Artigo
| Thermoplastic starch-ucuuba biocomposites: effect of buriti pulp oil on lignocellulosic fiber dispersion |
|
Marcelo F. L. OliveiraI; Valdir F. Veiga-JuniorII; Marcia G. OliveiraI,; Fernanda Cristina F. BragaI,*
I. Instituto Nacional de Tecnologia (INT), Divisão de Materiais (DIMAT), 20081-312 Rio de Janeiro - RJ, Brasil Received: 04/01/2024 *e-mail: fernanda.braga@int.gov.br The use of residual biomass in biocomposite production aligns with environmental goals set by solid waste policies. Starch, a low-cost and renewable natural polymer, often faces challenges with mechanical strength and water vapor permeability. Ucuuba cake, an Amazonian agro-industrial byproduct, offers fiber reinforcement to enhance thermoplastics. Buriti pulp oil, also from the Amazon, acts as a processing aid, improving thermal stability and reducing hydrophilicity. This study explores buriti oil as a partial substitute for glycerol in thermoplastic starch (TPS) biocomposites with ucuuba fibers. Characterization showed that buriti pulp oil, rich in oleic and palmitic acids, enhances hydrophobicity, reducing water absorption. It also lowered final torque during processing, facilitating flow and homogeneity. Mechanical tests indicated a marginal increase in strength but decreased elongation at break. Scanning electron microscopy (SEM) revealed smoother surfaces and better fiber adhesion. Rheological and thermal analyses confirmed the role of buriti pulp oil as a processing aid and thermal stabilizer. This research underscores the potential of renewable resources and industrial residues in sustainable polymer development. INTRODUCTION The environmental imbalances caused by the Industrial Revolution, combined with rising living standards over the centuries, have weakened ecosystems through climate change, increased solid waste generation, and resource depletion, among other issues.1 Indeed, while industrialization has spurred socio-economic benefits, increased competitiveness, and technological advancement, it has also led to significant environmental challenges.2,3 With the advent of the new century, global efforts are focused on reducing synthetic polymer waste due to growing environmental awareness of its impacts.1,4-7 In response, Brazil has enacted the National Solid Waste Policy (Law No. 12.305/10), effective from August 2, 2010, which outlines actions to address the mismanagement of solid waste (domestic, industrial, electronic, etc.) with a focus on sustainable development.7-9 To address the high demand for energy and materials from fossil resources, renewable and bio-based raw materials have garnered increasing interest from the scientific community, as evidenced by a surge in patents and publications, particularly for biomass-derived materials.1,2 Within this context, a new, more eco-efficient, sustainable, and environmentally conscious industrial approach has emerged.10 This aims to avoid scenarios similar to conventional plastics (petroleum derivatives), which currently make up about one-fifth of urban waste.4,5,7 Biomass has thus become a viable alternative due to its renewable and environmentally friendly nature. According to European Directive 2009/28/EC, biomass is defined as the biodegradable fraction of products, waste of biological, plant, and/or animal origin, as well as industrial and municipal waste.11-13 Biomass has the potential to serve as an energy source, contributing approximately 12% to the global renewable energy mix, with a range of 40 to 50% in developing countries.12,13 Starch aligns well with the biomass profile and has been extensively studied as a source for biodegradable polymers.14,15 It is fully compatible with the environment, does not produce toxic waste, and is readily available, making it a cost-effective and economically viable material.16,17 As a polysaccharide, starch exists in granular form in various plant parts and exhibits different characteristics depending on its botanical source, such as cereals, legumes, and tubers.16-18 Typically, the granular structure of starch has a crystallinity of about 15-45%. When exposed to high temperatures and shear forces, the granules can be processed into thermoplastic starch (TPS) by disrupting their structure, which leads to molecular chain disruption and subsequent intermolecular rearrangement.4,19 Unlike traditional thermoplastics, starch does not follow conventional processing methods due to its strong hydrogen bonds, which prevent melting. Therefore, additional treatments, such as chemical modification or plasticization, are necessary to facilitate processing through extrusion, injection, or compression molding.6 Plasticization is crucial for breaking down the crystalline structure of native starch, enabling the production of thermoplastic starch.20 The term "plasticizer" was universally defined in 1951 by the International Union of Pure and Applied Chemistry (IUPAC) as a substance or material incorporated into a thermoplastic and/or elastomeric matrix that facilitates processing, enhances flexibility, and increases the elongation of the final product.21-23 Chemically, a plasticizer works by weakening the intermolecular forces between bonds, reducing crystallinity, and thereby increasing the mobility of molecular segments, which results in greater plasticity of the material.22,23 In the case of starch, the plasticizer aids in breaking down its granules, assisted by shear and temperature, leading to the formation of thermoplastic starch. This starch is typically plasticized with formamide, urea, glycerol, or other plasticizers. However, some research18,24-26 indicates that combining two types of plasticizers during processing can improve the performance of the final product more effectively than using just one, addressing the challenges associated with this type of material. Vegetable oils offer a promising route for developing polymeric materials from renewable sources due to their availability, inherent biodegradability, and low toxicity.27 The biodiversity of the Amazon region provides a range of plant species with significant potential for various applications. Buriti pulp oil, extracted from the fruit of the buriti palm (Mauritia flexuosa L. f.), is primarily found in the central part of Brazil and the southern Amazon lowlands. This oil contains fatty acids, tocopherols, and carotenes, which impart photoluminescent properties to materials, along with high thermal stability and antimicrobial activity.28-29 In parallel, to address the limitations of conventional thermoplastic starches, such as low mechanical strength, high water vapor permeability, and high cost, new research20 avenues have emerged focusing on incorporating industrial residues into biodegradable films. The reuse of industrial waste from renewable sources has been extensively studied, resulting in numerous scientific publications, patents, and commercially available products. Agro-industrial waste, in particular, offers significant potential for beneficial applications for both humans and the environment.30 Sources of fiber from agro-industrial residues (e.g., bagasse, fruit peels, vegetable scraps) have been utilized to create higher value-added products, extending beyond traditional uses such as animal feed and fertilizers. However, many Brazilian plant species from the Amazon region and their respective fibers remain underexplored in terms of their properties and potential applications.30-32 The ucuuba, also known as ucuhuba or Virola, is a species native to the Amazon, primarily found in floodplains and "igapós".33 In Brazil, it is concentrated in the Amazon Basin, extending across the states of Amazonas, Pará, Maranhão, Ceará, and Goiás, where it thrives in flooded areas, riverbanks, streams, holes, and paranás, often in locations prone to flooding.34 Despite its prevalence, little is known about the species and its potential applications when used as waste. This work aims to develop TPS biocomposites from agro-industrial residues, providing an alternative to mitigate the environmental impact of agro-industries and plastic packaging sectors. The study evaluates the influence of buriti pulp oil on the physical and mechanical properties of TPS / ucuuba biocomposites. To our knowledge, there are no previous reports in the literature on using this vegetable oil as a partial substitute for glycerol in the TPS matrix in the presence of lignocellulosic fibers.
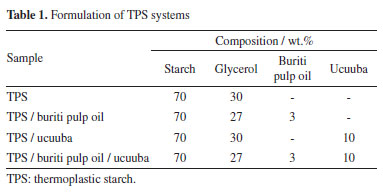
EXPERIMENTAL Materials The corn starch used in the experiment, containing 27% amylose and 73% amylopectin (Amidex 3001), was obtained from Ingredion® Co. (Brazil) and used as received. The moisture content of the starch was 11% (m/m). Glycerol, used as a plasticizer, was purchased from Alphatec Química Fina Ltda. (Brazil) and was used without further purification or drying. Ucuuba fibers (an Amazonian agro-industrial residue) and buriti pulp oil were donated by Mega Pack Plásticos Sociedade S. A. (Brazil). Fatty acids composition of buriti pulp oil The fatty acids in buriti pulp oil were identified using high-performance gas chromatography with a flame ionization detector (GC-FID). The chromatographic analysis was performed using a Varian gas chromatograph, model CP 3800, equipped with a flame ionization detector, split/splitless inlet, and a 20M Carbowax column (30 m × 0.25 mm internal diameter with 0.25 µm film thickness, Agilent Technologies). The temperature program was set to 100 ºC for 5 min, increasing at a rate of 7 ºC min-1 to 210 ºC with a split ratio of 1:10, and the detector temperature was 250 ºC. Identifications were made by comparing retention times with those of a standard mixture. The injection volume was 1.0 mL, and peak areas of fatty acid methyl esters were determined by normalization. Preparation of biocomposites Initially, the ucuuba fibers were dried, milled, and sieved to obtain a fiber length predominantly around 0.42 mm. The starch was thoroughly premixed with 27 wt.% glycerol and 3 wt.% buriti pulp oil, with or without 10 wt.% ucuuba fiber, manually until a homogeneous mixture was achieved at 23 ºC. The mixtures were then processed at 120 ºC in a Haake Rheodrive 4 Polylab OS internal mixer, equipped with roller rotors running at 60 rpm. The mixing time for all preparations was set to 4 min to avoid starch degradation. Laminates of both the matrix and biocomposites were obtained using a hydraulic press (Marconi) with a heating system and controlled cooling apparatus. The processing conditions were: temperature of 120 ºC, applied pressure of 493 kgf cm-2, and pressurization time of 10 min. Table 1 presents these formulations.
Processabillity Torque-time curves were measured to analyze the processing behavior of the biocomposites and specifically to evaluate the effect of buriti pulp oil on melt flowability. These torque results for the TPS and its biocomposites reflect the flow behavior and, consequently, the viscosity of the systems. Mechanical properties Samples were cut using a CEAST pneumatic die punch machine, and the tensile testing samples were shaped like dumbbells. After cutting, the samples were pre-conditioned at 23 ± 2 ºC and 60 ± 2% relative humidity before being tested on a Universal Testing Machine (model DL 3000, Instron Inc). Testing was conducted at a constant crosshead speed of 10 mm min-1 with a 10 kN load cell, following the DIN 53504 standard.35 Five measurements were taken for each composition, and the average value was recorded. Contact angle measurement The surface hydrophobicity of TPS and its biocomposites was characterized by contact angle measurements using a pocket goniometer (model PGX+). Scanning electron microscopy (SEM) Samples for SEM analysis were quickly plunged into liquid nitrogen for about 40 min before fracturing. They were then coated with a thin layer of gold using the sputter deposition method (Emitec equipment, model SC 7620) for 2 min at 20 mA. Micrographs were taken at 1000× magnification with a FEI scanning electron microscope (model Inspect S 50) under low vacuum conditions at an accelerating voltage of 15 kV. Rheological measurements Rheological properties were measured with an oscillatory shear parallel plate rheometer (model Anton Paar, MCR301) with a 25 mm diameter plate and a constant gap of 1 mm, at 150 ºC. Measurements were taken over a frequency range from 100 to 0.1 rad s-1 and a strain of 0.01%, which is within the linear viscoelastic region. The storage modulus (G') and complex viscosity (h*) versus angular frequency were reported for each composition. Thermal properties Thermal stability of the TPS and its biocomposites was assessed using thermogravimetric analysis (TGA). Thermal events were studied with a Netzsch thermal analyzer (model STA 409) under a nitrogen atmosphere (flow rate 60 mL min-1). Approximately 10 mg of films were heated in aluminum crucibles from 40 to 600 ºC at a heating rate of 20 ºC min-1. All measurements were performed in triplicate. Statistical analysis To evaluate the statistical significance of the impact of the additives on the hydrophobic and mechanical properties of TPS biocomposites, a one-way analysis of variance (ANOVA) was performed. Subsequently, Tukey's honest significant difference (HSD) test was applied for pairwise comparisons. Data analysis was conducted using Jamovi software, version 2.6.44 (The Jamovi Project, Australia, 2022), considering a significance level of 5%.
RESULTS AND DISCUSSION Buriti pulp oil characterization Table 2 presents the profile of fatty acids present in buriti pulp oil, and it is possible to verify the main constituents observed were oleic (C18:1 cis) and palmitic (C16:0) acids. This behavior was already expected, since vegetable oil has hydrophobic character, which is directly related to its structural constitution: triglycerides formed between glycerol and a variety of fatty acids.
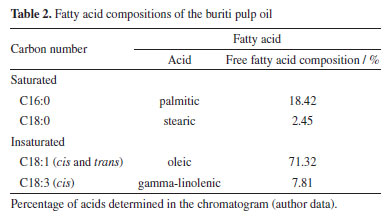
Studies have shown that the lipid composition of buriti oil influences its interaction with starch, forming a stable starch-lipid binary complex. Oleic acid, the major component of the buriti oil, has a long chain (C18) and is unsaturated, which makes it less soluble in water.36 The relationship between chain size and degree of unsaturation is directly related to the complexation index in starch and fatty acid systems, influencing in the hydrophilicity of the resulting material.37,38 Processabillity Figure 1 depicts the torque-time curve profiles of the compositions during the processing of each TPS systems.
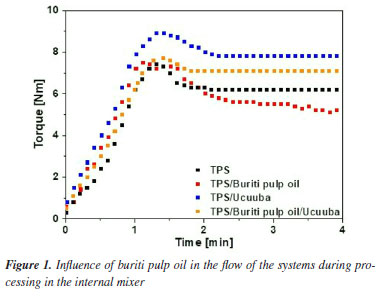
The torque obtained during processing is an extremely important parameter as it is directly related to viscosity of systems, the rate of dissipated mechanical energy to obtain a homogeneous mixture and the time required to reach this state. This characteristic of homogeneity is achieved when the value of torque was stabilized.34,39,40 It can be seen that during processing, all starch compositions have a similar tendency to increase the maximum torque as a function of the component load into the mixing chamber, requiring a high shear force to rotate the rotors in the presence of the premix. After this, there is a gradual reduction in values until complete stabilization after 4 min. This observed behavior is attributed to the action of water and glycerol. These plasticizers play a fundamental role in starch processing, acting synergistically to promote plasticization and gelatinization. Water, considered the primary plasticizer, forms hydrogen bonds with the hydroxyl groups of glucose units in starch. These bonds weaken intermolecular interactions, disrupting crystalline structures and leading to the gelatinization of starch granules. This process increases the mobility of polymer chains, facilitating material deformation and fluidity. Glycerol, on the other hand, functions as a secondary plasticizer, penetrating the amorphous regions of starch. Its three hydroxyl groups form hydrogen bonds with starch, reducing van der Waals forces and increasing intermolecular distance. This interaction provides the material with enhanced flexibility and plasticity.4,41 However, the influence on processability is clearly noticeable by replacing part of glycerol with buriti pulp oil (3 wt.%). The addition of buriti pulp oil led to a reduction in the final torque of the systems, suggesting its performance as a processing aid, facilitating the flow and wettability of the melt, beyound promoting a more homogeneous mixture with lower energy expenditure. Chemically analyzed, buriti pulp oil effectively weakened the molecular interactions within the starch, which facilitated the destruction of the crystalline structure and the subsequent melting (or fusion) of the starch, under shear and temperature. Mechanical properties Table 3 shows the mechanical properties of the TPS matrix and its biocomposites with the partial replacement of glycerol by 3 wt.% of buriti pulp oil.
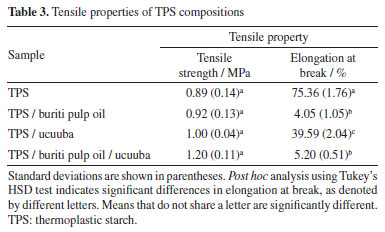
A significant reduction in elongation at break was observed in systems with buriti pulp oil. This behavior may be associated with the presence of water in the easily evaporated glycerol (hydrophilic), which made part of the plasticizer unavailable to interact freely with the starch chains and thus become extremely glassy.42 The tensile strength results demonstrated low variability, suggesting consistency among the different compositions. To quantitatively assess the impact of buriti pulp oil, a statistical analysis was conducted. One-way ANOVA revealed a significant decrease in elongation at break (p < 0.05) for compositions containing buriti pulp oil. Tukey's post hoc test confirmed that samples with buriti pulp oil (group b) exhibited significantly lower elongation compared to pure TPS (group a). While tensile strength remained relatively unaffected, the results suggest that buriti pulp oil acts as a more pronounced plasticizer, compromising the ductility of the composites. Hydrophobicity Wettability is a physicochemical property typically characterized by the technique of measuring the contact angle of a wetting liquid (usually water) on a substrate and aims to qualify the type of molecular interaction between these two interfaces. Depending on the acting forces (cohesive or dispersed) and the type of surface obtained (roughness and/or porosity), it is possible to predict the degree of hydrophilicity of the material through the angle formed by the drop and the surface of the material.43 Starch-based materials are known to absorb water due to the presence of hydroxyl groups. To mitigate this tendency, vegetable oils, such as buriti pulp oil and ucuuba, were incorporated into TPS. As shown in Figure 2 and Table 4, the addition of these oils significantly increased the contact angle of the biocomposites, indicating enhanced hydrophobicity.
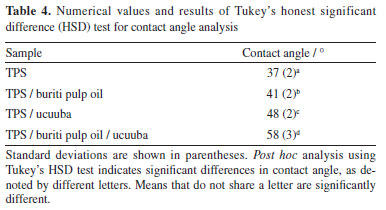
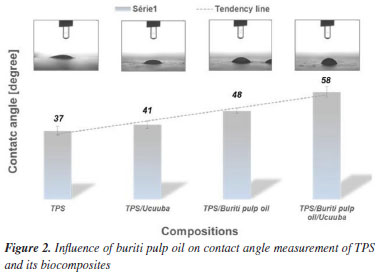
To quantitatively assess the impact of the additives on the wettability of TPS biocomposites, a statistical analysis was performed. The mean and standard deviation of the contact angle values were analyzed using one-way ANOVA and Tukey's HSD test at a 95% confidence level. The results indicated significant differences (p < 0.05) among the samples, suggesting that the additives significantly influenced the contact angle. A Tukey post hoc analysis revealed that the combined addition of buriti and ucuuba oil (group d) resulted in a significant increase in the hydrophobicity of the biocomposites, compared to the other groups (a, b, c) as shown in Table 4. This result suggests a synergistic effect between the two additives, reinforcing the hypothesis that the combination of both would lead to a greater increase in hydrophobicity. Scanning electron microscopy (SEM) The observed morphologies (Figure 3) are consistent with the results of the hydrophobicity test, carried out by measuring the contact angle. Substituting a portion of glycerol with 3 wt.% buriti pulp oil (Figures 3b and 3d) resulted in smoother and more homogeneous surface morphologies. This suggests that vegetable oil enhanced starch plasticization, breaking down granules and leading to a more cohesive matrix. This improved cohesion can be attributed to two factors: (i) the formation of a stable starch-lipid complex and (ii) the reduced solubility of starch matrices stabilized with buriti pulp oil.36,37 The stability of the formed complex led to a reduction in the free volume of the matrix, consequently hindering the permeability of volatile products during sample preparation through the compression molding process. This enhanced the effectiveness of hydrogen bond interactions, making it difficult for hydroxyl groups to interact with water molecules and, thus, increasing the permeability of vegetable oil between the starch chains.38,43-45
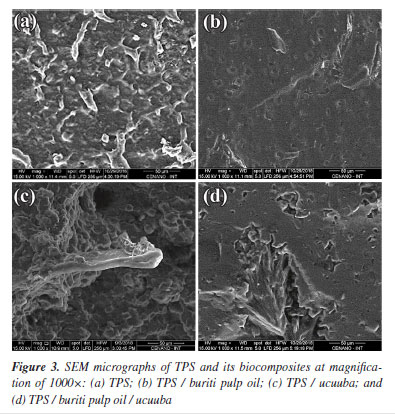
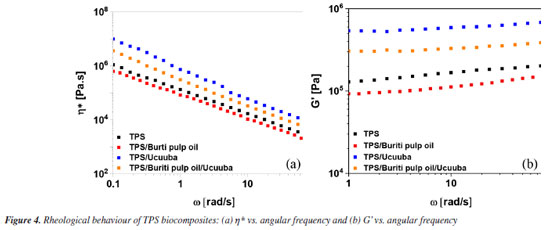
The micrographs also reveal improved fiber adhesion in the TPS matrix when glycerol / buriti pulp oil is present, a phenomenon not observed in systems without vegetable oil in the composition. The TPS / ucuuba sample (Figure 3c) exhibited voids at the matrix-fiber interface, suggesting lower adhesion due to the phenomenon known as pull-out. These voids were caused by incomplete starch breakdown, resulting in the presence of intact carbohydrate granule residues within the continuous phase of the thermoplastic starch (Figure 3a) that were unable to encapsulate the ucuuba fiber, as observed in Figure 3c. Consequently, local stress concentrations increased, promoting premature mechanical failure of the biocomposite. Rheological properties Rheological evaluation has been utilized to better understand processability parameters for scale-up operations to pilot and industrial production levels, in addition to aiding in the comprehension of morphology, interfacial properties, volume fraction, among other aspects.46 According to Aichholzer and Fritz,47 in a study conducted in the late 1980s, glycerol was already extensively used as a plasticizer in starch processing, acting as an additive that facilitates the breakdown of starch particles. This process fragments the crystalline structure of polysaccharides, transforming them into highly amorphous thermoplastic structures. This disruption of the starch crystalline phase occurs through a combination of operational parameters (mechanical and thermal) via multiple chemical and physical reactions, which are possible only in the presence of a plasticizer. This leads to the breakdown of molecular bonds and, consequently, the irreversible swelling of the granules with destruction of their crystalline spherulitic structures. Generally, the conversion of starch to a thermoplastic involves the following stages: plasticizer diffusion, granule expansion, gelatinization, decomposition, melting and crystallization.41 In this context, the rheological properties of the systems were evaluated to determine how the addition of the co-plasticizer (buriti pulp oil) affects viscosity and elasticity in the melt, as illustrated in Figure 4.
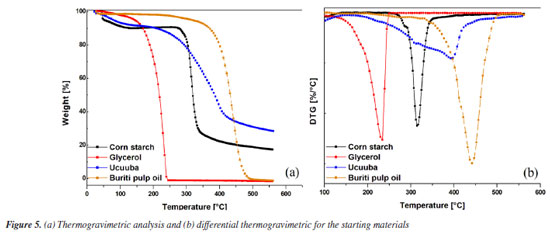
As shown in Figure 4a, the complex viscosity of the TPS system exhibited a slight reduction in the presence of buriti pulp oil. However, this behavior is unlikely to be associated with any molecular interaction between the oil (nonpolar) and starch (polar), due to their distinct chemical characteristics. It can therefore be inferred that the oil functioned as an external plasticizer, migrating to the surface and forming a lubricant layer that reduced flow resistance during processing. Additionally, it is important to note that the viscosity curve tends to decrease with increasing shear rate, which is expected as it falls within the linear viscosity region. This behavior indicates that the material possesses pseudoplastic characteristics, where the polymer chains orient themselves in the direction of flow, resulting in reduced viscosity. As anticipated, the incorporation of ucuuba (vegetable fiber) into thermoplastic starch compositions increases the viscosity of the material due to the disruption of matrix flow and restricted polymer chain mobility. Thermal properties Thermal stability is a crucial factor to consider, as it allows for the prediction of processing conditions without subjecting the material to thermal degradation. Therefore, the starting materials were analyzed to provide a more concrete discussion of thermoplastic starch and its biocomposites. For this purpose, corn starch, glycerol, buriti pulp oil, and ucuuba were initially evaluated (Figure 5), as well as TPS and its biocomposites (Figure 6).
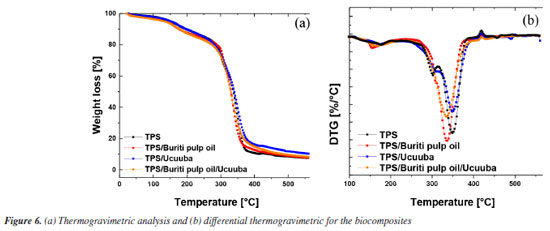
The starting materials (Figure 5) were characterized for their thermal stability, with all thermograms showing an initial weight loss corresponding to dehydration and possible volatilization of low molar mass components (below 150 ºC). This initial weight loss is expected due to the moisture content in the starting materials, which is attributed to the presence of hydroxyl groups in their structures that can absorb and retain moisture. Corn starch (Figure 5a) exhibited a degradation stage starting around 283 ºC, reaching its maximum degradation at 315 ºC. This degradation is attributed to the breakdown of amylose and amylopectin, followed by the decomposition of carbonaceous residues, as reported by López et al.48 For the plasticizers, glycerol and buriti pulp oil showed single degradation steps around 231 and 403 ºC, with maximum degradation temperatures of 268 and 447 ºC, respectively, indicating that buriti pulp oil has higher thermal stability compared to glycerol. Ucuuba fiber showed two degradation stages: (i) around 275 ºC, likely related to the degradation of short-chain organic compounds, and (ii) around 380 ºC, attributed to the thermal degradation of medium and long carbon chain compounds. The TPS compositions and their biocomposites were also evaluated, as shown in the thermograms of Figure 6. The thermoplastic starch (TPS) degradation steps consist primarily of two stages: (i) water loss and glycerol removal associated with material hydrophilicity (below 150 ºC); (ii) thermal degradation of the starch fractions (amylose and amylopectin) starting around 295.6 ºC, followed by carbonaceous degradation, reaching a maximum degradation temperature of 301.4 ºC. The TPS / ucuuba biocomposite exhibited a slight increase in thermal stability due to the incorporation of vegetable fibers during the processing of the thermoplastic starch matrix. The initial degradation temperature of the TPS / ucuuba biocomposite increased by about 5.7 ºC compared to TPS (301.3 vs. 295.6 ºC), and an additional degradation stage was observed above 335 ºC, which includes both TPS carbonaceous products and degraded ucuuba lignocellulose. This increase in the initial degradation temperature of the TPS matrix is likely related to the synergistic interactions between the components of the processed system. A similar increase was observed when adding 3 wt.% of buriti pulp oil as a glycerol substitute (TPS / buriti pulp oil). However, the increase was more pronounced (around 11 ºC), demonstrating the effectiveness of buriti oil as a thermal stabilizer by raising the initial degradation temperature compared to TPS (Tonset (2) TPS = 295.6 ºC and Tonset (2) TPS / buriti oil = 306.8 ºC). This behavior was expected due to the thermal and photo-protective properties of buriti oil, which enhance the stability of the thermoplastic starch matrix. Regarding the biocomposites, no significant difference was found when using buriti oil as a plasticizer in combination with glycerol, as the increase of 1.7 ºC falls within the error range of the analysis equipment. This may be related to the lower degradation temperature of lignocellulosic fibers.
CONCLUSIONS This study addresses environmental issues related to waste management and plastic pollution by focusing on biopolymers and agricultural and industrial waste streams. The addition of buriti pulp oil to thermoplastic starch (TPS) served as an effective processing aid, reducing final torque and improving flow and mixture homogeneity. It also facilitated the breakdown of starch crystalline structures under shear and temperature. Mechanically, replacing glycerol with buriti pulp oil marginally increased strength but decreased elongation at break, possibly due to the fact that water content of glycerol reduces plasticizer availability. The oil also reduced the hydrophilicity of the TPS matrix, enhancing water resistance and reducing hydrolysis and biodegradation. SEM analysis showed that buriti pulp oil led to smoother and more homogeneous surfaces, indicating better starch plasticization. Rheological tests confirmed that buriti pulp oil acted as an external plasticizer, reducing flow resistance and enhancing matrix cohesion. Thermal stability improved with buriti pulp oil, as evidenced by a higher initial degradation temperature. This suggests that the thermal and photo-protective properties of the buriti oil enhance the stability of starch-based biocomposites. These findings demonstrate the benefits of buriti pulp oil in enhancing the processing and thermal properties of starch-based biocomposites, offering advantages over glycerol alone.
ACKNOWLEDGEMENTS The authors acknowledge Ingredion® Co. (Brazil) for providing Amidex 3001 and Mega Pack Plásticos Sociedade S. A. (Brazil) for supplying the buriti pulp oil. Additionally, thanks are extended to MCTI/SISNANO/INT-CENANO-CNPq for process No. 442604/2019, which supported the acquisition of the SEM images.
REFERENCES 1. Vilaplana, F.; Strömberg, E.; Karlsson, S.; Polym. Degrad. Stab. 2010, 95, 2147. [Crossref] 2. Asada, R.; Stern, R.; Ecological Economics 2018, 149, 120. [Crossref] 3. Oliveira, M. F. L.; Braga, F. C. F.; Leite, M. C. A.; Oliveira, M. G.; Macromol. Symp. 2016, 367, 42. [Crossref] 4. Ramírez, M. G. L.; Satyanarayana, K. G.; Iwakiri, S.; de Muniz, G. B.; de Tanobe, V.; Flores-Sahagun, T. S.; Carbohydr. Polym. 2011, 86, 1712. [Crossref] 5. Oliveira, M. F. L.; China, A. L.; Oliveira, M. G.; Leite, M. C. A.; Mater. Lett. 2015, 158, 25. [Crossref] 6. Müller, P.; Renner, K.; Móczó, J.; Fekete, E.; Pukánszky, B.; Carbohydr. Polym. 2014, 102, 821. [Crossref] 7. Parveen, N.; Naik, S. V. C. S.; Vanapalli, K. R.; Sharma, H. B.; Sci. Total Environ. 2024, 945, 173893. [Crossref] 8. Presidência da República Casa Civil; Lei No. 12.305, de 02 de agosto de 2010, Institui a Política Nacional de Resíduos Sólidos; Altera a Lei No. 9.605, de 12 de fevereiro de 1998; e dá Outras Providências; Diário Oficial da União (DOU), Brasília, seção 1, de 03/08/2010, p. 3. [Link] accessed in April 2025 9. Bruhn, N. C. P.; Viglioni, M. T. D.; Nunes, R. F.; Calegario, C. L. L.; J. Cleaner Prod. 2023, 421, 138503. [Crossref] 10. Lino, F. A. M.; Ismail, K. A. R.; Castañeda-Ayarza, J. A.; Energy Nexus 2023, 11, 100232. [Crossref] 11. European Union; Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009; On the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC; Official Journal of the European Union, 2009, L 140, p. 16-62. [Link] accessed in April 2025 12. Khalil, H. P. S. A.; Bhat, A. H.; Yusra, A. F. I.; Carbohydr. Polym. 2012, 87, 963. [Crossref] 13. de Corato, U.; de Bari, I.; Viola, E.; Pugliese, M.; Renewable Sustainable Energy Rev. 2018, 88, 326. [Crossref] 14. Campos, A.; Sena Neto, A. R.; Rodrigues, V. B.; Luchesi, B. R.; Mattoso, L. H. C.; Marconcini, J. M.; Ind. Crops Prod. 2018, 124, 149. [Crossref] 15. Saepoo, T.; Sarak, S.; Mayakun, J.; Eksomtramage, T.; Kaewtatip, K.; Carbohydr. Polym. 2023, 299, 120221. [Crossref] 16. Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R.; Carbohydr. Polym. 2017, 157, 1094. [Crossref] 17. Rosa, M. F.; Chiou, B.; Medeiros, E. S.; Wood, D. F.; Williams, T. G.; Mattoso, L. H. C.; Orts, W. J.; Iman, S. H.; Bioresour. Technol. 2009, 100, 5196. [Crossref] 18. Ma, X.; Yu, J.; Kennedy, J. F.; Carbohydr. Polym. 2005, 62, 19. [Crossref] 19. Wang, C. Z.; Li, F. Y.; Wang, L. M.; Li, J. F.; Guo, A. F.; Zhang, C. W.; Liu, P.; RSC Adv. 2015, 5, 49824. [Crossref] 20. Fourati, Y.; Magnin, A.; Putauxc, J. L.; Boufia, S.; Carbohydr. Polym. 2019, 229, 2020. [Crossref] 21. Araújo, D. J. C.; Machado, A. V.; Vilarinho, M. C. L. G.; Waste Biomass Valorization 2019, 10, 2863. [Crossref] 22. Jennings, T. C.; Starnes, W. H. In PVC Handbook; Wilkes, C. E.; Summers, J. W.; Daniels, C. A., eds.; Hanser Verlag: München, 2005, ch. 4. 23. Jia, P.; Xia, H.; Tang, K.; Zhou, Y.; Polymers 2018, 10, 1303. [Crossref] 24. Vieira, M. G. A.; da Silva, M. A.; dos Santos, L. O.; Beppu, M. M.; Eur. Polym. J. 2011, 47, 254. [Crossref] 25. Barbosa, O. L.; Oliveira, M. F. L.; Braga, F. C. F.; Monteiro, N. M.; Oliveira, M. G.; Veiga-Junior, V. F.; Polymers 2024, 16, 3037. [Crossref] 26. Nykänen, V. P. S.; Härkönen, O.; Nykänen, A.; Hiekkataipale, P.; Ruokolainen, J.; Ikkala, O.; Green Chem. 2014, 16, 4339. [Crossref] 27. Xia, Y.; Larock, R. C.; Green Chem. 2010, 12, 1893. [Crossref] 28. Pardauil, J. J. R.; Souza, L. K. C.; Molfetta, F. A.; Zamian, J. R.; Rocha Filho, G. N.; da Costa, C. E. F.; Bioresour. Technol. 2011, 102, 5873. [Crossref] 29. Leão, K. M. M.; Reis, L. V. C.; Speranza, P.; Rodrigues, A. P.; Ribeiro, A. P. B.; Macedo, J. A.; Macedo, G. A.; Biotechnol. Rep. 2019, 24, 365. [Crossref] 30. Franco, C. R.; do Nascimento Filho, W. B.; Rev. Virtual Quim. 2015, 7, 1968. [Crossref] 31. Teixeira, R. S.; Santos, S. F.; Christoforo, A. L.; Payá, J.; Savastano Junior, H.; Lahr, F. A. R.; Cem.Concr. Compos. 2019, 102, 134. [Crossref] 32. Sarasini, F.; Fiori, V.; J. Cleaner Prod. 2018, 195, 240. [Crossref] 33. Serra, J. L.; Rodrigues, A. M. C.; de Freitas, R. A.; Meirelles, A. J. A.; Darnet, S. H.; da Silva, L. H. M.; Food Res. Int. 2019, 116, 12. [Crossref] 34. Limas, J. D.; Silva, B. M. S.; Moraes, W. S.; Rev. Arvore 2007, 31, 37. [Crossref] 35. Deutsches Institut für Normung; DIN 53504-1:Testing of Rubber - Determination of Tensile Strength at Break, Tensile Stress at Yield, Elongation at Break and Stress Values in a Tensile Test, Berlin, 2017. [Link] accessed in April 2025 36. Chumsri, P.; Panpipat, W.; Cheong, L. Z.; Chaijan, M.; Foods 2022, 11, 2430. [Crossref] 37. Chang, F.; He, X.; Fu, X.; Huang, Q.; Jane, J. L.; J. Agric.Food Chem. 2014, 62, 7862. [Crossref] 38. Bohórquez-Ayala, M.; Rojano-Quiroz, D.; González-Cuello, R.; García-Zapateiro, L.; Ortega-Toro, R.; Rev. Mex. Ing. Quim. 2020, 20, 423. [Crossref] 39. Pang, A. L.; Ismail, H.; Bakar, A. A.; Iran. Polym. J. 2018, 27, 87. [Crossref] 40. Seggiani, M.; Cinelli, P.; Balestri, E.; Mallegni, N.; Stefanelli, E.; Rossi, A.; Lardicci, C.; Lazzeri, A.; Materials 2018, 11, 772. [Crossref] 41. Niazi, M. B. K.; Zijlstra, M.; Broekhuis, A. A.; Eur. Polym. J. 2013, 49, 1861. [Crossref] 42. Giuri, A.; Colella, S.; Listorti, A.; Rizzo, A.; Corcione, C. E.; J.Therm. Anal. Calorim. 2018, 134, 549. [Crossref] 43. Khadijah, H.; Azilah, A.; Azren, A.; Mohd, A.; Nurul, A.; J. Res. Updates Polym. Sci. 2024, 13, 1. [Crossref] 44. da Costa, D. S.; Takeuchi, K. P.; da Silva, R. M.; de Oliveira Filho, J. G.; Bertolo, M. R. V.; Belisário, C. M.; Egea, M. B.; Plácido, G. R.; Polysaccharides 2022, 3, 121. [Crossref] 45. de Oliveira Filho, J. G.; Bertolo, M. R. V.; Fernandes, S. S.; Lemes, A. C.; Silva, G. C.; Bogusz Junior, S. B.; de Azeredo, H. M. C.; Mattoso, L. H. C.; Egea, M. B.; J. Food Chem. 2024, 434, 137454. [Crossref] 46. Braga, F. C. F.; Furtado, C. R. G.; Oliveira, M. G.; Macromol. Symp. 2014, 343, 70. [Crossref] 47. Aichholzer, W.; Fritz, H. G.; Starch/Staerke 1998, 50, 77. [Crossref] 48. López, O. V.; Ninago, M. D.; Lencina, M. M. S.; García, M. A.; Andreucetti, N. A.; Ciolino, A. E.; Villar, M. V.; Carbohydr. Polym.
Associate Editor handled this article: Marcela M. Oliveira |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access