Artigo
| Sustainable use of boiler ash to purify biodiesel and waste frying oil: a proposal of circular economy |
|
Rhalid AkelI; Fernanda S. LiraI; Vinicius C. LunaI; Matheus Z. MarquesII; Leonardo G. VasconcelosII; Marilza CastilhoI; I. Grupo de Eletroquímica e Novos Materiais (GENMAT), Departamento de Química, Instituto de Química, Universidade Federal de Mato Grosso, 78060-900 Cuiabá - MT, Brasil Recebido: 11/15/2024 *e-mail: adriano.siqueira@ufmt.br Innovations in biodiesel purification pave the way for lower production costs and a more sustainable future. This study explores the remarkable potential of biodiesel boiler waste plants, both directly and in modified forms, for the purification process of soy methyl biodiesel (BMS). It extends to the inclusion of waste frying oil (ORF) samples. The untreated and HCl-treated biomass underwent a thorough characterization utilizing simultaneous thermogravimetry-differential thermal analysis (TG-DTA) and thermogravimetric-differential scanning calorimetry (TG-DSC) coupled with Fourier transform infrared spectroscopy (FTIR), infrared spectroscopy, X-ray fluorescence (EDX), and inductively coupled plasma optical emission spectroscopy (ICP OES). FTIR and EDX analyses revealed a significant presence of SiO2 in the biomass, which can effectively regulate moisture and reduce impurities during biodiesel production. Acidity, saponification value, water, and ester content were measured to assess the role of the residue in biodiesel purification. Both untreated and HCl-treated waste showed impressive efficiency in water adsorption and removing organic impurities in ORF and BMS. This confirms that boiler ash derived from rice husk fuel is a powerful adsorbent for impurities such as water and organic compounds in BMS and ORF, showing great promise as a reusable resource in biodiesel production facilities. INTRODUCTION Biodiesel shines as a remarkable alkyl ester derivative of long-chain fatty acids derived from renewable vegetable oils and animal fats, serving as a powerful alternative to fossil fuels in compression ignition engines. The biodiesel production chain is vital across multiple sectors, fostering job creation, driving regional socio-economic growth, decreasing our dependence on natural resources, and offering environmental benefits such as reduced greenhouse gas emissions, improved air quality, and preserving biodiversity.1-5 Feedstocks such as soybean, sunflower, and palm oils are predominantly used in biodiesel synthesis. Moreover, used cooking oils, or oil recovery fats (ORF), have been investigated as viable substitutes for refined vegetable oils, presenting benefits like mitigating food safety issues. However, a critical challenge in biodiesel production remains to achieve high product purity while concurrently reducing the generation of low-value by-products. Water presents a substantial challenge in biodiesel production, introducing a range of complications that can affect both the processing and storage phases. The presence of water can lead to microbial growth, which contaminates the biodiesel and contributes to sludge development. This thick, undesirable residue can impede flow and clog filters. Additionally, water exposure can accelerate corrosion in storage tanks, particularly in metal components, making careful management essential to maintain the integrity of the infrastructure. This moisture can also cause freezing issues within storage tanks, potentially disrupting the biodiesel supply chain during colder months. Beyond the production and storage phases, water in biodiesel poses serious risks to engine performance. It can lead to clogging, which restricts fuel flow, and it can accelerate wear on delicate aluminum and zinc components found within engines, ultimately compromising longevity and efficiency. Moreover, another critical by-product of the transesterification process that must be carefully removed is residual alcohol. These leftover alcohols represent an inefficiency in production and significantly impact the properties of the biodiesel. They can decrease the viscosity and density of the biofuel, potentially altering its performance characteristics and making it less suitable for use in engines. Therefore, addressing both water and residual alcohols is vital for producing high-quality biodiesel.1,6,7 At present, there are two main methods used for biodiesel purification: the wet method, which involves washing with water, and the dry method, which makes use of adsorbent materials. The wet method is the more commonly used technique, as it effectively dissolves glycerol and short-chain alcohols in water. However, one disadvantage of the wet purification method is that it produces a significant amount of wastewater, which requires appropriate treatment for reuse or disposal. Conversely, the dry purification method avoids the creation of liquid waste and has lower energy requirements by eliminating the need for distillation and other processes. Adsorbents such as magnesium silicates, silica, and ion exchange resins are frequently employed, as they have a strong affinity for impurities in biodiesel.5,8,9 Fadhil et al.10 embraced innovation by utilizing activated charcoal from tea residue to absorb impurities from biodiesel sourced from ORFs. Their efforts paid off with an impressive methyl ester yield of approximately 94%, surpassing the traditional washing method with acidified water, which yielded around 88%. De Paula et al.6 also sought to unlock the potential of ORFs, producing biodiesel through conventional transesterification and exploring dry washing techniques with bauxite, attapulgite, and bentonite. Bauxite and bentonite shone brightly among these, effectively removing glycerol and soap. Understanding the significance of silica in dry purification processes, it is heartening to explore a sustainable alternative through rice husk biomass. Nano-silica (SiO2) derived from rice husks has significant potential to enhance biodiesel performance. This nanomaterial can significantly improve combustion characteristics, reduce emissions, and enhance engine efficiency when added as an additive.11 Many industries, particularly those producing vegetable oil, rely on rice husks as a fuel source for their energy needs. The use of rice husks as boiler fuel represents an interesting reuse of biomass, but the generation of its ashes represents a disposal problem. Using this ash within the biodiesel production plant as a purifying agent for biodiesel impurities is an important initiative to reduce costs and improve the system. By considering this eco-friendly option, we can foster a more sustainable future while supporting industries seeking to minimize their environmental impact. Together, we can make a difference in our economy and planet.12-15 This study demonstrates the valuable application of rice husk ash, which is rich in silica and sourced from biodiesel production boilers. Through a simple treatment process, this ash can eliminate water and organic impurities from biodiesel, particularly methylated soybean biodiesel. Additionally, it holds great potential for purifying soybean oil used in frying, paving the way for its sustainable reuse.
EXPERIMENTAL The residue, consisting of the by-product from burning a combination of rice husk and eucalyptus bark, was collected directly from the operational furnaces of the boilers from the Bio-Oleo Industry. The collected material was mixed and placed in an oven at 120 ºC for 24 h. Afterward, the sample was spread out on a bench, remixed, and then quartered. A portion from each quadrant was used (RST) or treated with hydrochloric acid (RT) as needed. A mixture of 10 g of boiler biomass and 60 mL of distilled water was prepared to form the untreated mixture (RST). The boiler waste underwent treatment by adding hydrochloric acid (6 and 1 mol L-1) to the RST. Hydrochloric acid (HCl) was added until the pH values 1 (RT1), 3 (RT3), 5 (RT5), and 7 (RT7), respectively. The RST, RT1, RT3, RT5, and RT7 suspensions were stirred for 2 h, filtered using Whatman No. 41 paper, and washed with 100 mL of distilled water. The filtrates were characterized and used in purification tests for methylated soybean biodiesel (BMS) and residual soybean oil used in frying (ORF). The ORF was obtained by purchasing soybean oil from a local business, and the authors themselves conducted frying simulations to mimic its real-world usage. The simulation was carried out with the oil used to fry food 10 times and stored in a plastic bottle. The purification tests were performed using 5 g of RST, RT1, RT3, RT5, and RT7 in 40 mL of impure oil (BMS and ORF). The suspensions were magnetically stirred for 20 min at 700 rpm. The oil was then separated using a syringe system, as illustrated in Figure 1. Subsequently, the filtrates were dried and characterized using Fourier transform infrared spectroscopy (FTIR), simultaneous thermogravimetry-differential thermal analysis (TG-DTA), inductively coupled plasma optical emission spectroscopy (ICP OES), X-ray fluorescence (EDX), and thermogravimetric-differential scanning calorimetry (TG-DSC) coupled with FTIR. During the purification tests, the BMS and ORF were assessed for water content, saponification value, acid number, and ester content. After use for BMS and ORF purification, the residues were called RSTI, RT1I, RT3I, RT5I, RT7I and RSTII, RT1II, RT3II, RT5II, and RT7II, respectively.

Thermal analysis The TG-DTA curves were obtained using the Shimadzu DTG-60H simultaneous. The samples were placed in a 70 μL alumina crucible, with an approximate sample mass of 7 mg. The heating rate was set at 20 ºC min-1, under a dry synthetic air atmosphere with a flow rate of 100 mL min-1, within a temperature range of 30-800 ºC. The TG-DSC curves were obtained using an alumina crucible (70 μL) with a sample mass of approximately 7 mg. The heating rate was set to 20 ºC min-1, and the experiments were conducted under a dry air atmosphere with a flow rate of 100 mL min-1 within a temperature range of 30-1000 ºC. For the evolved gases analysis (EGA), experiments were performed using a thermogravimetric analyzer, Mettler TG-DSC 1, coupled with a mid-infrared absorption spectrometer equipped with Fourier transform, iS10 Nicolet FTIR spectrometer. The transfer line consisted of a 120 cm long stainless-steel tube with a 2 mm internal diameter, maintained at 250 ºC. The FTIR measurements were conducted using a DTGS detector within a specially developed gas cell, also heated to a constant temperature of 250 ºC. Both the interferometer and gas cell compartments were purged with N2. FTIR spectra were recorded in the 4000-600 cm-1 region, comprising 16 scans per spectrum at a resolution of 4 cm-1. Infrared analysis Absorption spectra in the infrared region were obtained using a spectrophotometer, model iS5, with 4 cm-1 resolution, in the region between 4000-600 cm-1, using the attenuated total reflectance (ATR) technique with Ge Crystal. Metal content The samples were digested using a Berghoff microwave digestion system, with 0.5 g of biomass in 10 mL of aqua regia. The digested material was then diluted in a 50.00 mL volumetric flask and analyzed using an ICP OES spectrometer. The ICP OES analyses were obtained using the inductively coupled plasma atomic emission spectroscopy iCAP74 Duo model by Thermo Scientific. The analysis was performed with RF power set to 1250 W, plasma flow rate at 12 L min-1, auxiliary flow rate at 0.5 L min-1, radial flow rate at 0.5 L min-1, and air flow rate for the nebulizer at 0.5 L min-1. The exposure time was 30 s, and the detector operated in axial and radial modes. The experiments for analysis using fluorescence spectroscopy and energy dispersive X-ray (EDX) were conducted using Shimadzu equipment, of the Rayny model EDX-700/800 series. The samples were deposited in the sample holder until approximately 1 cm of the container height was filled. The analyses were performed with a 5 mm collimator and beam ranging from 50 (Ti-Sc channel, 0.02 step) to 15 keV (Na-Sc channel, 0.01 step), with a measurement time of 100 s, using an X-ray tube of Rh. These experiments were carried out in an inert atmosphere (He). Water content The water content of the purified biodiesel was determined according to the guidelines specified in ASTM D 6304.16 The analysis was carried out using a Karl-Fischer Kem Kyoto model MKC-610 titrator.17 Acid number This parameter was evaluated following EN 14104 standards,18 with results expressed in mg KOH mg-1 for each sample.19 Titration was performed in triplicate using a standardized NaOH solution at 0.01 mol L-1 as the titrant. The preparation of the titrant involved mixing 2.0 g of BMS or ORF with 25 mL of ethyl ether and methanol in a 2:1 ratio, using a phenolphthalein indicator. The acidity index was determined using Equation 1. 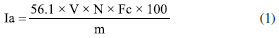 Ia is the acid number, V is the volume (mL) of standardized NaOH, N is the normal concentration of the NaOH solution, Fc is the correction factor of the NaOH solution, and m is the mass of the sample (g). Saponification value The saponification value was determined by the ratio of the mass (mg) of KOH required to saponify 2.0 g of the BMS or ORF sample, following the protocols outlined by the Adolfo Lutz Institute.20 Equation 2 represents this process. The sample was combined with 25 mL of 4% (m/v) alcoholic potassium hydroxide solution in a 250 mL round-bottomed flask and heated using a reflux system for 1 h. Afterward, the mixture was allowed to settle for 10 min. The mixtures were titrated using standardized HCl at 0.5 mol L-1 in the presence of a phenolphthalein indicator. 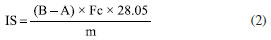 IS represents the saponification index expressed in mg KOH g-1 of sample, A is the HCl volume used in the titration, B is the HCl volume used in the blank titration, Fc is the correction factor of the HCl solution 0.5 mol L-1, and m is the mass of the sample (g). Ester content The ester content was evaluated according to Knothe.21 The 1H NMR (nuclear magnetic resonance) analyses of the samples were carried out using Bruker 500 MHz nuclear magnetic resonance equipment, equipped with magnet (model Bruker magnet system AscendTM 500), console (model Bruker Avance III HD); probe (model BBO 500MHz S1-5mm); temperature variation unit (model BCU 1-40/50); amplifiers (model HPPR II Preamplifier), workstation (model HP Z420 Workstation); acquisition and processing software (model BrukerTopSpin 3.6.2) and 1H NMR tubes (brand NORELL, 300 MHz, dimensions: 178 × 5 mm). To acquire the 1H NMR spectra, 25.0 ± 0.2 mg of sample was weighed on an analytical balance in a 1 mL volumetric flask, and then the volume of the flask was topped up with deuterated chloroform (CDCl3-d3, Sigma-Aldrich). After solubilization, 600 µL of the sample solution was transferred with a micropipette to the 1H NMR tubes for subsequent spectra acquisition.
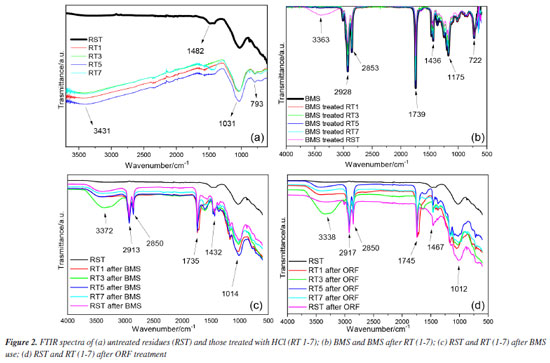
RESULTS AND DISCUSSION Biomass Fly ashes are typically classified according to their pH value and the ratio of calcium to sulfur into three main categories: acidic ash (with a pH range of 1.2 to 7), mildly alkaline ash (pH 8-9), and strongly alkaline ash (pH 11-13). When the acidity of rice straw and eucalyptus chips ashes (RST) suspended in distilled water was analyzed, the resulting pH value of 9 indicated that it falls within the mildly alkaline range, suggesting it has moderate alkalinity.22 Due to the inherent alkalinity of rice straw ash (RST), it was subjected to a controlled treatment with HCl to adjust its pH to specific values of 1, 3, 5, and 7. This adjustment aimed to investigate whether altering the acidity of the biomass would lead to changes in the material. The goal was to determine if these modifications could enhance the potential of ash as a purification agent for biodiesel, exploring whether such alterations could improve its effectiveness in removing impurities during the biodiesel production process. FTIR spectra In the FTIR spectra of RST, RT1, RT3, RT5, and RT7 (Figure 2), bands are observed at 3431 cm-1, corresponding to the O - H stretching of the silanol group and siloxanes (Si - O - Si - O), which may also be attributed to OH vibrations of water molecules adsorbed from the atmosphere. The bands corroborate the presence of silica in the biomass at 1031 and 793 cm-1, indicative of Si - O - Si stretching. This silica likely originates from the utilization of rice husks as boiler fuel. Furthermore, a band around 1482 cm-1 observed in samples RST, RT7, and RT5 is attributed to the presence of sodium carbonate.11
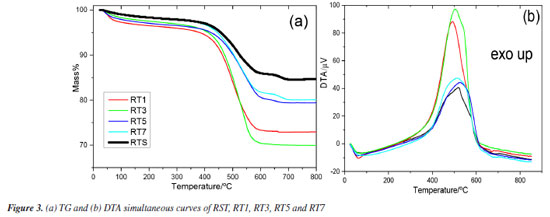
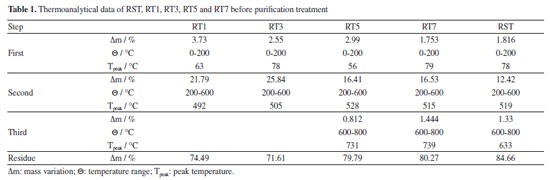
Thermal analysis The thermal analysis of the biomass reveals two distinct thermogravimetric profiles, see Figure 3. Samples RT1 and RT3 exhibit three steps of thermal decomposition, whereas RST, RST5, and RT7 samples demonstrate four consecutive steps, see Figure 3 and Table 1. In the first step of thermal decomposition, all samples undergo dehydration, with RST and RT7 demonstrating the smallest mass loss. This indicates that these materials retain more moisture content than others. The second step, characterized by oxidative degradation, occurs at different temperature ranges across the samples. For RT3, RT5, RT7, and RST, thermal degradation is observed between 200 and 600 ºC, while RT1 shows a broader degradation ranging from 200 to 800 ºC. This thermal decomposition is likely associated with the decomposition of organic constituents in the biomass, such as cellulose, hemicellulose, and a portion of lignin. These compounds, which are not completely decomposed during the initial boiler process, undergo further degradation under high temperatures, releasing volatile gases and contributing to the overall mass loss.23
Samples RT5, RT7, and RST show a distinct third mass loss between 600 and 700 ºC, likely due to the decomposition of carbonates present in these materials. This observation is consistent with the FTIR analysis results, confirming a higher ash content in these samples, likely linked to carbonate minerals that decompose at elevated temperatures, releasing gases like CO2. In contrast, RT1 and RT3 exhibit higher heat release during their oxidative decomposition, which can be attributed to the absence of carbonates in these samples. Without carbonates to effectively absorb energy during degradation, we experience a notable increase in heat release as the thermal oxidation process occurs with organic and inorganic components. These differences in thermal behavior suggest that the lower pH values in the treated materials may promote the leaching of inorganic compounds, thereby influencing the thermal stability and composition of the ash. An HCl test tube confirmed the carbonates formed at 600 ºC. The thermoanalytical profiles observed in the TG-DSC curves exhibited similarities to those found in the TG-DTA curves, as illustrated in Figure 2S (presented in Supplementary Material). Furthermore, Figure 3S (Supplementary Material) provides a detailed FTIR spectrum of the gases released during the thermal analysis, illustrating the significant liberation of water molecules during the initial phase of thermal decomposition. This release is a crucial indicator of the breakdown processes of the material under heat. Moreover, at a temperature of 550 ºC, carbon dioxide (CO2) detection is compelling evidence of the ongoing thermal decomposition of residual organic matter embedded within the biomass, highlighting the complex interactions that occur during the heating process. EDX and ICP OES analysis The EDX analyses conducted on samples RST, RT1, RT3, RT5, and RT7 yielded valuable insights into the normalized results of the respective oxides of inorganic components, as detailed in Table 2. Notably, sample RT1 showed a significant concentration of SiO2 at 51.98%. This finding likely stems from the leaching of various inorganic compounds, with carbonates playing a predominant role. The identification of aluminum oxide in RT3 suggests the potential for formation of Al(OH)3 precipitate during treatment at pH 3.
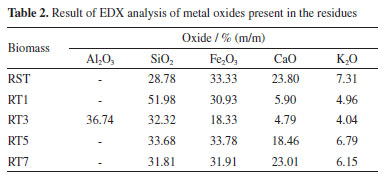
Additionally, the increased calcium content observed in RST, RT5, and RT7 supports the presence of calcium carbonate, and the lower pH values further reinforce the notion of enhanced leaching of metals and inorganic materials from the waste. Complementary ICP OES analysis (see Table 1S, Supplementary Material) of RST confirmed the presence of aluminum, iron, and calcium while revealing lower concentrations of manganese, chromium, copper, sodium, and phosphorus, which were not detected in the EDX analyses. This comprehensive approach offers a clearer understanding of the inorganic composition and behavior during processing. Purification process for methylated soybean biodiesel and waste frying oils The assessment of using untreated and acid-treated waste in the purification of BMS and ORS involved characterizing the materials and evaluating the oil parameters. Both untreated and acid-treated waste were employed, and the physicochemical parameters of the oils were assessed both before and after the purification processes. FTIR spectra In the FTIR spectra of the BMS and ORF samples, both before and after undergoing the purification system, see Figures 2 and 1S (Supplementary Material), characteristic bands appear at 1300 and 1000 cm-1, corresponding to the C - O bonds. These bands may be associated with two coupled vibrations, C -C(=O) - O and O -C -C.24,25 Also, the spectral analysis reveals bands within the frequency range of 3000 to 3500 cm-1. These bands indicate hydrogen bonding associated with the - OH functional group, which strongly suggests the presence of water and/or alcohol within the sample. Furthermore, we observe distinct bands between 1739 and 1750 cm-1, characteristic of saturated aliphatic esters. These frequencies are linked to the stretch of the carbonyl group ( -C=O) in esters, confirming the expected structural features.24 The comparative analysis of the FTIR spectra for treated versus untreated biochar derived from BMS reveals that the boiler waste purification process did not significantly alter the physicochemical properties of the biodiesel. This finding indicates that the overall characteristics, which can affect the performance and quality of the biodiesel, remain largely intact despite the treatment. However, an intriguing detail emerged from the spectrum of the treated BMS. A specific absorption band was observed at 3363 cm-1, absent in the untreated sample. This band suggests the presence of a potential interaction between the BMS and certain inorganic components that were not effectively leached out during the acid treatment process. This interaction might provide valuable information about the chemical environment and reinforce the possibility that residual inorganic materials could influence the properties and functionality of the treated biomass. Such insights could be crucial for understanding the efficiency and potential applications of treated biochar in biodiesel production and other fields. In the FTIR spectra of the biomass treated with the oils, new bands revealing the presence of adsorbed material emerged, particularly a band at 3332 cm-1. The unwashed waste retained bands linked to the remaining BMS and ORF, adding complexity and making it challenging to discern the bands related to potential impurities within the waste. TG-DSC/FTIR for residues used in BMS The TG-DSC analyses of the boiler waste used in the BMS purification process (RSTI, RT1I, RT3I, RT5I, and RT7I) are presented in Figure 4. These analyses reveal that the thermal decomposition of these materials occurs in the fifth step for the RT1I, RT3I, and RT7I samples and the fourth step for the RT5I and RSTI samples, confirming the presence of adsorbed material. The first step is losing water and/or alcohol (oil impurities), consistent with observations from waste not used in the purification process. However, subsequent steps indicate organic materials, suggesting the presence of BMS or ORS and other organic components that may be related to impurities retained in the waste.
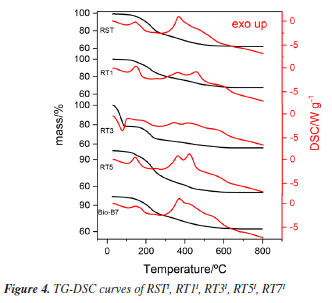
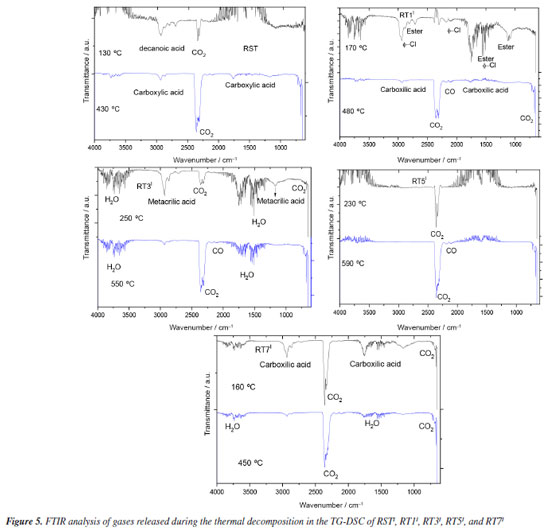
In the EGA of thermal decomposition of RSTI (Figure 5), decanoic acid (DA) was primarily detected at 130 ºC, characterized by bands at 2940 cm-1 and - OH groups characterized by bands at 1186, 1767, and 2928 cm-1.26 In the EGA of RT1I (Figure 5), chlorinated aromatic compounds (Ar-Cl) were detected, characterized by bands at 1539 and 2714 cm-1, and stearate groups characterized by bands at 1086 and 1106 cm-1. The greater amount of HCl reinforces the presence of chlorides used to treat this waste.11
In the EGA of RT3I (Figure 5), methacrylic acid (MA) was indicated, characterized by bands at 1168 and 2938 cm-1, related to OH groups.27 This compound may be a by-product of oil degradation and adsorbed in the residue. In the products released during the thermal decomposition of RT7I (Figure 5), hexadecanoic acid (HDA) was observed, characterized by bands at 1160 and 2940 cm-1. Furthermore, the EGA of RT1I (Figure 5) suggested the presence of impure organic components adsorbed to the residue. This is reinforced by the detection of aldehyde, ester, and carboxylic acid groups at temperatures below 200 ºC when the sample was heated to 170 ºC. The CO gases with bands at 2107 to 2185 cm-1 and CO2 with bands at 2358 cm-1 are attributed to the incomplete decomposition of organic matter. At temperatures above 500 ºC, only CO2 was detected. The observed thermal decompositions of the residues after BMS purification indicate an estimated adsorption capacity of 22% (m/m). TG-DSC/FTIR fot residues used in ORF The TG-DSC analyses of the boiler residues (RSTII, RT1II, RT3II, RT5II, RT7II) used in the ORS purification process are depicted in Figure 4S and Table 2S (Supplementary Material). These analyses reveal that the thermal decomposition of these materials occurs in the second step for samples RT1II, RT5II, RT7II, and RSTII, and in the fourth step for sample RT3II. The first step is associated with the release of water, with RT3II showing a higher quantity in this regard. RT3II proved to be the most adsorbent of organic materials contained in the oil, exhibiting 4 decomposition steps, with the third step showing the greatest loss of mass. Additionally, there was a significant presence of carboxylic acids adsorbed in the process, likely resulting from the oxidation of esters in the soybean oil. Furthermore, the RSTII sample (Figure 5S, Supplementary Material) also showed the presence of adipic acid, as evidenced by bands at 2943, 1746, and 1152 cm-1 observed when the material was heated to 360 ºC. Additionally, CO and CO2 were detected, resulting from the oxidative decomposition of organic matter. Assessment of BMS and ORF post-purification process The purification process of the BMS samples resulted in a remarkable decrease in acid numbers, as highlighted in Table 3. These values consistently aligned with those outlined in Brazilian National Agency for Petroleum, Natural Gas and Biofuels (ANP) Resolution No. 920/2023.28 Although Brazil currently lacks specific regulations for ORFs, it is inspiring to see that Technical Report No. 1129 from the National Health Surveillance Agency (ANVISA) advocates for best frying practices to uphold the quality of oil and products. Moreover, Collegiate Board Resolution - RDC 21630 sets a clear standard by establishing a maximum frying temperature of 180 ºC. ANVISA recognizes the acidity index of virgin refined soybean oil as acceptable, with a commendable maximum limit of 0.3%.
The saponification values for BMS and ORF showed impressive stability, measuring 190 and 188 mg KOH g-1, respectively. Notably, these values remained consistent even after the biomass purification process, indicating adherence to current legislation. Regarding water content, both BMS and ORFs initially exceeded the limit set by ANP Resolution No. 920/202328 (200 ppm). However, the implementation of RT1, RT3, and RST effectively reduced the water content in both BMS and ORFs. Specifically, RT1 halved the water content in BMS, while RST achieved comparable results for ORFs. This improvement can be compared to the purification process utilizing 2% silica, highlighting the effectiveness of these methods in enhancing the quality of the samples.31,32 The NMR analysis on the samples revealed intriguing spectral signals within the chemical shift range of d 4.1-4.3 ppm. These signals indicate the protons found in mono-, di-, or triacylglycerols, suggesting the presence of these important lipid classes. A distinct signal at d 2.3 ppm was also noted, characteristic of protons attached to -CH2 groups near the methyl ester functional group. This finding points to the structural complexity of the compounds under investigation. A prominent singlet observed at d 3.6 ppm further corroborated the existence of methyl ester groups ( -CO2CH3) within the samples. By examining the intensity values of these signals, we were able to quantitatively determine the concentration of ester in each sample, offering valuable information about their chemical makeup.21 The findings presented in this study demonstrate that the various biomass-derived materials, specifically the BMS samples - namely Bio-RST, Bio-RT1, Bio-RT3, Bio-RT5, and Bio-RT7 - exhibited no significant physicochemical changes post-purification. This observation is illustrated in Figure 6S (Supplementary Material), which highlights the consistency of their properties throughout the purification process. Furthermore, an analysis of the gases emitted during the thermal decomposition of these materials revealed the presence of residual hydrochloric acid (HCl). This residual acid indirectly influences the acidity levels of the oils treated with different derivatives, specifically RT1, RT3, and RT5. Notably, these treatments successfully reduced the acidity level of the oils. It is essential to highlight that while alterations in acidity were observed, the resulting acidity levels remained within the desirable parameters for biodiesel oils. Consequently, the potential use of these waste materials should not be discounted solely based on their acidity changes. As summarized in Table 3S (Supplementary Material), all biodiesel samples derived from these treatments comply with the stringent standards outlined by ANP Resolution No. 920/2023,28 which dictates that the ester content must be at least 96.5% methyl esters. This compliance signifies that there were no substantial physical and chemical alterations in the properties of the biodiesel after the purification processes utilizing boiler waste, ensuring the viability of these materials for biodiesel production.
CONCLUSIONS The biodiesel production sector is on a transformative journey towards independence from non-renewable sources, embracing sustainable alternatives in its production chain. Evaluating boiler waste from biodiesel production furnaces has unveiled exciting possibilities, particularly when 12% (m/m) of this waste was integrated into 40 mL of BMS or ORFs. Characterization data highlights the potential of these waste materials as effective impurity adsorbents, resulting in the creation of sustainable materials with an impressive average silica content of 51% (m/m), while preserving the essential properties of BMS and ORF. A remarkable achievement has been the reduction of water content, even though the initial levels already complied with ANP Resolution No. 920/202328 standards. Since excess water can compromise storage and engine performance, the significant decrease in water content in the samples is a noteworthy breakthrough. The use of RT1 for BMS and RST for ORF led to reductions of up to 38% in initial water values. This indicates that boiler biomass is a viable and cost-effective option for biodiesel purification, particularly in recycling used frying oils. Considering the need for energy cost-efficiency and the pursuit of sustainable solutions, a pioneering system for direct purification of RST is proposed. Alternatively, constructing a fractionated column utilizing RST, RT1, RT3, RT5, and RT7 could eliminate water and organic molecule impurities from the oil, heralding a new era in biodiesel innovation while promoting a circular economy.
SUPPLEMENTARY MATERIAL Supplementary data are available free of charge at http://quimicanova.sbq.org.br as PDF file.
ACKNOWLEDGMENTS This work has been partly supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant 438886/2018-6) and by Universidade Federal do Mato Grosso (UFMT, financial support and fellowships). The Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT) partly financed this study to support research in the state of Mato Grosso (number 40292.574.21598.07052018). The authors would like to thank the Thermal Analysis Laboratory (LATIG) of the Analytical/Inorganic Chemistry Department of IQ/UNESP-Araraquara, SP.
REFERENCES 1. Manikandan, S.; Sakthivel, L.; Parthiban, A.; Baiju, R. C.; Subramanian, S.; Catal. Lett. 2024, 154, 6049. [Crossref] 2. Reis, M. A.; dos Santos, B. L. B.; Crestani, L.; Silva, L. F. O.; Martinello, K. B.; Mohandoss, S.; Ahmad, N.; Vieira, Y.; Dotto, G. L.; Sep. Purif. Technol. 2024, 347, 127626. [Crossref] 3. Meher, L. C.; Sagar, D. V.; Naik, S. N.; Renewable Sustainable Energy Rev. 2006, 10, 248. [Crossref] 4. Bateni, H.; Saraeian, A.; Able, C.; Biofuel Res. J. 2017, 4, 668. [Crossref] 5. Chozhavendhan, S.; Singh, M. V. P.; Fransila, B.; Kumar, R. P.; Devi, G. K.; Curr. Res. Green Sustainable Chem. 2020, 1-2, 1. [Crossref] 6. de Paula, A. J. A.; Krügel, M.; Miranda, J. P.; Rossi, L. F. S.; da Costa Neto, P. R.; Quim. Nova 2011, 34, 91. [Crossref] 7. Atadashi, I. M.; Aroua, M. K.; Abdul Aziz, A. R.; Sulaiman, N. M. N.; Renewable Sustainable Energy Rev. 2012, 16, 3456. [Crossref] 8. Atadashi, I. M.; Aroua, M. K.; Aziz, A. A.; Renewable Energy 2011, 36, 437. [Crossref] 9. Kubota, T.; de Araújo, M. V. G.; Vieira, J. V. F.; da Silva, T. A.; Ramos, L. P.; Zawadzki, S. F.; J. Polym. Res. 2013, 20, 48. [Crossref] 10. Fadhil, A. B.; Dheyab, M. M.; Abdul-Qader, A. Q. Y.; J. Assoc. Arab Univ. Basic Appl. Sci. 2012, 11, 45. [Crossref] 11. Kapile, F. A.; Bereczky, A.; Lukács, K.; Ntalikwa, J. W.; Kivevele, T. T.; Biofuels, Bioprod. Biorefin. 2024, 18, 1437. [Crossref] 12. Yadav, M.; Dwibedi, V.; Sharma, S.; George, N.; J. Environ. Chem. Eng. 2022, 10, 108550. [Crossref] 13. Dong, Z.; Qu, N.; Jiang, Q.; Zhang, T.; Han, Z.; Li, J.; Zhang, R.; Cheng, Z.; J. Environ. Chem. Eng. 2024, 12, 112690. [Crossref] 14. Saceda, J. J. F.; De Leon, R. L.; Rintramee, K.; Prayoonpokarach, S.; Wittayakun, J.; Quim. Nova 2011, 34, 1394. [Crossref] 15. Ahmaruzzaman, M.; Prog. Energy Combust. Sci. 2010, 36, 327. [Crossref] 16. ASTM D6304: Standard Test Method for Determination of Water in Petroleum Products, Lubricating Oils, and Additives by Coulometric Karl Fischer Titration, Philadelphia, 2016. 17. Fregolente, P. B. L.; Wolf Maciel, M. R.; Oliveira, L. S.; Braz. J. Chem. Eng. 2015, 32, 895. [Crossref] 18. EN 14104: Fat and Oil Derivatives - Fatty Acid Methyl Esters (FAME), Determination of Acid Value, European Committee for Standardization: Berlin, 2003. 19. Lobo, I. P.; Ferreira, S. L. C.; da Cruz, R. S.; Quim. Nova 2009, 32, 1596. [Crossref] 20. Instituto Adolfo Lutz; Métodos Físico-Químicos para Análise de Alimentos; Zenebon, O.; Pascuet, N. S.; Tiglea, P., eds.; Instituto Adolfo Lutz: São Paulo, 2008. [Link] accessed in April 2025 21. Knothe, G.; Trans. ASAE 2001, 44, 193. [Crossref] 22. Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A. A.; Abri, A.; Sattler, M.; Techapaphawit, S.; Case Studies in Construction Materials 2019, 11, e00263. [Crossref] 23. Azat, S.; Korobeinyk, A. V.; Moustakas, K.; Inglezakis, V. J.; J. Cleaner Prod. 2019, 217, 352. [Crossref] 24. Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P.; Appl. Energy 2011, 88, 2050. [Crossref] 25. Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P.; Kamaronzaman, M. F. F.; Kahar, H.; Hassan, N.; Hanafi, M. F.; Sapawe, N.; Mater. Today: Proc. 2020, 31, 329. [Crossref] 26. Gurgel, W. P.; Correa, A.; Santos, C. C.; Santos, A. O.; Saraiva, G. D.; Freire, P. T. C.; Nogueira, C. E. S.; Moreira, S. G. C.; de Sousa, F. F.; Spectrochim. Acta, Part A 2023, 287, 122068. [Crossref] 27. Rufino, E. S.; Monteiro, E. E. C.; Polymer 2000, 41, 4213. [Crossref] 28. Agência Nacional do Petróleo, Gás Natural e Biocombustíveis (ANP); Resolução ANP No. 920, de 04 de abril de 2023, Estabelece a Especificação do Biodiesel e as Obrigações Quanto ao Controle da Qualidade a Serem Atendidas pelos Agentes Econômicos que Comercializem o Produto em Território Nacional; Diário Oficial da União (DOU), Brasília, Brasil, 2023. [Link] accessed in April 2025 29. Agência Nacional de Vigilância Sanitária (ANVISA); Informe Técnico No. 11, de 05 de outubro de 2004, Dispõe sobre a Utilização e Descarte de Óleos e Gorduras Utilizados para Fritura; ANVISA: Brasil, 2004. [Link] accessed in April 2025 30. Agência Nacional de Vigilância Sanitária (ANVISA); Resolução RDC No. 216, de 15 de setembro de 2004, Dispõe sobre Regulamento Técnico de Boas Práticas para Serviços de Alimentação; Diário Oficial da União (DOU), Brasília, Brasil, 2004. [Link] accessed in April 2025 31. Faccini, C. S.; da Cunha, M. E.; Moraes, M. S. A.; Krause, L. C.; Manique, M. C.; Rodrigues, M. R. A.; Benvenutti, E. V.; Caramão, E. B.; J. Braz. Chem. Soc. 2011, 22, 558. [Crossref] 32. Manique, M. C.; Faccini, C. S.; Onorevoli, B.; Benvenutti, E. V.; Caramão, E. B.; Fuel 2012, 92, 56. [Crossref]
Associate Editor handled this article: Boniek G. Vaz |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access