Artigo
| Hirtinol A: a new limonoid from the seeds of Trichilia hirta Linneaus (Meliaceae) |
|
Renata Rodrigues da Silva RobainaI,* I. Laboratório de Ciências Químicas, Universidade Estadual do Norte Fluminense Darcy Ribeiro, 28013-602 Campos dos Goytacazes - RJ, Brasil Received: 01/30/2025 *e-mail: renatarobainadasilva@gmail.com Species of the genus Trichilia (Meliaceae) produce a significant variety of secondary metabolites. Limonoids, the largest part of these compounds, have attracted considerable attention from the scientific community, due to their structural diversity and biological activity. One species of this genus, Trichilia hirta Linneaus, has been the subject of several studies, highlighting its potential as a source of bioactive molecules, due to its varied biological activities, including insecticidal, anticancer, and antifungal properties. This suggests that T. hirta is a promising source of bioactive compounds. In the pursuit of isolating and identifying limonoids with bioactive potential, the T. hirta species was selected for a phytochemical investigation, using different liquid chromatography methods for the separation and purification of compounds (column chromatography, medium-pressure liquid chromatography and high-performance liquid chromatography), together with spectroscopic techniques, such as nuclear magnetic resonance and mass spectrometry, for structural elucidation. This approach led to the isolation and identification of a new cedrelone-class limonoid, designated as Hirtinol A (1), in addition to a mixture of 21α-melianone (2) and 21β-melianone (3), and the glycoside schumaniofioside A (4), the first chromone identified in a Trichilia species. INTRODUCTION The genus Trichilia (Meliaceae) currently comprises 110 accepted species.1 The species in this genus are small to medium-sized trees, found in Tropical America, Africa, and the Indo-Malaysia region,2,3 that have a wide range of biological activities.4-8 The ethanolic extract of the branches and leaves of T. hirta exhibited moderate activity against third-instar Aedes aegypti larvae,9 and the extract of the fruits of this species was active against Candida spp.10 A polysaccharide-rich fraction obtained from the aqueous extract of T. hirta leaves displayed selective antiproliferative activity against human cancer cells,11 like the aqueous extract of the roots.12 Studies also demonstrated that the limonoid hirtin, a compound isolated from T. hirta, exhibits insecticidal activity against Peridroma saucia Hübne.13 Its seeds, rich in oils, are used as hair cream by women in southern Mexico and Guatemala and have an anti-lice effect.3 Different classes of secondary metabolites are produced by Trichilia species, such as monoterpenes, sesquiterpenes, diterpenes, triterpenes, steroids, coumarins, flavonoids, phenolic acids, amino acids, and lactones, however, limonoids are the most abundant of these.14 Limonoids are highly oxidized triterpenes, commonly defined as triterpene derivatives with a side chain converted into a furan ring by losing four carbon atoms.15 Given their structural diversity and wide range of biological activities, these compounds have attracted the interest of the scientific community.16,17 According to Passos et al.,18 227 different limonoids were identified in different Trichilia species between 1996 and 2020, of which 44.5% have a rearranged skeleton, 35.2% have intact rings, and 20.3% are of the seco-ring type. Among limonoids, protolimonoids, triterpenes, sesquiterpenes, steroids, and esters, 23 compounds have currently been isolated from the fruit, bark, wood, and leaves of T. hirta.19-23 Aiming to isolate and identify new compounds with potential biological activities, the T. hirta species was selected for phytochemical study. This research resulted in the isolation and identification of a new limonoid (1), as well as previously known compounds (2-4).
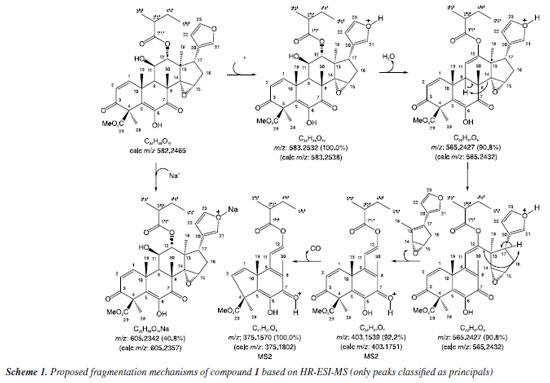
EXPERIMENTAL Plant material The seeds of T. hirta, identified by Domingos A. Folli, were collected at the Linhares-ES, Brazil, in June 2023 (SISGEN: AC8E4F3). The voucher specimen, code CVRD 12022, is in the Vale Natural Reserve. Extraction and isolation T. hirta seeds were dried at room temperature until a constant weight was reached. The seeds were powdered (6.28 kg), extracted at room temperature by maceration in hexane (3 extractions of 7 days each), and methanol (3 extractions of 7 days each), and evaporated at reduced pressure to obtain the hexanic (535.0 g) and methanolic (1.925 kg) extracts. A 104.0 g portion of the methanolic extract was fractionated using column chromatography (CC) with a dichloromethane/methanol gradient (1:0 to 0:1) and silica gel, yielding 13 fractions (THSM 1-THSM 13). The fraction THSM 3 (43.3792 g) was subjected to CC using silica gel and hexane/ethyl acetate gradient (1:0 to 0:1), yielding 12 new fractions (THSM 3.1-THSM 3.12). 1.200 g of the THSM 3.11 fraction (6.1246 g) was subjected to medium-pressure liquid chromatography (MPLC) using silica gel C-18 as the stationary phase and methanol/water gradient as the mobile phase (3:7 to 1:0 in 70 min and 1:0 for 20 min) at a flow rate of 15 mL min-1 with monitoring at 220, 254, and 365 nm. The beginning, middle, and end of each observed peak were collected separately, yielding 14 fractions (THSM 3.11.1-THSM 3.11.14). The THSM 3.11.4 fraction (41.6 mg) was purified by high-performance liquid chromatography (HPLC) using a C-18 column and methanol/water gradient (6:4 to 1:0 in 20.00 min, 1:0 for 7.00 min, 1:0 to 6:4 in 2 min, 6:4 for 10.00 min), with a flow rate of 3 mL min-1, on a semi-preparative scale, which resulted in the isolation of the limonoid Hirtinol A (1) (10.2 mg). The fraction THSM 12 (2.4943 g) was subjected to CC using silica gel and dichloromethane/methanol gradient 1:0 to 0:1 (THSM 12.1-THSM 12.9), leading to the isolation of compound 4 in the fraction THSM 12.9 (414.5 mg). A crystalline precipitate formed in the hexanic extract (12.1832 g) was separated and purified by CC with silica gel and hexane/ethyl acetate in gradient (1:0 to 0:1) yielding 8 fractions (THSH A.1-THSH A.8). Compounds 2 and 3 were identified in fraction THSH A.6 (2.5848 g). General experimental procedures Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker ASCEND 500 spectrometer (500 MHz for 1H and 125 MHz for 13C), using CDCl3 as the solvent. High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) data were acquired with a Bruker Daltonics micrOTOF-Q II mass spectrometer, using the positive ion analysis mode. Column chromatography (CC) was carried out on silica gel (0.063-0.200 mm, Merck). The solvents used in the extraction and isolation steps were purchased from Synth (Diadema, Brazil), of analytical and HPLC grade. MPLC separation steps were carried out in BÜCHI C-620 equipment with a C-605 binary pump, C-660 automatic collector, and C-640 UV-Vis detector, utilizing a glass column 15/460-04432 and silica gel C-18. HPLC purification was carried out on a Kromasil100A semi-preparative C-18 column (4.6 × 250 mm, 5 μm) using Shimadzu LC-20AD equipment, with a 500 μL loop and SPD-M20A UV-Vis detector. Infrared spectra were recorded with a Shimadzu FT-IR 8300 spectrophotometer. Spectroscopic data Hirtinol A (1) Yellow amorphous solid; IR (KBr, disc) νmax / cm−1 3417, 2949, 2879, 1734, 1787, 1633, 1504, 1458, 1381, 1350, 1234, 1186, 1157, 1122, 1074, 1035, 977, 923, 871, 846, 790, 731, 684, 650, 601, 576, 540, 514, 493; 1H NMR (500 MHz, CDCl3) d 6.89 (d, J 10.2 Hz, H-1), 6.12 (d, J 10.2 Hz, H-2), 2.70 (m, H-9), 4.18 (t, J 5.6 Hz, H-11), 5.12 (sl, H-12), 3.99 (sl, H-15), 2.34 (dd, J 13.7 and 6.7 Hz, Ha-16), 2.02 (dd, J 13.7 and 11.1 Hz, Hb-16), 2.94 (dd, J 11.7 and 6.6 Hz, H-17), 0.78 (s, 3H-18), 1.67 (s, 3H-19), 7.15 (m, H-21), 6.09 (m, H-22), 7.31 (m, H-23), 1.84 (s, 3H-28), 1.43 (s, 3H-30), 3.77 (s, 3H-Me), 2.29 (m, H-2'), 1.02 (d, J 6.8 Hz, 3H-3'), 1.62 (m, Ha-4'), 1.32 (m, Hb-4'), 0.84 (t, J 0.85, 3H-5'); 13C NMR (125 MHz, CDCl3) d 151.31 (C-1), 126.4 (C-2), 195.6 (C-3), 59.30 (C-4), 129.3 (C-5), 142.0 (C-6), 196.4 (C-7), 46.0 (C-8), 43.8 (C-9), 40.5 (C-10), 73.0 (C-11), 81.6 (C-12), 45.0 (C-13), 68.2 (C-14), 56.5 (C-1), 31.6 (C-16), 42.0 (C-17), 15.7 (C-18), 26.1 (C-19), 121.7 (C-20), 140.1 (C-21), 111.1 (C-22), 142.9 (C-23), 22.7 (C-28), 170.3 (C-29), 53.0 (Me), 175.8 (C-1'), 41.2 (C-2'), 16.5 (C-3'), 25.5 (C-4'), 11.6 (C-5'); (+)-HR-ESI-MS m/z 605.2342 [M + Na]+ (calcd. m/z 605.2357, Δm/z = 2.6 ppm), 583.2532 [M + H]+ (calcd. m/z 583.2538, Δm/z = 1.0 ppm), product ions: m/z 565.2427 (90.8%), 403.1539 (92.2%) and m/z 375.1570 (100.0%).
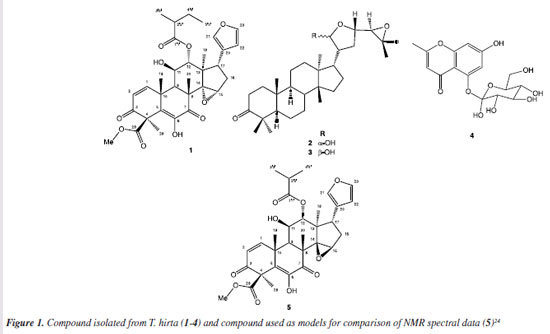
RESULTS AND DISCUSSION A new limonoid named Hirtinol A (1), a mixture of 21α-melianone (2) and 21β-melianone (3), and glycoside schumaniofioside A (4) (Figure 1), were isolated and identified from the hexanic and methanolic extracts of T. hirta seeds. Compound structures were established based on one- (1H and 13C) and two-dimensional NMR data (attached proton test (13C-APT), heteronuclear multiple bond correlation (HMBC), correlation spectroscopy (1H-1H-COSY) and nuclear Overhauser enhancement spectroscopy (1H-1H-NOESY)), HR-ESI-MS, and comparisons with literature data.24-26
Compound 1 was isolated as a yellow amorphous solid. The IR spectrum (Figure S33) obtained for compound 1 displayed characteristic absorptions of hydroxyl groups (3217 cm-1), esters carbonyls (1834 and 1787 cm-1) and α,β-unsaturated carbonyls (1633 cm-1). The 13C-APT NMR spectrum reveals 32 signals corresponding to carbon atoms, of which, using the HSQC (heteronuclear single quantum coherence) correlation map (Figure 14S, Supplementary Material), 12 were identified as non-hydrogenated [(C)12: 5 sp3, one oxygenated at dC 68.2 ppm (C-14), and 7 sp2 corresponding to 2 olefins at dC 129.3 (C-5) and 121.7 (C-20) ppm, 1 oxygenated olefin at dC 142.0 ppm (C-6), 2 α,β-unsaturated carbonyls at dC 195.6 (C-3) and 196.4 (C-7) ppm, and 2 ester carbonyls at dC 175.8 (C-1') and 170.3 (C-29) ppm], 11 as methynes [(C)11: 6 sp3, among which 3 are oxygenated with dC/dH at 73.0/4.18 (CH-11), 81.6/5.12 (CH-12) and 56.5/3.99 (CH-15) ppm, and 5 sp2 including 2 oxygenated at dC/dH 140.1/7.15 (CH-21) and 142.9/7.31 ppm], 2 as methylenes (C-16 and C-4'), and seven methyls, including one oxygenated at dC/dH 53.0/3.77 ppm. These data led to the deduction of the molecular formula (C)4(C-O)(C=C)(C-O)(C=O)4(CH)3(HC-O)3(CH)3)(HC-O)2(CH2)2(CH3)3 (O-CH3)=C32H36O12. After considering the presence of an epoxide ring [C-O-CH: dC 68.2 ppm (C-14), and dC/dH 56.5/3.99 ppm (C-15)], an ether [(HC-O)2=(HC-O-CH), [dC/dH 140.9/7.15 ppm (C-21) and 142.9/7.31 ppm (C-23)], and 2 hydroxyl groups [dC/dH 73.0/4.18 ppm (C-11) and dC 142.0 ppm (C-6)] it is possible to propose the molecular formula C32H38O10, corroborated by the HR-ESI-MS spectrum (Figure 3S, Supplementary Material) which shows the peak corresponding to the molecular formula C32H39O10+ ([M + H]+) at m/z 583.2532 (calcd. m/z 583.2538, Δm/z = 1.0 ppm) (Scheme 1).
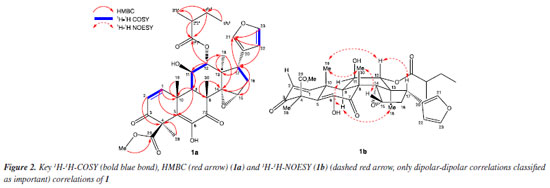
Comparisons to literature data24 and results observed in HMBC (Figure 2; Table S1, Supplementary Material) allowed the identification of the compound as a limonoid with A, B, C, and D rings intact. The molecular formula C32H38O10 indicates a degree of unsaturation equal to 14, compatible with 4 carbonyls, 4 double bonds, and 6 rings. The signals observed in the 13C-APT and 1H spectra at dC/dH 121.7/- (C-20), 140.1/7.15 (C-21), 111.1/6.09 (C-22), and 142.9/7.31 ppm, and the long-distance correlations (3JHC) of H-23 and H-22 with C-21 (Figure 2) allow the identification of a furan ring.
The presence of two doublets in the 1H NMR spectrum, at dH 6.89 ppm, (H-1), and dH 6.12 ppm (H-2), both with J 10.2 Hz, in addition to the characteristic signals of olefinic carbons at dC 151.3 and 126.4 ppm, referring to C-1 and C-2, and the carbonyl signal at dC 195.6 (C-3), associated with the long-distance correlations (2JHC and 3JHC) of H-2, H-3, and 3H-28 with C-3 (Figure 2) confirm the presence of an α,β-unsaturated carbonyl in ring A. The methyl ester at position 29 is characterized by the presence of the signals at dC/dH 170.3/- ppm (C-29) and 53.0/3.77 ppm (MeO) and by the 3JCH correlation of the MeO hydrogens with C-29 (Figure 2). The observed signals in 1H and 13C-APT NMR spectra at dC/dH 81.6/5.12 ppm (CH-12) and dC 196.4 ppm (C-1') together with the correlations of H-12, 2H-4' (2JHC), and H-2' (3JHC) with C-1', 3H-3' (3JHC) and 3H-5' (2JHC) with C-4', and H-2' (2JHC) with C-3' (Figure 2) suggest the presence of a 2-methyl butyl ester at C-12. The diosphenol group (α,β unsaturated keto-enol) in ring B, characteristic of cedrelone class limonoids,27 can be identified by the signals in the 13C-APT NMR spectrum with chemical shifts at dC 129.3 ppm, attributed to the olefinic carbon C-5, dC of 142.0 ppm, referring to the oxygenated olefinic carbon C-6 and dC 196.4 ppm, attributed to the carbonyl in C-7, as well as the long-distance correlations (2JHC and 3JHC) of H-9 and H-1 with C-5. The 1H-1H-NOESY spectrum, together with literature data24 enabled the relative configuration of limonoid 1 to be established. The dipolar interactions of H-17 (dH 2.94, dd, J 11.0, 6.6 Hz) and 3H-19 (dH 1.67, s) with H-12 (dH 5.12, sl), H-15 (dH 3.99, sl) and 3H-19 (dH 1.67, s) with 3H-30 (dH 1.43, s), and of 3H-18 (dH 0.78, s) and H-11 (dH 4.18, t, J 5.6 Hz) with H-9 (dH 2.70, sl) indicate the epoxide ring in C-14/C-15, the ester group in C-12 and the furan ring in the α position, and the OH group in C-11 in the β position (Figure 2).
CONCLUSIONS The phytochemical study of T. hirta seeds, employing different liquid chromatography techniques, resulted in the isolation of a new cedrelone-type limonoid, named Hirtinol A (1). A mixture of 21α-melianone (2) and 21β-melianone (3), and the glycosylated chromone, glycoside schumaniofioside A (4) were also isolated and identified. This paper is the first report of a chromone identified in a Trichilia species. Limonoids, structured similar to compound 1, presenting the hydroxyl group linked to C11, showed significant activities against Lepdoptera species,24 indicating a possible potential of the new limonoid as an insecticide.
SUPPLEMENTARY MATERIAL One- (1H and 13C) and two-dimensional (HSQC, HMBC, 1H-1H-COSY, and 1H-1H-NOESY) NMR data, and HR-ESI-MS data are available on http://quimicanova.sbq.org.br, in the form of a PDF file, with free access.
DATA AVAILABILITY STATEMENT All data are available in the text.
ACKNOWLEDGMENTS The authors are grateful to the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), Universidade Federal Rural do Rio de Janeiro (UFRRJ), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - finance code 001.
REFERENCES 1. Trichilia P. Browne, https://wfoplantlist.org/taxon/wfo-4000038940-2024-12?page=1, accessed in May 2025. 2. Pennington, T. D.; Styles, B. T.; Blumea 1975, 22, 419. [Link] accessed in May 2025 3. Pennington, T. D.; Styles, B. T.; Taylor, D. A. H.; A Monograph of Neotropical Meliaceae; The New York Botanical Garden Press: New York, 1981. 4. Passos, M. S.; Nogueira, T. S. R.; Robaina, R. R. S.; Calixto, S. D.; Simão, T. L. B. V.; Muzitano, M. F.; Lassounskaia, E.; Braz-Filho, R.; Vieira, I. J. C.; Phytochemistry 2023, 214, 113818. [Crossref] 5. Gomes, A. F.; Moreira, B. O.; Yatsuda, R.; de Souza, É. P.; David, J. M.; Alves, C. Q.; Macedo, G. E. L.; Correia, S. J.; de Paula, V. F.; Nat. Prod. Res. 2021, 35, 4789. [Crossref] 6. Ji, K.-L.; Cao, D.-H.; Li, X.-F.; Guo, J.; Zhang, P.; Xu, Y.-K.; Phytochem. Lett. 2015, 14, 234. [Crossref] 7. Liu, S.-B.; Mei, W.-L.; Chen, H.-Q.; Wang, J.; Wang, Z.-N.; Dai, H.-F.; Arch. Pharm. Res. 2018, 41, 1170. [Crossref] 8. Armelle, T. T.; Pamela, N. K.; Pierre, M.; Müller, I. B.; Marat, K.; Sass, G.; Ephrem, N. A.; Med. Chem. 2016, 12, 655. [Crossref] 9. Díaz Castillo, C. F.; Morelos Cardona, S. M.; Carrascal Medina, M.; Pájaro González, Y.; Gómez Estrada, H.; Rev. Cubana Plant. Med. 2012, 17, 256. [Link] accessed in May 2025 10. Sierra, J. D.; Contreras, O. I.; Angulo, A. A.; Indian J. Nat. Prod. Resour. 2022, 13, 170. [Crossref] 11. Hernández, E.; Gonzáles, B.; Díaz, A.; Gonzáles, M.; Morris, H. J.; Delgado, L.; Martínez, C. E.; Bol. Latinoam. Caribe Plant. Med. Aromat. 2013, 12, 176. [Link] accessed in May 2025 12. Soza, E. H.; Duharte, A. B.; Portuondo, D.; Ortega, V. T.; González, N. M.; Quevedo, H. J. M.; Manrique, C. E. M. M.; Bol. Latinoam. Caribe Plant. Med. Aromat. 2010, 9, 457. [Link] accessed in May 2025 13. Xie, Y. S.; Isman, M. B.; Gunning, P.; MacKinnon, S.; Arnason, J. T.; Taylor, D. R.; Sanchez, P.; Hasbun, I. C.; Towers, G. H. N.; Biochem. Syst. Ecol. 1994, 22, 129. [Crossref] 14. Vieira, I. J. C.; Terra, W. S.; Gonçalves, M. S.; Braz-Filho, R.; Am. J. Anal. Chem. 2014, 5, 91. [Crossref] 15. Taylor, D. A. H. In Progress in the Chemistry of Organic Natural Products; Herz, W.; Grisebach, H.; Kirby, G. W., orgs.; Springer Vienna: Vienna, 1984, ch. 1. 16. Waterman, P. G.; Phytochemical Potential of Tropical Plants; Springer Science+Business Media: New York, 1993. 17. Fang, X.; Di, Y. T.; Hao, X. J.; Curr. Org. Chem. 2011, 15, 1363. [Crossref] 18. Passos, M. S.; Nogueira, T. S. R.; Azevedo, O. A.; Vieira, M. G. C.; Terra, W. S.; Braz-Filho, R.; Vieira, I. J. C.; Phytochem. Rev. 2021, 20, 1055. [Crossref] 19. Cortez, D. A. G.; Vieira, P. C.; Fernandes, J. B.; da Silva, G. F. G. F.; Ferreira, A. G.; Phytochemistry 1992, 31, 625. [Crossref] 20. Chan, W. R.; Taylor, D. R.; Chem. Commun. (London) 1996, 206. [Crossref] 21. Chauret, D. C.; Durst, T.; Arnason, J. T.; Sanchez-Vindas, P.; San Roman, L.; Poveda, L.; Keifer, P. A.; Tetrahedron Lett. 1996, 37, 7875. [Crossref] 22. Vieira, I. J. C.; Azevedo, O. A.; De Souza, J. J.; Braz-Filho, R.; Gonçalves, M. S.; De Araújo, M. F.; Molecules 2013, 18, 2589. [Crossref] 23. Vieira, M. G. C.; Braz-Filho, R.; Vieira, I. J. C.; Nat. Prod. Commun. 2019, 14, 1. [Crossref] 24. Simmonds, M. S. J.; Stevenson, P. C.; Porter, E. A.; Veitch, N. C.; J. Nat. Prod. 2001, 64, 1117. [Crossref] 25. Xu, G.-H.; Kim, J.-A.; Kim, S.-Y.; Ryu, J.-C.; Kim, Y.-S.; Jung, S.-H.; Kim, M.-K.; Lee, S.-H.; Chem. Pharm. Bull. 2008, 56, 839. [Crossref] 26. Kumar, V.; Gupta, M.; Gandhi, S. G.; Bharate, S. S.; Kumar, A.; Vishwakarma, R. A.; Bharate, S. B.; Tetrahedron Lett. 2017, 58, 3974. [Crossref] 27. Tan, Q.-G.; Luo, X.-D.; Chem. Rev. 2011, 111, 7437. [Crossref]
Guest Editor handled this article: Antonio E. M. Crotti |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Química Nova
Publicações da Sociedade Brasileira de Química
Caixa Postal: 26037
05513-970 São Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access