Artigo
| Chemical composition, antioxidant potential, and cholinesterase inhibitory activity of essential oils from three Thunbergia species growing wild in northeast Brazil |
|
Alice M. S. de Almeida; Tchiara M. A. Tenório; Lucas V. B. Rodrigues; Carolina A. de Araujo; Claudio A. G. da Camara* Departamento de Química, Universidade Federal Rural de Pernambuco, 52171-030 Recife - PE, Brasil Received: 02/01/2025 *e-mail: claudio.camara@ufrpe.br Essential oils from the leaves and flowers of Thunbergia grandiflora,Thunbergia alata and Thunbergia erecta occurring in urban fragments of the Atlantic Forest in the state of Pernambuco, Brazil, were analyzed by GC-FID (gas chromatography with flame ionization detector) and GC-MS (gas chromatography mass spectrometry). A total of 76 compounds were identified, representing more than 95% of the oils. Differences in the chemical profile were found among the oils. Germacrene D (leaves: 0.61 ± 0.02 to 20.49 ± 0.78%; flowers: 0.84 ± 0.10 to 21.71 ± 0.77%) was the only sesquiterpene identified in abundance in all oils. The chemical compositions of the oils were compared using principal component analysis and hierarchical cluster analysis, which revealed significant differences. All three species exhibited antioxidant and cholinesterase activity. The oil from leaves of T. erecta achieved the best results on the DPPH (2,2-diphenyl-1-picrylhydrazyl) test and with the ABTS (2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) radical. The oil of T. erecta exerted a significant inhibition effect on the enzymes AChE (acetylcholinesterase), and BuChE (butyrylcholinesterase). Moreover, none of the oils tested showed toxicity against Artemia salina. INTRODUCTION The family Acanthaceae Juss. encompasses more than 200 genera and 4000 species distributed on all continents.1 Among the genera, Thunbergia is native to tropical regions of Africa, Madagascar and Australia and occurs in different biomes of Brazil.2 Species of Thunbergia are commonly found in the state of Pernambuco (northeast Brazil), predominantly on the edge of Atlantic Forest fragments.3 Thunbergia grandiflora Roxb., Thunbergia alata Bojer ex Sims. and Thunbergia erecta T. Anderson, are commonly known as the Bengal clockvine, black-eyed Susan vine and bush clockvine, respectively. Besides the use of different parts of these plants as folk remedies by local communities for the treatment of malaria, diabetes, diarrhea, fever and cough,3,4 these species are used as ornamental plants and widely ground in gardens due to their brightly colored flowers and characteristic aromas. According to the literature, leaf extracts have been used to synthesize silver nanoparticles (AgNPs) and are reported to exhibit various bioactive properties, including antibacterial effects,5 nephroprotective properties,6 antitumor activity,7 cytotoxicity, anticholinesterase activity,8 and antioxidant effects.9,10 Phytochemical investigations of the leaves and flowers of species of this genus have revealed the presence of polyphenols, flavonoids, iridoid glycosides, fatty acids, anthocyanin and derivates of cinnamic acid.11-13 However, few studies have focused on the volatile chemical composition of these species. To the best of our knowledge, T. alata and T. erecta have not previously been investigated for the biological activities or chemical constituents of their essential oils. Terpenoids compounds present in essential oils can act as free radical scavengers and antilipoperoxidants, in addition to protecting collagen against manipulation caused by the superoxide anion radical.14,15 Other compounds in essential oils have demonstrated potential as lipoxygenase inhibitors, combining antioxidant and anticholinesterase activities, characteristics that may be promising for the treatment of Alzheimer's disease (AD).15,16 AD is a neurodegenerative disease characterized by reduced levels of acetylcholine (ACh) in the brain, resulting in memory impairment, emotional changes, and personality changes in advanced stages.17,18 ACh, the main neurotransmitter of cholinergic transmission, is regulated by acetylcholinesterase (AChE), an enzyme essential for maintaining its levels in a healthy brain.19 Thus, inhibition of AChE is a promising therapeutic strategy to alleviate the symptoms of AD.20 Approved treatments include the natural alkaloids galantamine and rivastigmine,21 as well as synthetic drugs such as donepezil, which are widely used for symptomatic treatment. However, the search for more effective natural compounds has attracted increasing interest worldwide. With this focus, and giving continuity to the chemical and biological contribution of aromatic plants that occur in the state of Pernambuco, this work offers a comparative study of the chemical composition of essential oils from the leaves and flowers of Thunbergia grandiflora,Thunbergia alata and Thunbergia erecta occurring in fragments of the Atlantic Forest in Pernambuco (Brazil), and reports their antioxidant activity, cytotoxicity and the potential inhibition of the enzymes acetylcholinesterase and butyrylcholinesterase in the oil from the leaves.
EXPERIMENTAL Plant material The fresh leaves and flowers of T. grandiflora, T. alata and T. erecta were collected from three adult plants of each species during the flowering period in the Federal Rural University of Pernambuco (UFRPE), in a fragment of Atlantic Forest, (8º01'05.2"S / 34º56'50.6''W) in the morning period in February 2019 (registered in National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) under No. 335599). The plants were identified by botanist Dr. Maria R. C. S. de Melo (Federal Rural University of Pernambuco). Voucher of all samples were mounted and deposited in the Vasconcelos Sobrinho Herbarium of the UFRPE, under numbers: 54150 Thunbergia grandiflora Roxb., 54149 Thunbergia alata Bojer ex Sims. and 54154 Thunbergia erecta T. Anderson. Chemicals All monoterpene (α-pinene) and sesquiterpenes (β-elemene, β-caryophyllene, aromadendrene, α-humulene, allo-aromadendrene, germacrene D, bicyclogramacrene, spathulenol and caryophyllene oxide) used in the identifications of volatile components were purchased from Sigma-Aldrich. Instrumental analysis The essential oils from fresh leaves (100 g) and flowers (100 g) were separately isolated using a modified Clevenger-type apparatus and hydrodistillation for 2 h. The oil layers were separated and dried over anhydrous sodium sulfate, stored in hermetically sealed glass containers, and kept at low temperature (-5 ºC) until analysis. Total oil yields were expressed as percentages (g 100 g-1 of fresh plant material). All experiments were carried out in triplicate. Quantitative gas chromatography (GC) analyses were carried out using a PerkinElmer Clarus 500 GC apparatus equipped with a flame ionization detector (FID) and a non-polar DB-5 fused silica capillary column (30 m × 0.25 mm × 0.25 μm) (J & W Scientific). The oven temperature was programmed from 60 to 240 ºC at a rate of 3 ºC min-1. The injector and detector temperatures were 260 ºC. Hydrogen was used as the carrier gas at a flow rate of 1 mL min-1 in split mode (1:30). The injection volume was 1.0 µL of diluted solution (1/100) of oil in n-hexane. The amount of each compound was calculated from GC-FID peak areas in the order of DB-5 column elution and expressed as a relative percentage of the total area of the chromatograms. The qualitative gas chromatography-mass spectrometry (GC-MS) analyses were carried out using a Varian 220-MS IT GC system with a mass selective detector, mass spectrometer in EI 70 eV with a scan interval of 0.5 s and fragments from 40 to 550 Da. fitted with the same column and temperature program as that for the GC-FID experiments, with the following parameters: carrier gas = helium; flow rate = 1 mL min-1; split mode (1:30); injected volume = 1 µL of diluted solution (1/100) of oil in n-hexane. Identification of the components was based on GC-MS retention index with reference to a homologous series of C8-C40 n-alkanes calculated using the Van der Dool and Kratz equation22 and by computer matching against the mass spectral library of the GC-MS data system (NIST 11 and Wiley, version 17) and co-injection with authentic standards as well as other published mass spectra.23 Area percentages were obtained from the GC-FID response without the use of an internal standard or correction factors. Principal component analysis Principal component analysis (PCA) based on the complete data set (three different plant samples for each species) was conducted to evaluate the chemical variation of essential oils of leaves and flowers of T. grandiflora,T. alata and T. erecta. The GC-MS data were exported in ASCII format to Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) to produce a data matrix of sample versus metabolite peak with associated peak areas. All the analyses were performed using Unscrambler® software, version 9.5.24 A similarity graph was generated by the distance of the correlation coefficient using hierarchical cluster analysis (HCA) with the aid of BioDiversity Pro, version 2.25 Acetylcholinesterase and butyrylcholinesterase activity The acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibition tests were performed using the colorimetric method described by Ellman,26 with modifications. Solutions containing 50 μL of phosphate buffer 50 mM, pH 7.4, 125 μL of dithionitrobenzoic acid (DTNB, 3 mM in phosphate buffer, pH 7.4), 25 μL of the enzymes acetylcholinesterase (AChE from electric eel, 1 U mL-1 in phosphate buffer, pH 7.4) or butyrylcholinesterase (BuChE from equine serum, 1 U mL-1 in phosphate buffer, pH 7.4) and different concentrations (0.78-50 µg mL-1) of the essential oils from the leaves of T. grandiflora,T. alata,T. erecta and the positive control were diluted in MeOH and added to each well of a 96-well microplate, followed by incubation for 15 min at 25 ºC. Next, 25 μL of acetylthiocholine iodide (ACTI, 15 mM in phosphate buffer, pH 7.4) or 25 μL of butyrylcholine iodide (BTChI, 15 mM in phosphate buffer, pH 7.4) were added. AChE and BuChE activity were analyzed in an ELISA EZ Read 2000 microplate reader with absorbance at 405 nm every 13 s, five times. Donepezil was used as the positive control. All samples were analyzed in triplicate. After normalization of the data, the nonlinear regression curve was created with the aid of the GraphPad Prism, version 7.01 (GraphPad Software, San Diego, CA, USA) statistical program. Toxicity against Artemia salina Leach The brine shrimp lethality bioassay was performed following the reported procedure by Meyer et al.27 with modifications. The growth medium was prepared with filtered sea water in a small tank divided into two compartments. The shrimp eggs were added to the covered compartment and a lamp was placed above the open side of the tank to attract hatched shrimps through perforations in the partition wall. After 48 h, the shrimps mature as nauplii (A. salina) and were ready for the assay. Stock solutions of the test were prepared by dissolving oil in Tween 80® 0.5% (v/v), and adding sea water to complete 5 mL of the total volume. Appropriate volumes were then added to tubes with sea water containing 10 nauplii to afford different concentrations of essential oil and positive control. The essential oils of leaves from T. grandiflora,T. alata and T. erecta ranged from 50.0 to 650.0 µg mL-1, respectively. Each concentration was tested in triplicate. Citoxan concentration was from 1 to 15 μg mL-1 (cyclophosphamide) and was used as positive control. The negative control samples contained sea water and Tween 80®. After 24 h of incubation under light, the number of dead and survivor brine shrimps in each tube was counted. Mortality data obtained from these experiments were submitted to Probit analysis using the SAS software, version 9.0 (SAS Institute Inc., Cary, North Carolina, USA), for the determination of the lethal concentration for 50% nauplius population (LC50), with a 95% confidence level for all experiments. Toxicity of the oils was compared by the method described by Dolabela28 as a basis: LC50 < 80 μg mL-1 was considered highly toxic; LC50 between 80 and 250 μg mL-1 was considered moderately toxic; and LC50 > 250 μg mL-1 was considered non-toxic. Antioxidant assay DPPH• radical scavenging The antioxidant activity of the essential oils from leaves of T. grandiflora,T. alata and T. erecta was performed against the free radical DPPH following the methodology of Araujo et al.29 Stock solutions were prepared from the extracts and methanol fraction at several concentrations (0.10 to 5.0 µg mL-1). Through preliminary analysis, appropriate quantities of stock solutions of the samples and 450 µL of the solution of DPPH• (23.6 mg mL-1 in EtOH) were transferred to 0.5 mL Eppendorf tubes and the volume was completed with EtOH, following homogenization. Samples were sonicated for 30 min and the amount of DPPH• was recorded on a UV-Vis (Biochrom EZ Read 2000) device at a wavelength of 517 nm in a 96-well plate. The scavenger activity of the free radicals DPPH• was expressed in terms of EC50 (concentration of antioxidant required to reduce the original amount of the radicals by 50%); lower values indicate the better antioxidant potential. Ascorbic acid was used as a positive control and all concentrations were tested in triplicate. Radical cation ABTS•+ The determination of antioxidant activity of the essential oils from leaves of T. grandiflora,T. alata and T. erecta against the radical cation ABTS•+ was carried out following the methodology described by Araujo et al.,29 in a UV-Vis (Biochrom EZ Read 2000) device, using Trolox as the standard compound. The starting concentrations of the sample solutions were 0.1-1.0 µg mL-1, with the addition of 450 µL of the radical ABTS•+ solution to give final concentrations of 2.5-100.0 µg mL-1 of the samples. Samples were protected from light and sonicated for 6 min. The absorbance of the samples and the positive control were measured at a wavelength of 734 nm using a 96-well plate microplate. The scavenger activity of the radical cation ABTS•+ were expressed in terms of EC50, lower values indicate the better antioxidant potential. Each concentration was tested in triplicate. The antiradical efficiency was established using linear regression analysis and the 95% confidence interval (p < 0.05) was obtained using the statistical program GraphPad Prism, version 5.0 (GraphPad Software, San Diego, CA, USA). Statistical analysis The results were expressed as mean ± standard deviation (SD). The data were submitted to analysis of variance (ANOVA) using one-way with comparison of means by Tukey's test from Statistica software, version 7.0.30 The differences were considered significant at p < 0.05. All experiments were performed in triplicate (n = 3).
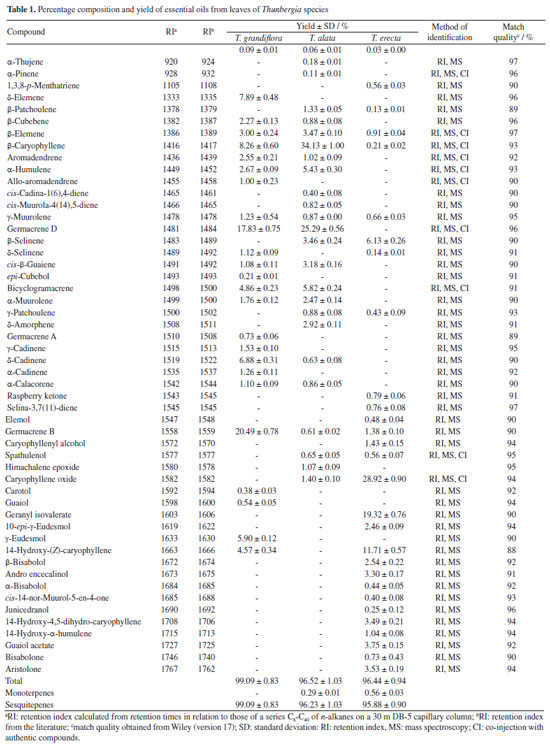
RESULTS AND DISCUSSION The steam distillation of the leaves and flowers furnished yellowish oils with citric aromas. The highest yield was obtained from the leaves and flowers of T. grandiflora (0.09 ± 0.01 / 0.07 ± 0.01%, respectively) (Tables 1 and 2).
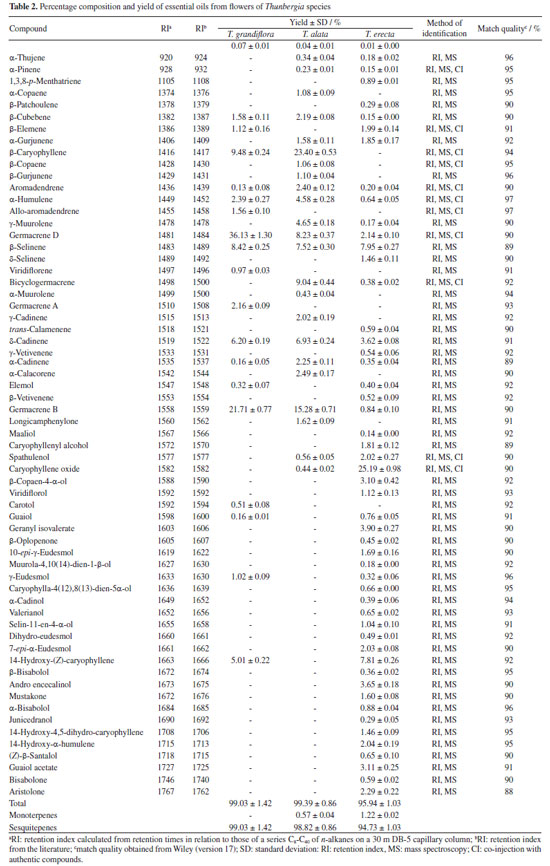
All oils investigated showed monoterpenes and sesquiterpenes in their compositions. Sesquiterpenes were predominant in the leaves and flowers oils of T. grandiflora (99.09 ± 0.83 / 99.03 ± 1.42%, respectively). Sesquiterpenes were the major constituents in the oils from the leaves and flowers of T. erecta (95.88 ± 0.90 / 94.73 ± 1.03%, respectively) and T. alata (96.44 ± 0.94 / 95.94 ± 1.03%, respectively) (Tables 1 and 2). Twenty-four compounds were identified in the leaf oil and eighteen were identified in the flower oil of T. grandiflora, representing 99.09 ± 0.83 / 99.03 ± 1.42% of the oils, respectively. Germacrene B (20.49 ± 0.78%), germacrene D (17.83 ± 0.75%) and β-caryophyllene (8.26 ± 0.60%) were the major constituents in the leaf oil, whereas germacrene D (36.13 ± 1.30%), germacrene B (21.71 ± 0.77%), β-caryophyllene (9.48 ± 0.24%) and β-selinene (8.42 ± 0.25%) were the major constituents in the flower oil. These results differ from those reported by Mbachu and Moronkola,31 where 3-octanol (45.96%) and 3,7-dimethyl-1,6-octadien-3-ol (13.22%) were found as the major constituents of the flower oil from T. grandiflora collected in Nigeria. 3-Octanol was also found in the leaf oil from T. grandiflora in the present analysis, but at a small proportion (0.09 ± 0.01%). Moreover, the major constituents found in the T. grandiflora oils investigated herein were not detected in the analysis of the species collected in Nigeria. Twenty-four and twenty-three compounds were identified in the leaf and flower oils from T. alata, representing 96.52 ± 1.03 / 99.39 ± 0.86% of the oils, respectively. β-Caryophyllene (leaves: 34.13 ± 1.00%; flowers: 23.40 ± 0.53%), germacrene D (leaves: 25.29 ± 0.56%; flowers: 8.23 ± 0.37%), germacrene B (flowers: 15.28 ± 0.71%) and bicyclogermacrene (flowers: 9.04 ± 0.44%) were the major constituents in both oils. Twenty-eight and fifty-one compounds were identified in the leaf and flower oils from T. erecta, representing 96.44 ± 0.94 / 95.94 ± 1.03% of the oils, respectively. Caryophyllene oxide (leaves: 28.92 ± 0.90%; flowers: 25.19 ± 0.98%), geranyl isovalerate (leaves: 19.32 ± 0.76%), 14-hydroxy-(Z)-caryophyllene (leaves: 11.71 ± 0.57%; flowers: 7.81 ± 0.26%) and β-selinene (leaves: 6.13 ± 0.26%; flowers: 7.95 ± 0.27%) were the major constituents in the leaves and flowers oils. The chemical composition of the oils investigated herein revealed significant differences among the species of Thunbergia. For instance, only germacrene B was identified in all the oils samples analyzed. The variability in the chemical profile of the Thunbergia oils was confirmed by principal component analysis (PCA) and hierarchical cluster analysis (HCA). Figures 1 and 2 display the PCA (scores and loadings) and HCA data of the GC-MS analyses of the essential oils from the leaves and flowers of the three species of Thunbergia.
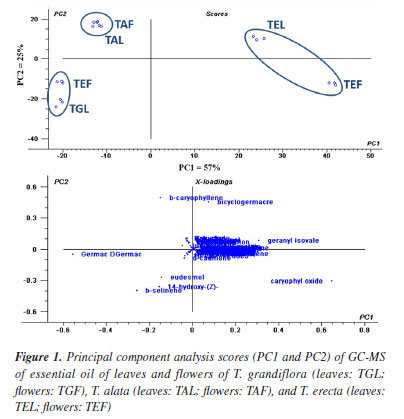
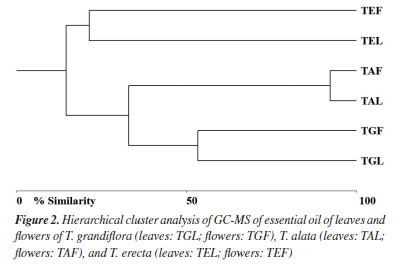
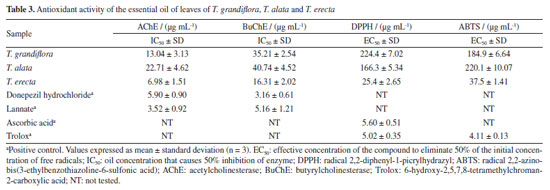
The analysis led to the separation of the oils into three distinct groups referring to the species studied. Seventy-three percent of the variability in the leaf and flower oils were explained by the first two components: PC1 (57%) and PC2 (25%). The separation among the groups determined by PCA of the leaf and flower oils mainly occurred due to the variation in the percentages of the compounds identified in the oils from T. grandiflora (germacrene B: 20.49 ± 0.78% in the leaf oil and 21.71 ± 0.77% in the flower oil, and germacrene D: 17.83 ± 0.75% in the leaf oil and 36.13 ± 1.30% in the flower oil), T. alata (β-caryophyllene: 34.13 ± 1.00% in the leaf oil and 23.40 ± 0.53% in the flower oil) and T. erecta (caryophyllene oxide: 28.92 ± 0.90% in the leaf oil and 25.19 ± 0.98% in the flower oil). The HCA revealed high similarity in the composition of the leaf and flower oils from the same species. The greatest similarity (92.14%) was found for the leaf and flower oils from T. alata. The lowest similarity (21.21%) was observed between the leaf and flower oils of T. erecta. This low similarity may be explained by the significant differences in the proportions of geranyl isovalerate (leaves: 19.32 ± 0.76%; flowers: 3.90 ± 0.27%) and the presence of numerous compounds in the flower oil that were not detected in the leaf oil. Studies on the determination of the chemical compounds of essential oils from species of the genus Thunbergia are scarce. Olaoluwa et al.32 found phytol (4.10%), i-propyl hexadecanoate (3.19%) and diisooctyl-1,2-benzenedicarboxylate (3.18%) as the major constituents of the leaf oil as well as methoxyl-phenyl-oxime (23.45%), Z-11-hexadecenoic acid (12.28%) and n-hexadecanoic acid (11.66%) as the major constituents of the essential oil from the stems of Thunbergialaevis collected in Nigeria. However, none of these constituents were found in the present analysis of species that occurred in the Atlantic Forest biome in the state of Pernambuco, Brazil. The in vitro inhibitory effect of essential oils from the leaves of Thunbergia species on the enzymes acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) is presented in Table 3.
The toxicity test with Artemia salina revealed low mortality (< 10%) at the concentration of 1000 μg mL-1. Therefore, the mean lethal concentration was not estimated. Among the three oils tested, the greatest inhibitory effect was observed for T. erecta oil, with a more pronounced inhibition of the AChE enzyme compared to BuChE. Furthermore, in the tests with AChE, T. erecta oil demonstrated an inhibitory effect equivalent to that observed for the positive control donepezil hydrochloride. On the other hand, the positive control lannate presented an inhibitory effect approximately 2 and 3 times greater than T. erecta oil in the tests with AChE and BuChE, respectively. The methanolic extract of T. grandiflora leaves collected in Bangladesh is reported33 to have AChE and BuChE enzyme inhibition properties. However, the effectiveness in preventing AChE and BuChE enzymes observed for the ethanolic extracts were approximately 19 and 9 times less active than the essential oil of the plant reported in the present study. Another study34 reports that the aqueous extract of Thunbergia laurifolia leaves collected in Thailand revealed significant potential to inhibit AChE in mice exposed to lead in in vivo and in vitro models, indicating that dietary supplementation of T. laurifolia may have benefits in alleviating learning deficit and memory loss due to lead exposure. Numerous studies35,36 have reported the anticholinesterase activity of sesquiterpenes, including those identified as the major constituents of Thunbergia oil. Essential oils have been documented as inhibitors of other targets, such as triphosphatases (ATPases) and the gamma-aminobutyric acid (GABA) receptor.37,38 All oils exhibited antioxidant activity in the DPPH and ABTS tests (Table 3). The best results in the ABTS and DPPH tests were found for T. erecta oil. The oil was approximately six times more active than T. alata and T. grandiflora oils in the ABTS and DPPH tests, respectively. The results found for T. erecta oil can be explained by the presence of a higher percentage of compounds from the oxygenated sesquiterpene class in its composition (84.35%). Among these compounds, the presence of caryophyllene oxide in high percentages was identified in the oil, as well as other oxygenated sesquiterpenes that are reported39,40 to have antioxidant properties. Antioxidant properties are also reported for ethanolic extracts of different Thunbergia species, such as T. laurifolia collected in Thailand,34 T. erecta collected in Egypt41,42 and India,13 T. grandiflora collected in Bangladesh33 and T. coccinea collected in Saudi Arabia.43 In these studies, the observed antioxidant potential is mainly attributed to the presence of phenolic compounds in the extracts. Thus, due to their composition rich in sesquiterpenes, the essential oils of the analyzed Thunbergia species may exert a multifunctional action, including antioxidant and cholinesterase inhibitory properties. This combination of effects may contribute to neuronal protection and prevention of damage caused by environmental stressors, making them a promising alternative for the development of new therapeutic strategies.
CONCLUSIONS This is the first report of the chemical composition of the essential oils from the leaves and flowers of Thunbergia grandiflora,T. alata and T. erecta that occur in an urban fragment of the Atlantic Forest in the Brazil. The GC-MS analysis of the oils from these three species revealed a new chemotype for the genus, that sesquiterpenes were the predominant chemical class. The chemical analysis revealed both qualitative and quantitative differences among the different species, which were confirmed by principal component analysis and hierarchical cluster analysis. The oils from the leaves and flowers of T. alata exhibited a high degree of similarity (92.14%), as all compounds found in the flower oil were also found in the leaf oil. In contrast, significant differences were found in the composition of the leaf and flower oils from T. erecta. Furthermore, all three species demonstrated antioxidant and cholinesterase activity. Among them, the leaf oil of T. erecta exhibited the strongest antioxidant effects in the DPPH and ABTS assays and significantly inhibited AChE and BuChE enzymes. However, none of the tested oils showed toxicity against Artemia salina, highlighting their potential for safe therapeutic applications.
SUPPLEMENTARY MATERIAL Supplementary data are available free of charge at http://quimicanova.sbq.org.br as PDF file.
ACKNOWLEDGMENTS The authors are grateful to Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); productivity scholarship: CNPq grant PQ-2 306514/2022-2, FACEPE grant IBPG-1996-1.06/22; IBPG-0276-1.06/24; APQ-16021.06/24; APQ-0991-1.06/24.
REFERENCES 1. Reis, A. S.; Gil, A. S. B.; Kameyama, C.; Rodriguesia 2017, 68, 887. [Crossref] 2. Flora do Brasil, http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB21729, accessed in April 2025. 3. Jeruto, P.; Lukhoba, C.; Ouma, G.; Otieno, D.; Mutai, C.; J. Ethnopharmacol. 2008, 116, 370. [Crossref] 4. Cho, Y. C.; Kim, Y. R.; Kim, B. R.; Cho, S.; Int. J. Mol. Med. 2016, 38, 1596. [Crossref] 5. Mathew, A.; Thomas, S.; J. Nanosci. Nanotechnol. 2019, 5, 669. [Crossref] 6. Farag, F. S. A. A.; El-Mordy, F. M. A.; Ibrahim, M. H.; Gad, E. S.; Soliman, R. H.; Anwar, H. M; Cell Biochem. Biophys. 2024, 82, 2813. [Crossref] 7. Jetawattana, S.; Boonsirichai, K.; Charoen, S.; Martin, S. M.; Asian Pac. J. Cancer Prev. 2015, 16, 4357. [Crossref] 8. Uddin, M. J.; Russo, D.; Rahman, M. M.; Uddin, S. B.; Halim, M. A.; Zidorn, C.; Milella, L.; Evidence-Based Complementary and Alternative Medicine 2021, 2021, 9995614. [Crossref] 9. Sultana, K.; Chatterjee, S.; Roy, A.; Chandra, I.; Med. Aromat. Plants 2015, 4, 2167. [Crossref] 10. Naowaboot, J.; Nanna, U.; Chularojmontri, L.; Tingpej, P.; Pannangpetch, P.; Asian Pac. J. Trop. Biomed. 2021, 11, 97. [Crossref] 11. Ibrahim, M. T.; El Wafa, S. A. A.; Sleem, A. A.; J. Pharmacogn. Phytochem. 2017, 6, 43. [Link] accessed in May 2025 12. Damtoft, S.; Frederiksen, L. B.; Jensen, S. R.; Phytochemistry 1994, 35, 1259. [Crossref] 13. Vankar, P. S.; Srivastava, J.; Int. J. Food Eng. 2010, 6, 1. [Crossref] 14. Chen, J. H.; Ho, C. T.; J. Agric. Food Chem. 1997, 45, 2374. [Crossref] 15. Frota, L. S.; Alves, D. R.; Freitas, L. S.; Lopes, F. F.; Marinho, M. M.; Marinho, E. S.; Morais, S. M. D.; J. Braz. Chem. Soc. 2022, 33, 446. [Crossref] 16. Morais, S. M.; Lima, K. S. B.; Siqueira, S. M. C.; Cavalcanti, E. S. B.; Souza, M. S. T.; Menezes, J. E. S. A.; Trevisan, M. T. S.; Rev. Bras. Plant. Med. 2013, 15, 575. [Crossref] 17. Ahmed, F.; Ghalib, R. M.; Sasikala, P.; Ahmed, K. M.; Pharmacogn. Rev. 2013, 7, 121. [Crossref] 18. Elufioye, T. O.; Obuotor, E. M.; Agbedahunsi, J. M.; Adesanya, S. A.; Biol.: Targets Ther. 2017, 11, 107. [Crossref] 19. Dhanasekaran, S.; Perumal, P.; Palayan, M.; J. Appl. Pharm. Sci. 2015, 5, 12. [Crossref] 20. Mushtaq, G.; Greig, N. H.; Khan, J. A.; Kamal, M. A.; CNS Neurol. Disord.: Drug Targets 2014, 13, 1432. [Crossref] 21. Mehta, M.; Adem, A.; Sabbagh, M.; International Journal of Alzheimer's Disease 2012, 1, 728983. [Crossref] 22. Van Den Dool, H.; Kratz, P. D.; J. Chromatogr. A 1963, 11, 463. [Crossref] 23. Adams, R. P.; Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Espectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, 2007. 24. Unscrambler®, version 9.5; CAMO Process AS, Norway, 2007. 25. McAlece, N.; Gage, J. D. G.; Lambshead, P. J. D.; Paterson, G. L. J.; BioDiversity Professional Statistics Analysis Software; Scottish Association for Marine Science and the Natural History Museum: London, 1997. 26. Ellman, G. L.; Courtney, D.; Andies, V.; Featherstone, R. M.; Biochem. Pharmacol. 1961, 7, 88. [Crossref] 27. Meyer, B. N.; Ferrigni, N. R.; Putnam, L. B.; Jacobsen, L. B.; Nichols, D. E.; Mclaughlin, J. L.; J. Med. Plants Res. 1982, 45, 31. [Crossref] 28. Melo, L. F. A.; da Camara, C. A. G.; Oliveira, L. L. D. S. S.; Modesto, J. C. A.; Pérez, C. D.; Biotemas 2012, 25, 145. [Crossref] 29. Araujo, C. A.; da Camara, C. A.; de Moraes, M. M.; de Vasconcelos, G. J.; Pereira, M. R.; Zartman, C. E.; Nat. Prod. Res. 2021, 36, 436. [Crossref] 30. Statistica, version 7.0; StatSoft, Tulsa, OK, USA, 2004. 31. Mbachu, K. A.; Moronkola, D. O.; J. Adv. Med. Pharm. Sci. 2017, 15, 57. [Crossref] 32. Olaoluwa, O. O.; Moronkola, D. O.; Kwenga, S.; Mgbeoji, O.; World J. Pharm. Res. 2016, 5, 224. [Link] accessed in April 2025 33. Uddin, M. J.; Alam, M. N.; Biswas, K.; Rahman, M. A.; Cogent Food Agric. 2016, 2, 1256929. [Crossref] 34. Phyu, M. P.; Tangpong, J.; BioMed. Res. Int. 2013, 2013, 186098. [Crossref] 35. Faruque, M.; Nazmi, K.; van Splunter, A.; Laine, M. L.; Bikker, F. J.; Biomed. Pharmacother. 2023, 168, 115699. [Crossref] 36. Alimi, D.; Hajri, A.; Jallouli, S.; Sebai, H.; Vet. Parasitol. 2023, 322, 110028. [Crossref] 37. Guo, Z.; Ma, Z.; Feng, J.; Zhang, X.; Agric. Sci. China 2009, 8, 1492. [Crossref] 38. Zahran, H. E. M.; Abou-Taleb, H. K.; Abdelgaleil, S. A. M.; J. Asia-Pac. Entomol. 2017, 20, 133. [Crossref] 39. Nogueira Neto, J. D.; de Sousa, D. P.; de Freitas, R. M.; Rev. Cienc. Farm. Basica Apl. 2013, 34, 125. [Link] accessed in April 2025 40. El-Gawad A.; Ind. Crops Prod. 2016, 80, 36. [Crossref] 41. Farag, F. S. A. A.; Anwar, H. M.; Aboushousha, T.; Mohammed, H. S.; Ismail, L. D. M.; Appl. Biochem. Biotechnol. 2023, 195, 5881. [Crossref] 42. Refaey, M. S.; Abdelhamid, R. A.; Elimam, H.; Elshaier, Y. A.; Ali, A. A.; Orabi, M. A.; Bioorg. Chem. 2021, 108, 104643. [Crossref] 43. Zaman, G. S.; Alshahrani, M. Y.; Barua, P.; Aladel, A.; Zaman, F. A.; Ahmad, I.; Saeed, M.; Cell. Mol. Biol. 2021, 67, 56. [Crossref]
Guest Editor handled this article: Ivo José C. Vieira |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access