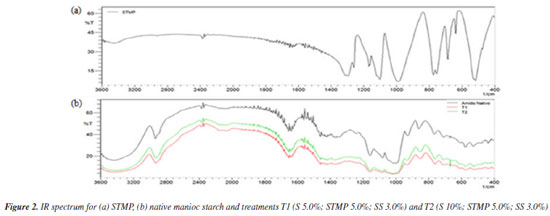
Infrared spectroscopy (IR) Figure 2a shows STMP spectra, this compound being employed in the present study as crosslinking agent, and in Figure 2b the native manioc starch and crosslinked starches T1 and T2 spectra can be seen. Crosslinked starches samples enable us to observe some of the main starch bands and those of sodium trimetaphosphate, exhibiting, however, modified intensity and in certain cases, a shift. From the STMP spectrum (Figure 2a) it is possible to observe the main characteristic bands: at 520 cm-1, the band related to the out-of-plane vibrational stretching for the phosphorus links of the kind (O-P-O) and/or (P=O); at 685 cm-1, due to the (O-P-O) symmetrical stretching; bands at the region between 745 and 785 cm-1 are related to the (P-O-P) symmetrical stretching; between 995-1085 cm-1 are bands due to the (P-O-P) asymmetric vibrational stretching; bands at 990 and 1120 cm-1 can also be attributed to (P-O-P) symmetrical stretching; the band in the region of 1165 cm-1 can be attributed to the (P-O-P) symmetrical stretching; bands in the region of 1240 to 1320 cm-1 refer to the asymmetrical stretching of the (P=O) link.42 Figure 2b shows the infrared spectrum of native manioc starch used in the experiment. In the region between 3200 and 3600 cm-1 the presence of a wide band resulting from the hydroxyl group stretching vibrations -O-H (axial deformation), associated by hydrogen bonds can be seen. At approximately 2860 cm-1 are the stretching vibrations related to C-H bonds. In the region between 1440-1480 cm-1 the angular deformation of the CH2 group can be seen. Bands positioned in the regions of 920 and 1160 cm-1 are due to C-O and C-C bond stretching. Stretching of glycosidic bonds (α-1,4 C-O-C) are observed in the region of 1050 to 900 cm-1, more specifically, ring symmetric and asymmetric axial deformations at 1040 and 1160 cm-1, respectively. The starch spectrum shows two weak bands (shoulders) at approximately 1047 and 1022 cm-1, which are attributed to axial deformations of primary alcohols C-OH and to CH2.42
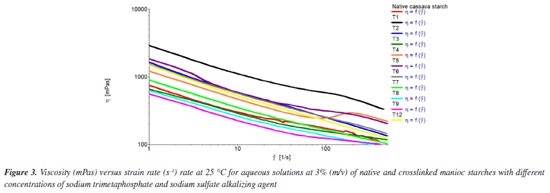
According to Smith,43 these bands can be associated with the starch crystalline ordination (increasing proportionately with the polymer crystallinity), and with the amorphous characteristic of starch (increasing with the reduction in crystallinity), respectively. In the region around 1050 and 1100 cm-1, stretching of primary and secondary alcohols O-H band of the starch ring is observed. The band seen at 1644 cm-1 is related to the presence of bonded water, which was also observed for starch samples in other works.44,45 In Figure 2b, the spectra of crosslinked samples T1 and T2 under different conditions can also be observed. The bands in the region of 1047 cm-1 related to the crystalline structure, and 1022 cm-1, related to the native starch amorphous structure, respectively, and already previously reported, are poorly defined, although being prominent. As for the native manioc starch, slight reduction in the band intensity in the 1047 cm-1 region was observed. This can be attributed to the crosslinked sample reduction in crystallinity degree, and a slight enlargement and reduction in the 1022 cm-1 band intensity, related to the increased polymer amorphous structure. According to Li et al.,46 changes in this organization after the crosslinking reaction could be proven by the intensity of these bands. For the crosslinked starches a slight enlargement and a lower intensity of the bands related to the hydroxyl groups unfolding in the region between 3200 and 3600 cm-1 was also observed, based on the SMPT crosslinking reaction with the starch ring hydroxyls. Such fact was also observed in the works by García et al.,47 and Peng et al.48 However, there was significant band reduction in the region of 1100 cm-1, caused by the starch-SMTP crosslinking process involving secondary alcohols hydroxyl groups (such as C4) of the glucose ring and primary alcohols (C6), reducing the intensity of these absorption bands. As relates to the intensity of the bands and their positions in relation to the main functional groups of the crosslinking agent - situated at 520 cm-1 (O-P-O) and/or (P=O); 685 cm-1 (O-P-O); 745 and 785 cm-1 (P-O-P); 995 and 1085 cm-1 (P-O-P); 990 and 1120 cm-1, (P-O-P); 1165 cm-1 (P=O); 1240 to 1320 cm-1 (P=O). P=O at 1210 cm-1 and P-O-C (810 cm-1) - their characterization was not effectively possible for the crosslinked starch samples since they were superimposed to those of native starch. A further factor would be the samples low crosslinking degree, as already reported in the works by Li et al.,46 and Peng et al.,48 However, very intense crosslinking is not desirable, since this could render the material insoluble. Rheometric analyses The viscosity versus strain rate curves of Figure 3 represent the rheological behavior of native manioc starch aqueous solutions and crosslinked starches obtained from the different treatments.
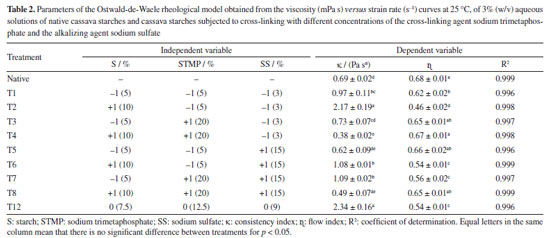
Significant changes were observed for the rheological behavior of crosslinked starches relative to native starch, with a trend to increased pseudoplasticity, and variable effects of crosslinking on viscosity. Indexes of consistency (k) and flow (ɳ), related to the viscosifying capacity and pseudoplasticity, respectively, are reported in Table 2.
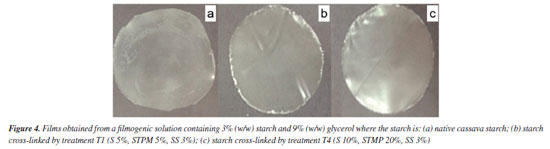
The highest k values were obtained for the T2 treatments, with the lowest concentrations of crosslinking and alkalizing agents relative to starch, and T12, which corresponds to the central spot (Table 2). Values for ɳ (flow behavior indices) were lower than unity (1) which characterizes non-Newtonian fluids with pseudoplastic behavior, that is, the farther from unity, the higher the pseudoplasticity, characterized by reduction in viscosity with increase in stress. The same behavior was observed by López et al.,49 in flow curves for starch and modified starches filmogenic suspensions. The T12 treatment samples, representing the central spot, and the T2 treatment sample exhibited the best results. Preparation and characterization of films Macroscopic and microscopic properties According to Figure 4 the cross-linking reaction was favorable for film formation. The cross-linked cassava starch films had better homogeneity, easiness of handling, continuity and brightness (Figures 4b and 4c) than the film made from native starch (Figure 4a).
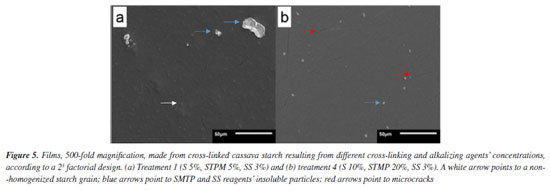
During the dehydration of the filmogenic solution to form the films, because of the driving force of water evaporation, a large number of expanded starch granules and their residues are randomly aggregated and compacted to make the films.50 In the case of the films obtained with cross-linked starches - T1 and T4, the cross-linking process disrupts the granules furthering the starch dissolution during the heating of the filmogenic solution, enabling more translucent and flexible films. However, the translucency can also be negatively affected by insoluble residues of the agents used in the cross-linking process. Various studies with edible films and coatings composed of native and chemically modified starches, whether associated with other polymers or not, have shown improvements in various characteristics.51 The appearance of films or macroscopic properties is not usually described, probably because it is considered a subjective analysis. However, according to Dang and Yoksan,52 the overall appearance is important with regard to consumer acceptance, and should be taken into account. In an unusual way Vicentino et al.,53 reported that the films of acetylated, acetylated oxidized, cationic, and etherified cassava starch showed greater homogeneity at the surface, with emphasis on the acetylated starch that showed better barrier properties, proving to be efficient for wrapping ‘Benitaka' grapes which increased their shelf life for an additional 12 days. A few SEM microscopy can be seen in Figure 5. Films surfaces have microcracks and insoluble particles derived from the cross-linking reagents.
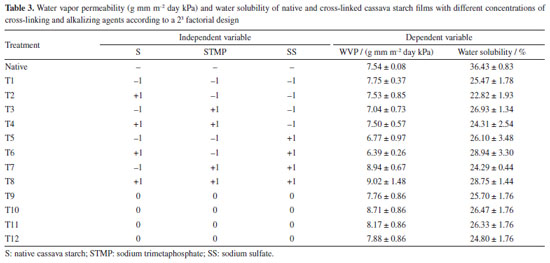
The film obtained using the T1 cross-linked starch (less STPM relative to starch and SS) exhibits non-homogenized starch granules, which impart a more irregular appearance, but in compensation smaller and less numerous microcracks; also, the insoluble particles are larger and more irregular. In turn, for the film obtained using the T4 cross-linked starch (more STPM relative to starch and SS), a homogeneous surface is observed, with particles resulting from smaller and better-distributed cross-linking. The microcracks, however, are larger and more numerous. The microcracks can be resolved by increasing the proportion of glycerol, used in this study as plasticizer. In other studies, granules and their fragments were also detected in films from other starch sources such as in Montaño-Leyva et al.,54 for wheat starch and in Zhang and Han55 for yellow field pea starch. Water vapor permeability (WVP) and solubility WVP is a measure of the ease with which water vapor can penetrate a material.56 In the case of films, lower permeability values are desirable to prevent the product inside the films from absorbing external moisture. Coatings are used to reduce loss of natural moisture of the coated product, such as in natura fruits. The differences in the respiratory activity of packaged vegetables may imply a greater or lesser need for a permeability barrier. According to Müller et al.,57 a material with higher permeability to water vapor may be suitable for fresh vegetables with a high respiratory rate, while a low-permeability film is beneficial for use on dehydrated products. Table 3 shows the results for WVP data obtained in the present study; the interactions of these responses are shown in Figure 6. Compared to the control film, obtained with native cassava starch, the test films had variable values, evidencing the influence of the concentration of reagents used in the crosslinking process. The T5 and T6 films, obtained from starches reticulated with low concentration of STMP and high concentration of SS, presented the lowest values. Comparing these films with the T3 and T4 films, obtained with the highest STMP concentration, a similar effect can be seen on WVP caused by the highest SS concentration (T5 and T6), the latter being, however, much more economic and safer. However, a simultaneous use of both the highest STMP and SS concentration are contraindicated due to increase in WVP values, as can be seen in the T7 and T8 films.
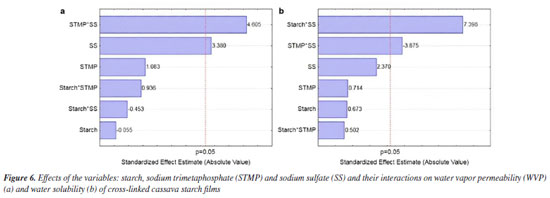
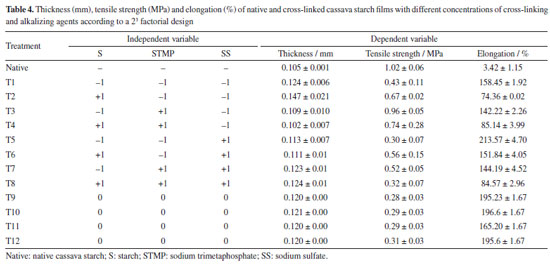
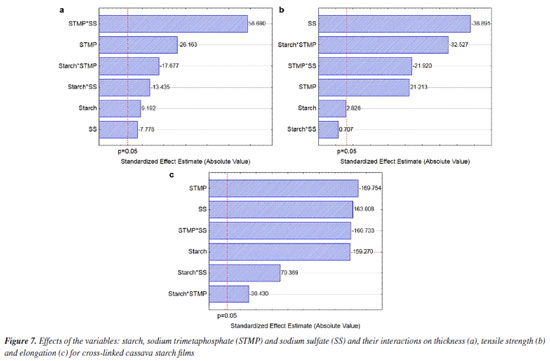
Figure 6 shows the effects of starch and concentrations of the STMP and SS reagents and their interactions on water vapor permeability (WVP). The STMP*SS interaction had a greater unwelcome positive effect on water vapor permeability (Figure 6a). The WVP value for the films produced with the cross-linked starches was reduced and varied between 6.39 (T6) and 9.02 (T8) g mm m-2 day kPa. The mathematical models for the WVP and solubility obtained with the coded variables are shown in Equations 3 and 4, respectively. WPV - 7.79 - 0.02 × S + 1.02 × STMP + 0.32 × SS + 0.28 × S × STMP - 0.14 × S × SS + 1.38 × STMP × SS (3) Solubility = 25.96 + 0.36 × S + 0.38 × STMP + 1.28 × SS + 0.27 × S × STMP + 3.99 × S × SS - 2.09 × STMP × SS (4) Some chemical modifications of starch can increase WVP. Biduski et al.,56 using native sorghum starch submitted to a double modification (acid hydrolysis followed by oxidation) found increased WVP for films from modified starches. The values were 3.94, 4.99 and 5.31 g mm m-2 day kPa for the films prepared from native starch and 5.48, 5.70 and 5.97 g mm m-2 day kPa for films prepared from dual modified starches. These values were slightly lower (higher WVP barrier) than those obtained in the present study. The reduction in WVP can also be obtained by adding less hydrophilic substances. Chinma et al.58 evaluated the effects of temperature (10 to 40 ºC) and relative humidity (50 and 80%) on the WVP (g mm m-2 day kPa) of edible films prepared from manioc starch and soy protein concentrate. Films prepared from manioc starch only without soya protein addition exhibited water vapor permeability of 3.9 to 4.6 g mm m-2 day kPa without statistical difference as for temperature and relative humidity variation. These values are also lower than those found in the present work. Ghasemlou et al.59 assayed WVP for manioc starch films to which plant essential oils had been incorporated. For the control film, which did not contain any essential oil, WVP was around 7.8 g mm m-2 day kPa, this figure being close to those found in the present study. The cross-linking favorably reduced water solubility in all films; films achieved water solubility values between 22.82 and 36.43% (native starch). The higher significant effect was that of the starch*SS interaction (Figure 6b); higher concentrations of these reagents increase the solubility value. This corroborates the data for T6 and T8 films, which exhibited the higher values among the crosslinked films. Basiak et al.60 found values of 30.16, 44.76 and 14.52% for the solubility of films prepared with native starches from wheat, corn and potato, respectively, showing that films solubility depends on the starch source. In the present study the solubility of native cassava starch film was 36.43%, but cross-linking reduced it. The water solubility of biodegradable films is dependent on the type of application; films used in products with high moisture content must have low solubility values, while edible films must have higher water solubility.58 Thickness and mechanical properties The results obtained for thickness (mm), tensile strength (MPa) and elongation (%) of the films composed of cross-linked cassava starch are shown in Table 4 and the effects of starch, sodium trimetaphosphate (STMP) and sodium sulfate (SS) and their interactions on these responses are shown in Figure 7.
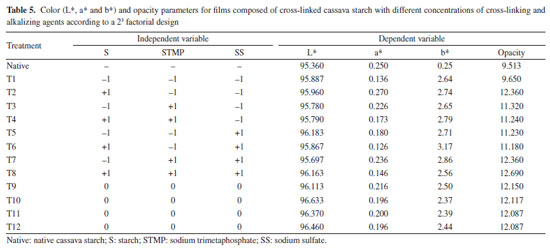
The cross-linking increased the film thicknesses, except for the T4 film, which had the lowest thickness, followed by the T3 film, both with the highest STMP and lowest SS concentrations. However, all of them were thin and varied between 0.102 and 0.147 mm thick, as shown in Table 4. According to Embuscado and Huber,61 biodegradable films are generally less than 0.300 mm thick. This is an important physical characteristic, as their use for packaging requires consideration of the type, volume, and weight of the food to be stored. The values obtained in this study were close to the values observed in the study by Pinto et al.,62 that used "pinhão" (edible seeds of Araucaria brasiliensis) starch, which were between 0.127-0.157 mm thick. All independent variables had significant effects on thickness (Figure 7a). The highest effect was observed for the positive STMP*SS interaction, increasing the film thickness. As in the response to WPV, the result of the interaction effect or simultaneous high addition of STMP*SS demonstrates the negative crosslinking power on starch. It is sure that cross-linking affects film thickness. However, as with the soaking power of crosslinked materials, high crosslinking level reduces the soaking power (water retention within the polymer network), while intermediate crosslink increases the soaking power.63 Higher water retention within the structure results in higher material thickness,63 which explains the higher value observed for T2 - treatment where the lowest SMTP and SS were used, associated with the higher value for starch. The formation of a loose network generates hygroscopic films of greater thickness. The mathematical models obtained for thickness, tensile strength and elongation with the coded variables are shown in Equations 5, 6 and 7, respectively. Thickness = 0.12 + 0.003 × S - 0.009 × STMP - 0.003 × SS - 0.006 × S × STMP - 0.005 × S × SS + 0.021 × STMP × SS (5) Tensile strength = 0.475 + 0.02 × S + 0.15 × STMP - 0.275 × SS - 0.23 × S × STMP + 0.005 × S × SS - 0.155 × STMP × SS (6) Elongation = 158.68 - 48.99 × S - 52.21 × STMP + 50.14 × SS - 9.36 × S × STMP + 21.65 × S × SS - 49.44 × STMP × SS (7) Tensile strength decreased by cross-linking (0.28 ± 0.03 to 0.96 ± 0.05 MPa), and elongation was intensively increased by cross-linking (74.36 ± 0.02 to 213.57 ± 4.70%) compared to films made from native cassava starch (1.02 ± 0.06 MPa and 3.42 ± 1.15%, respectively) (Table 4). For the tensile strength and elongation response variables, the starch*STMP and STMP*SS interactions showed negative effects (Figures 7b and 7c). The interaction of starch*SS was positive. The desired properties for packaging depend on the specific application. In general, packaging that requires extended elongation requires greater tensile strength to provide structural integrity to the packaged product. In other applications, such as in food coatings, a package with greater flexibility is desirable.64 The composition of the films and coatings generally includes plasticizers, compounds that improve the physical or mechanical properties including flexibility, strength and durability. The most commonly used plasticizers are glycerol and sorbitol.65,66 According to Zhang et al.,67 the use of chemically modified starch reduces the tensile strength and results in increased elongation at rupture. Gutiérrez et al.68 found 13.9 MPa for the tensile strength of a native manioc (3% m/v) and glycerol (1.9% m/v) film, the highest value reported in this paper. Lima et al.69 measured the tensile strength of bean starch (3%) films crosslinked with different concentrations of tetraethylorthosilicate (TEOS) (5, 20 and 40% m/m relative to starch) and glycerol addition (30% m/m relative to starch) and found intermediate values between 3.39 and 6.66 MPa. The authors observed increased tensile strength (6.66 MPa) for the 40% m/m TEOS films, while lower TEOS concentration films (5 and 20% m/m) and native starch (control) exhibited lower tensile strength, 3.46, 3.39 and 3.55 MPa, respectively, without any statistical difference among them. Zavareze et al.18 observed for native, oxidized potato starch films (3, 4 and 5% m/v) and glycerol (30% m/m relative to starch) figures between 3.53 and 9.12 MPa and demonstrated that oxidation increases films strength. It can be observed that the whole of the values found in the literature for tensile strength are higher than the values obtained in the present work. On the other hand, elongation values obtained for the crosslinked films are generally higher than those reported in the literature. By comparing elongation data, Zavareze et al.18 found, for native, oxidized potato films (3, 4 and 5% m/v) and glycerol (30% m/m relative to starch) elongation figures varying between 58 and 85% for native starch films, and between 38 and 56% for the films based on oxidized potato starch. Moraes et al.70 found elongation of 29% for a manioc starch (4% m/v) and glycerol (0.60% m/v) film. Lima et al.69 found for a bean starch (3% m/v), glycerol (0.30% m/v) and TEOS (40% m/m relative to starch) film elongation of 30.31%. These are lower values than most of those found in the present study, exception made to treatment 2 (74%), which is similar to the 85% value found by Zavareze et al.18 Sueiro et al.71 studied biodegradable films of cassava starch, pullulan and bacterial cellulose applied by casting methods and also observed a decrease in tensile strength (0.95 ± 0.19 to 1.41 ± 0.40 MPa) and an increase in elongation (56 ± 8 to 88 ± 4%) for the films with added glycerol (plasticizer) when compared to the control (4.82 ± 0.40 MPa and 33 ± 3%). In accordance to Mali et al.,41 and Seligra et al.,72 in general the starch films are broken and have low elongation, this being chiefly attributed to interactions of the sort of hydrogen bonds among polymer chains. Addition of compounds that reduce chain interactions by hydrogen bonds, such as plasticisers, increases starch films elongation. This effect was observed in this work, the addition of SMTP among starch chains making the films more flexible because of reduced hydrogen bonds. Color and opacity The results obtained for the color and opacity parameters of the crosslinked cassava starch composite films and the effects of the independent variables on these responses are shown in Table 5 and Figure 8, respectively.
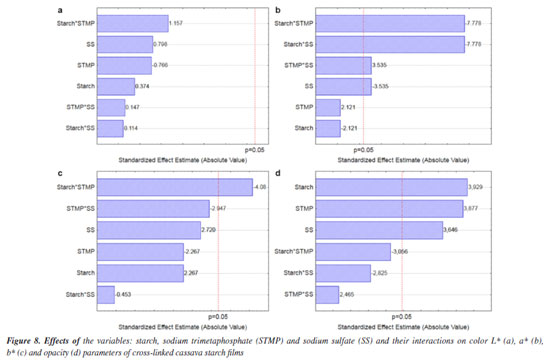
The L* parameter refers to luminosity (the closer to 100, the brightest) while the a* (the highest the positive value, the reddest; negative values mean green color) and b* parameters (the highest the positive value, the yellowest; negative values mean blue color) are the chromatic coordinates.41 The native cassava starch film was very bright (luminous),73 a bit opaque and yellowish white. The cassava starch cross-linking practically maintained the luminosity and, with the exception of the T1 film, the opacity slightly increased. Also small differences in the color were promoted, increasing the yellowish color of the films as shown in Table 5. The starch, STMP and SS variables individually assessed had no effect, as well as their interactions, on the luminosity response at the concentration ranges evaluated in the study (Figure 8a). In relation to opacity, the interactions also did not present significant effects at the evaluated ranges (Figure 8d). Meanwhile, the starch*STMP interaction had a negative, but desired, effect on the a* and b* color parameters (Figures 8b and 8c). The mathematical models obtained for the color parameters a* and b* and for opacity with the coded variables are shown in Equations 8, 9 and 10, respectively. For the L*color variable, it was not possible to generate an equation because independent variables did not show any significant effects (Figure 8a). Color a* = 0.19- 0.0015 × S + 0.015 × STMP - 0.025 × SS - 0.055 × S × STMP - 0.055 × S × SS + 0.025 × STMP × SS (8) Color b* = 2.65 + 0.10 × S - 0.10 × STMP + 0.12 × SS - 0.18 × S × STMP - 0.02 × S × SS - 0.13 × STMP × SS (9) Opacity = 11.74 + 0.77 × S + 0.76 × STMP + 0.71 × SS - 0.60 × S × STMP - 0.55 × S × SS + 0.48 × STMP × SS (10) Henrique et al.74 assayed the color for films from 3 and 5% commercial modified cassava starch (Amidomax 3500, Cargill®) and found, respectively, the following values: luminosity 86.24 and 84.09%, parameter a* 0.08 and 0.07 and parameter b* 0.07 and 0.05. The low values correspond to less luminous/light but practically colorless films, as compared with the values found for all of the films obtained in the present work. When carrying out a study on laminates of cassava starch and poly (vinyl alcohol) blends and evaluating the effect of the formulation on color and opacity, Zanela et al.75 found opacity from 31 to 56%, values higher that those found in the present study. Chen76 reported that the packaging opacity is the result of the morphology and chemical structure related to the composition of the material.
CONCLUSIONS All treatments resulted in films with better handling, continuity, and gloss compared to the film made from native starch. In general, crosslinking reduced water solubility and tensile strength, slightly increased thickness, greatly increased elongation, and slightly increased yellowing and opacity of the films. Treatment 1, which used the lowest levels of all reagents, presented the best results, with excellent macroscopic and microscopic appearance, fewer and smaller microcracks, low WVP, and intermediate values for solubility, thickness, tensile strength, and elongation. These results also qualify the resulting starch for use in coating formulations.
ACKNOWLEDGMENTS The authors are grateful to the Southern Microscopy Center (CEME-SUL), Rio Grande, RS, Brazil, for the microscopy analyses and the agro-industrial coordination of the Visconde da Graça campus of the Sul-Rio-Grandense Federal Institute for the valuable collaboration in chemical analyses. The authors also thank the CAPES and CNPq funding agencies for financial support.
REFERENCES 1. Dilkes-Hoffman, L. S.; Lane, J. L.; Grant, T.; Pratt, S.; Lant, P. A.; Laycock, B.; J. Cleaner Prod. 2018, 180, 325. [Crossref] 2. Assis, O. B. G.; Britto, D.; Forato, L. A.; O Uso de Biopolímeros como Revestimentos Comestíveis Protetores para Conservação de Frutas in natura e Minimamente Processadas; Embrapa, São Carlos, SP, 2009. [Link] accessed in February 2025 3. Borges, C. D.; Mendonça, C. R. B.; Zambiazi, R. C.; Nogueira, D.; Pinto, E. M.; Paiva, F. F.; Bioscience Journal 2013, 29, 1071. [Link] accessed in February 2025 4. Cazón, P.; Velazquez, G.; Ramírez, J. A.; Vázquez, M.; Food Hydrocolloids 2017, 68, 136. [Crossref] 5. Pigozzi, M. T.; Silva, V. M.; Mendes, F. Q.; de Oliveira, I. R. N.; e Moraes, A. R. F.; Lopes, E. A.; Ciência e Agrotecnologia 2021, 45, e019120. [Crossref] 6. Hassan, B.; Chatha, S. A. S.; Hussain, A. I.; Zia, K. M.; Akhtar, N.; Int. J. Biol. Macromol. 2018, 109, 1095. [Crossref] 7. Food and Drug Administration (FDA); Food Starch Modified: Code of Federal Regulation, vol. 3; FDA: Washington, 2007. 8. Elsabee, M. Z.; Abdou, E. S.; Mater. Sci. Eng., C 2013, 33, 1819. [Crossref] 9. LeCorre, D.; Bras, J. A. Dufresne, A.; Carbohydr. Polym. 2012, 87, 658. [Crossref] 10. de Azevêdo, L. C.; de Sá, A. S. C.; Rovani, S.; Fungaro, D. A.; Caderno de Prospecção 2018, 11, 351. [Crossref] 11. Wahyuningtiyas, N. E.; Suryanto, H.; Journal of Mechanical Engineering Science and Technology 2017, 1, 24. [Crossref] 12. Morgan, N. K.; Choct, M.; Anim. Nutr. 2016, 2, 253. [Crossref] 13. Hamad, K.; Kaseem, M.; Ko, Y. G.; Devi, F.; Polym. Sci., Ser. A 2014, 56, 812. [Crossref] 14. Ahmed, J.; Tiwari, B. K.; Iman, S. H.; Rao, M. A.; Starch-Based Polymeric Materials and Nanocomposites; Chemistry, Processing, and Applications, 1st ed.; CRC Press, 2012. 15. Li, B.; Wang, L.; Li, D.; Adhikari, B.; Mao, Z.; Carbohydr. Polym. 2012, 88, 912. [Crossref] 16. Zhong, Y.; Song, X.; Li, Y.; Carbohydr. Polym. 2011, 84, 335. [Crossref] 17. Sandhu, K. S.; Kaur, M.; Singh, N.; Lim, S.-T.; LWT - Food Sci. Technol. 2008, 41, 1000. [Crossref] 18. Zavareze, E. R.; Pinto, V. Z.; Klein, B.; El Halal, S. L. M.; Elias, M. C.; Prentice-Hernández, C.; Dias, A. R. G.; Food Chem. 2012, 132, 344. [Crossref] 19. El Halal, S. L. M.; Colussi, R.; Pinto, V. Z.; Bartz, J.; Radunz, M.; Carreño, N. L. V.; Dias, A. R. G.; Zavareze, E. R.; Food Chem. 2015, 168, 247. [Crossref] 20. Fonseca, L. M.; Gonçalves, J. R.; Halal, S. L. M.; Pinto, V. Z.; Dias, A. R. G.; Jacques, A. C.; Zavareze, E. R.; LWT - Food Sci. Technol. 2015, 60, 714. [Crossref] 21. Soares, G. A.; de Castro, A. D.; Cury, B. S. F.; Evangelista, R. C.; Carbohydr. Polym. 2013, 91, 135. [Crossref] 22. Bejenariu, A.; Popa, M.; Dulong, V.; Picton, L.; Le Cerf, D.; Polym. Bull. 2009, 62, 525. [Crossref] 23. Koo, S. H.; Lee, K. Y.; Lee, H. G.; Food Hydrocolloids 2010, 24, 619. [Crossref] 24. Costa Jr., E. S.; Mansur, H. S.; Quim. Nova 2008, 31, 1460. [Crossref] 25. Khan, B.; Niazi, M. B. K.; Jahan, Z.; Farooq, W.; Naqvi, S. R.; Ali, M.; Ahmed, I.; Hussain, A.; Food Packag. Shelf Life 2019, 19, 184. [Crossref] 26. Mathew, S.; Abraham, T. E.; Food Hydrocolloids 2008, 22, 826. [Crossref] 27. Fan, H. Y.; Duquette, D.; Dumont, M.-J.; Simpson, B. K.; Int. J. Biol. Macromol. 2018, 120, 263. [Crossref] 28. Iurckevicz, G.; Marques, P. T.; Lima, V. A.; Rev. Virtual Quim. 2017, 9, 1462. [Crossref] 29. Woo, K.; Seib, P. A.; Carbohydr. Polym. 1997, 33, 263. [Crossref] 30. Rutemberg, M. W.; Solarek, D. In Starch: Chemistry and Technology, 2nd ed.; Whistler, R. L.; Bemiller, J. N.; Paschall, E. F., eds.; Academic Press, 1984, p. 311. 31. Cuq, B.; Gontard, N.; Guilbert, S. In Active Food Packaging; Rooney, L. M., ed.; Springer: New York, 1995, p. 111. [Crossref] 32. Association of Official Analytical Chemists (AOAC); Official Methods of Analysis of the AOAC International, 19th ed.; AOAC: Gaithersburg, 2012. 33. Food and Agriculture Organization of the United Nations (FAO); FAOSTAT, Production; 2014, https://www.fao.org/faostat/en/#data, accessed in February 2025. 34. Demiate, I. M.; Dupuy, N.; Huvenne, J. P.; Cereda, M. P.; Wosiacki, G.; Carbohydr. Polym. 2000, 42, 149. [Crossref] 35. Gontard, N.; Guilbert, S.; Cuq, J.-L.; J. Food Sci. 1993, 58, 206. [Crossref] 36. Zamudio-Flores, P. B.; Torres, A. V.; Salgado-Delgado, L.; Bello-Péres, L. A.; J. Appl. Polym. Sci. 2010, 115, 991. [Crossref] 37. ASTM F2251-13: Standard Test Method for Thickness Measurement of Flexible Packaging Material; American Society for Testing and Materials, West Conshohocken, 2013. 38. ASTM D882-12: Standard Test Methods for Tensile Properties of Thin Plastic Sheeting; American Society for Testing and Materials, West Conshohocken, 2012. 39. Jangchud, A.; Chinnan, M. S.; J. Food Sci. 1999, 64, 153. [Crossref] 40. Hunterlab; The Color Management Company; Universal software, Reston, 1997. 41. Mali, S.; Grossmann, M. V. E.; García, M. A.; Martino, M. N.; Zaritzky, N.; J. Food Eng. 2006, 75, 453. [Crossref] 42. Silverstein, R. M.; Webster, F. X.; Kiemle, D. J.; Identificação Espectrométrica de Compostos Orgânicos; LTC: Rio de Janeiro, 2007. 43. Smith, B. C.; Infrared Spectral Interpretation: A Systematic Approach, 1st ed.; CRC Press, 1998. 44. Pavlovic, S.; Brandao, P. R. G.; Miner. Eng. 2003, 16, 1117. [Crossref] 45. Muscat, D.; Tobin, M. J.; Guo, Q.; Adhikari, B.; Carbohydr. Polym. 2014, 102, 125. [Crossref] 46. Li, B.; Wang, L.; Li, D.; Chiu, Y. L.; Zhang, Z.; Shi, J.; Chen, X. D.; Mao, Z.; J. Food Eng. 2009, 92, 255. [Crossref] 47. García, N. L.; Ribba, L.; Dufresne, A.; Aranguren, M. I.; Goyanes, S.; Macromol. Mater. Eng. 2009, 294, 169. [Crossref] 48. Peng, H.; Xiong, H.; Wang, S.; Li, J.; Chen, L.; Zhao, Q.; Eur. Food Res. Technol. 2011, 232, 693. [Crossref] 49. López, O. V.; Garcia, M. A.; Zaritzky, N. E.; Carbohydr. Polym. 2008, 73, 573. [Crossref] 50. Liu, Z.; Han, J. H.; J. Food Sci. 2005, 70, E31. [Crossref] 51. Shah, U.; Gani, A.; Ashwar, B. A.; Shah, A.; Wani, I. A.; Masoodi, F. A.; Int. J. Biol. Macromol. 2016, 84, 166. [Crossref] 52. Dang, M. K.; Yoksan, R.; Carbohydr. Polym. 2015, 115, 575. [Crossref] 53. Vicentino, S. L.; Floriano, P. A.; Dragunski, D. C.; Caetano, J.; Quim. Nova 2011, 34, 1309. [Crossref] 54. Montaño-Leyva, B.; Torres-Chávez, P.; Ramírez-Wong, B.; Plascencia-Jatomea, M.; Brown-Bojórquez, F.; Starch 2008, 60, 559. [Crossref] 55. Zhang, Y.; Han, J. H.; J. Food Sci. 2006, 71, E253. [Crossref] 56. Biduski, B.; da Silva, F. T.; da Silva, W. M.; El Halal, S. L. M.; Pinto, V. Z.; Dias, A. R. G.; Zavareze, E. R.; Food Chem. 2017, 214, 53. [Crossref] 57. Müller, C.; Yamashita, F.; Laurindo, J. B.; Carbohydr. Polym. 2008, 72, 82. [Crossref] 58. Chinma, C. E.; Ariahu, C. C.; Alakali, J. S.; J. Food Sci. Technol. 2015, 52, 2380. [Crossref] 59. Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M. J.; Khaksar, R.; Carbohydr. Polym. 2013, 98, 1117. [Crossref] 60. Basiak, E.; Lenart, A.; Debeaufort, F.; J. Sci. Food Agric. 2017, 97, 858. [Crossref] 61. Embuscado, M. E.; Huber, K. C.; Edible Films and Coatings for Food Applications; Springer: New York, 2009. 62. Pinto, V. Z.; Vanier, N. L.; Deon, V. G.; Moomand, K.; El Halal, S. L.; Zavareze, E. R.; Lim, L. T.; Dias, A. R.; Food Chem. 2015, 187, 98. [Crossref] 63. Cuq, B.; Gontard, N.; Cuq, J.-L.; Guilbert, S.; J. Food Sci. 1996, 61, 580. [Crossref] 64. Gontard, N.; Duchez, C.; Cuq, J.-L.; Guilbert, S.; Int. J. Food Sci. Technol. 1994, 29, 39. [Crossref] 65. Villadiego, A. M. D.; Soares, N. F. F.; Andrade, N. J.; Puschmann, R.; Minim, V. P. R.; Cruz, R.; Revista Ceres 2005, 300, 221. [Link] accessed in February 2025 66. Besinela Junior, E.; Monarim, M. M. S.; Camargo, M.; Mahl, C. E. A.; Simões, M. R.; da Silva, C. F.; Varia Scientia Agrárias 2010, 1, 131. [Link] accessed in February 2025 67. Zhang, Y.-R.; Wang, X.-L.; Zhao, G.-M.; Wang, Y.-Z.; Carbohydr. Polym. 2013, 96, 358. [Crossref] 68. Gutiérrez, T. J.; Tapia, M. S.; Pérez, E.; Famá, L.; Food Hydrocolloids 2015, 45, 211. [Crossref] 69. Lima, K. O.; Biduski, B.; Silva, W. M. F.; Ferreira, S. M.; Montenegro, L. M. P.; Dias, A. R. G.; Bianchini, D.; Eur. Polym. J. 2017, 89, 162. [Crossref] 70. de Moraes, J. O.; Scheibe, A. S.; Sereno, A.; Laurindo, J. B.; J. Food Eng. 2013, 119, 800. [Crossref] 71. Sueiro, A. C.; Faria-Tisher, P. C. S.; Lonni, A. A. S. G.; Mali, S.; Quim. Nova 2016, 39, 1059. [Crossref] 72. Seligra, P. G.; Jaramillo, C. M.; Famá, L.; Goyanes, S.; Carbohydr. Polym. 2016, 138, 66. [Crossref] 73. Ghorpade, V. M.; Gennadios, A.; Hanna, M. A.; Weller, C. L.; Cereal Chem. 1995, 72, 559. [Link] accessed in February 2025 74. Henrique, C. M.; Cereda, M. P.; Dupuy, N.; Agronomía Tropical 2007, 57, 25. [Link] accessed in February 2025 75. Zanela, J.; Reis, M. O.; Dias, A. P.; Mali, S.; Grossmann, M. V. E.; Yamashita, F.; Polímeros 2015, 25, 326. [Crossref] 76. Chen, H.; J. Dairy Sci. 1995, 78, 2563. [Crossref]
Associate Editor handled this article: Boniek G. Vaz |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access