Artigo
| Efficient extraction of 2'-Fucosyllactose from human milk: parameter optimization with surface response |
|
Elienae da Silva Gomes; Eloize Silva Alves; Patricia Daniele Silva Santos; Marcela de Souza Zangirolami; Oscar Oliveira Santos Laboratório de Química de Alimentos, Universidade Estadual de Maringá, Av. Colombo, 5790, 87020-900 Maringá-PR Brazil Received: 01/15/2025 *e-mail: oosjunior@uem.br 2'-Fucosyllactose (2'-FL) is the predominant oligosaccharide in human milk, responsible for important infant biological functions. Its quantification requires specific extraction and analysis methods, but current techniques use toxic solvents for extraction and are time-consuming. Therefore, this study optimized the extraction of 2'-FL from mature human milk, using water to eliminate the toxic solvents. Using response surface methodology, 27 experiments were evaluated by high-performance liquid chromatography coupled to mass spectrometry, with their concentrations calculated based on a standard curve. The optimal conditions included initial centrifugation time (15 min), water volume (3.0 mL), incubation time (90 min), and final centrifugation time (40 min), resulting in a maximum concentration of 3046 mg L-1. The method was validated in the other lactation phases, representing 1892.93 mg L-1 (transition) to 4247.33 mg L-1 (colostrum), showing high reproducibility and agreement with the literature. The proposed method was 50.43% faster and more economical than traditional methods, standing out as an efficient approach for the 2'-FL extraction. INTRODUCTION The human milk is composed of essential nutrients for neonatal development. Human milk contains proteins with antibacterial properties and immunological functions, and lipids responsible for the development of the brain and visual tissue.1 In addition, in particular bioactive compounds, contain the class of carbohydrates called oligosaccharides, which are versatile in digestion and perform several biological functions, also adding relevance to the nutritional profile of human milk.2-4 Thus, 2'-fucosyllactose (2'-FL) is one of the most abundant oligosaccharides in human milk and has attracted considerable interest due to its health benefits, especially in modulating the intestinal microbiota, with functions that promote adequate growth and reduce morbidity.5 The 2'-FL has been widely studied for its various physiological and biological activities. Studies5 have shown that its addition to infant formulas can provide benefits similar to those of breast milk, especially for babies who cannot be breastfed. Among its main advantages, its action as a prebiotic stands out, favoring the growth of beneficial bacteria, such as Bifidobacterium and Lactobacillus, which are essential for intestinal health. In addition, 2'-FL helps protect against gastrointestinal infections by acting as a receptor for pathogens, preventing their adhesion to the intestinal mucosa. Studies also indicate that the presence of oligosaccharides in human milk can contribute to cognitive development and reduce the incidence of respiratory diseases in babies who consume formulas enriched with this substance.6 In infant nutrition, commercially available fortified infant formulas added with 2'-FL are available, whose content has been shown to promote age-appropriate development and reduce morbidity rates and medication use, demonstrating its potential in infant nutrition.5 According to Soyyılmaz et al.,7 concentrations of oligosaccharides in human milk are influenced by the mother's lactation period corresponding to the colostrum (0-6 days), transition (7-14 days) and mature (> 15 days) stages, gestational age, and ethnicity.5-7 Extractions of oligosaccharides, such as 2'-FL, use processes that can be influenced by the choice of solvent. Selecting the appropriate extraction solvent is essential for optimizing the recovery and purity of this compound.8 The water is a highly polar solvent, which makes it efficient in the extraction of hydrophilic compounds, such as oligosaccharides. It is widely used for the extraction of naturally occurring oligosaccharides, with the following advantages: ecological, easy to handle, and low cost. In contrast, ethanol has an intermediate polarity, which allows good extraction of compounds with moderate solubility in water, such as some oligosaccharides. It has a good efficiency in the precipitation and purification of oligosaccharides, such as 2'-FL, since ethanol can precipitate other impurities, such as proteins, while keeping 2'-FL in solution.9 It has a higher value than water and requires care in handling, given its flammability degree. Methanol is a more polar solvent than ethanol and less polar than water, which makes it interesting for the extraction of specific oligosaccharides. It is highly toxic, making it difficult to use in the food and pharmaceutical industries. Furthermore, as it is more toxic, it requires complete and rigorous removal of the final product.10 The water extraction method used not only ensures efficiency but also helps to preserve the functional properties of 2'-FL, since the main goal is to maintain the probiotic properties and immunological benefits, thus being a fundamental solvent to guarantee these characteristics. Its biggest advantage is that 2'-FL can be extracted efficiently and at a lower cost.8 However, these methods commonly use organic solvents for extraction, which involve lengthy steps and often do not evaluate the multivariate effects during their optimization.2,11 Thus, chemometric methods based on response surface methodology (RSM) are primarily aimed at modeling and optimizing processes by analyzing both the individual effects and interactions between system variables, allowing for a more comprehensive and accurate understanding of process behavior. Unlike traditional optimization methods, which evaluate one factor at a time while keeping others constant, RSM enables the simultaneous optimization of multiple variables, reducing the number of experiments required and making the process more efficient, cost-effective, and faster. Additionally, this approach allows for the development of predictive mathematical models that provide a detailed analysis of individual effects and interactions between variables, resulting in better process optimization. Another important advantage of RSM is its applicability across various fields, such as chemical, biotechnological, aerospace, automotive, and food industries. Finally, the technique facilitates the graphical visualization of interactions between studied factors through response curves, simplifying data interpretation and enabling more assertive decision-making.12-15 In this context, this study aimed to optimize the extraction of 2'-fucosyllactose (2'-FL) from human milk, using a central composite design (CCD) combined with the response surface methodology (RSM). The proposed approach aimed to determine the ideal conditions of time and solvent volume to maximize the extraction of the compound, using exclusively water as the extraction medium. Thus, the study seeks an alternative to eliminate the use of toxic solvents, reduce process time and costs, and ensure high efficiency in the extraction of 2'-FL.
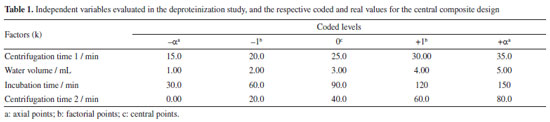
EXPERIMENTAL Reagents The compound 2'-fucosyllactose (2'-FL) with a high purity level (≥ 95%) used as the analytical standard was purchased from Sigma-Aldrich (USA). The mobile phase solvents included acetonitrile (high-performance liquid chromatography (HPLC) grade, J. T. Baker, Mexico), ammonium acetate (98%, J.T. Baker, Mexico), and ultrapure water Milli-Q generated by a Millipore Type I water system (Merck, Germany). Sampling The present study has been authorized by the local Research Ethics Committee, under certificate of presentation of ethical appreciation No. 93048818.0.0000.0104/2018, from the State University of Maringá (UEM, Maringá, Brazil). Samples of 600 mL of each lactation phase of human milk were collected from the human milk bank of the Regional University Hospital of Maringá, from individual donations of 9 different mothers, randomly recruited, considering colostrum (0 to 6 days postpartum), transitional (7 to 14 days postpartum) and mature (more than 15 days postpartum) human milk. The samples were homogenized in individual pools and stored in appropriate glass containers. The present study followed a specific protocol for collection and temperature defined by the human milk bank.16 For the experimental design of the extraction of the oligosaccharide 2'-FL, mature human milk was used, and for testing the applicability of the method, colostrum and transition human milk were used. Experimental design CCD is an experimental design approach that evaluates the influence and interaction of variables within a system. RSM is often used to optimize processes and understand how variables affect a specific response. To achieve greater extraction of 2'-FL, factors such as centrifugation time (min) (Table 1) at 6400 relative centrifugal force (RCF) × g were varied, following Huang et al.11 Additionally, the volume of water (mL) required for proper sample cleaning, as per Nijman et al.,17 and the incubation time (min) necessary for protein precipitation under low temperatures (6 ºC) were also considered.17,18 The experimental design was then employed using the Design Expert software.19 The axial points (±α) for the rotatable system (with a constant k < 5) were ±1.4142, used for calculating the quadratic terms, Table 1. The authors emphasize that a fixed volume of 5 mL of sample was used for each extraction.
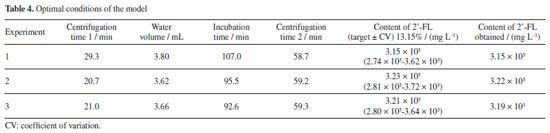
The extraction process was conducted according to the conditions established in the experimental design, with centrifugation performed using a refrigerated centrifuge model Harrier 18/80 (Sanyo MSE, UK). The aqueous phase supernatant was filtered through a polytetrafluoroethylene (PTFE) membrane using a 0.25 μm syringe filter (Filtrilo, Brazil). The concentration of the selected oligosaccharide was determined using liquid chromatography. Chromatographic conditions The experimental extractions were injected into a chromatographic system (UHPLC, Acquity Waters, Milford, USA) coupled to a triple quadrupole mass spectrometer equipped with electrospray ionization source (ESI-MS/MS, Xevo-TQ-XS, Waters, Milford, USA). The ACQUITY BEH Amide column (Waters 130 Å, 1.7 μm, 2.1 × 150 mm) was selected for evaluating the separation. Mobile phase A was acetonitrile, and mobile phase B was 50 mM ammonium acetate in water (H2O). The compound was separated by isocratic elution at 80/20 (v v-1) over total time of 3.00 min to establish column system equilibration. The total flow rate was set at 0.15 mL min-1 with a column oven temperature of 40 ºC, and the injection volume was 2 μL.20 The ESI-MS was operated in negative ion mode (ESI-). The remaining operating conditions were as follows: capillary voltage of 2 kV with desolvation temperature set to 500 ºC, cone voltage of 50 V, collision energy of 8 eV, and source temperature of 120 ºC. The nebulizing gas was generated by a nitrogen generator (Peak Scientific®, model NM32LA, Scotland), with cone gas and desolvation gas flows set at 50 and 800 L h-1, respectively. The gas pressure was programmed to 7.0 bar, using nitrogen gas. The induced dissociation was performed using multiple reaction monitoring (MRM) for both compound identification and quantification. Argon gas with a purity of 99.999% (White Martins, Brazil) was used as the collision gas at a pressure of 15,000 psi. Signals were recorded using MassLynx™ software version 4.1 (Waters, Milford, USA) after precursor ion fragmentation, which was automatically defined based on its intensity. Calibration curve The calibration curve was constructed using the high purity compound 2'-FL (≥ 95%, Sigma-Aldrich, USA) as a reference standard in the concentration range of 0.5-80 µg mL-1, with seven concentration points prepared using an acetonitrile:water solution (80:20 v v-1). The results obtained from triplicate analyses yielded a linear regression using the concentration range versus the area of the identified chromatographic peak, with a retention time 1.68 min. A correlation coefficient equal to R2 = 0.9987 and equation of the line equals y = 654.97x - 1269.9 were determined to calculate the equivalent endogenous concentration of 2'-FL in human milk in mg L-1 (Figure 1).
To quantify the 2'-FL content, the volume of dilution with water used in each experiment was considered. Statistical analysis The regression model equations, statistical significance (p < 0.05), lack of fit, and regression coefficients were assessed through analysis of variance (ANOVA) and multiple regression techniques using Desing Expert software.19
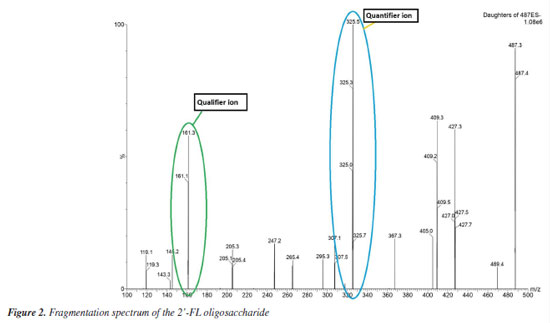
RESULTS AND DISCUSSION The optimal ion abundance values for the precursor-product ion were acquired at m/z 487 in negative mode. Under these ionization conditions, fragments of the analyte of interest were obtained. The quantifier ion with the highest ratio was at m/z 325, and the qualifier ion at m/z 161, Figure 2.
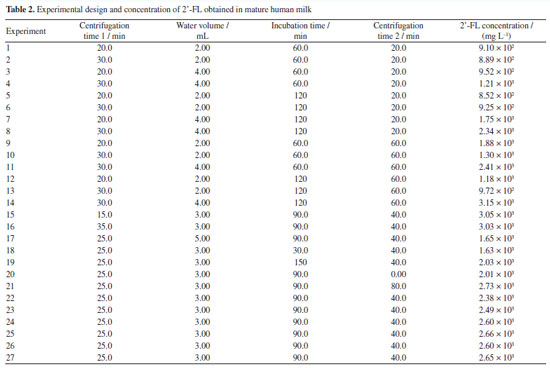
Experimental design and model optimization The results of the concentrations obtained from the extractions for each experiment of the experimental design are presented in Table 2.
Additionally, cone voltage and collision energy values of 30 and 8 V, respectively, were obtained. After optimizing the best conditions for ionization and identification of the analyte and its fragments, a standard of 1 µg mL-1 was injected, resulting in the spectrum at a retention time 1.68 min, Figure 3.
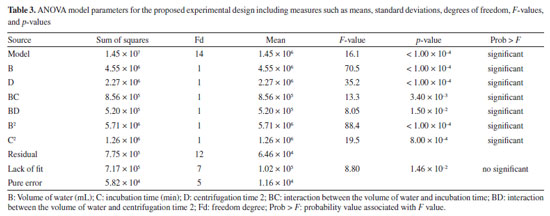
The optimization of the extraction conditions for the selected oligosaccharide concentration was conducted by assessing the effect of four independent variables: centrifugation time 1, water volume, incubation time, and centrifugation time 2. For this purpose, an experimental design using a central composite design was generated to obtain the optimal conditions. Experiment 15 proved to be highly significant due to its shorter centrifugation and incubation times, which are essential for saving time for both the analyst and the equipment. The experimental conditions were as follows: centrifugation time 1 - 15 min, water volume - 3.0 mL, incubation time - 90 min, and centrifugation time 2 - 40 min, resulting in a concentration of 3046 mg L-1 for the mature lactation phase. The use of analysis of variance (ANOVA) and response surface generated by Design Expert software19 demonstrated the model interaction among factors in the experiment, Table 3.
Among the models generated, the quadratic model was selected as it provides a more comprehensive and accurate representation of the relationships between the factors and the response, considering the nonlinear and interactive nature of the processes under study. The F-value of 16.05 indicates that the model is significant. There is only a 0.01% chance that an F-value of this size could occur due to noise. “Prob > F" values less than 0.05 indicate that the model terms are significant, while values greater than 0.05 indicate that the model terms are not significant. In this case, terms B, D, BC, BD, B2, and C2 are significant model terms. Values above 0.1000 indicate that the model terms are not significant. The lack of fit F-value of 8.80 suggests that the lack of fit is not significant. There is only a 1.46% chance that a lack of fit F-value could occur due to noise. The mathematical equation obtained for the concentration of 2'-FL in human milk is shown in Equation 1. Additionally, the coefficient of determination (R2 = 0.9493) and the variation (13.15%) indicate that the model is acceptable. X = 2561.50 + 588.45B + 350.01D + 256.67BC + 216.40BD - 597.46B2 - 220.55C2 (1)
where: X = concentration of 2'-FL; B = water volume; D = centrifugation time 2; BC = interaction between the volume of water and incubation time; BD = interaction between the volume of water and 2nd centrifugation time. Figure 4 presents an analysis of the accuracy or validity of the proposed model compared to experimental data, showing a depiction of the proximity values relative to the predicted values, which is crucial for evaluating the accuracy and reliability of the proposed model.
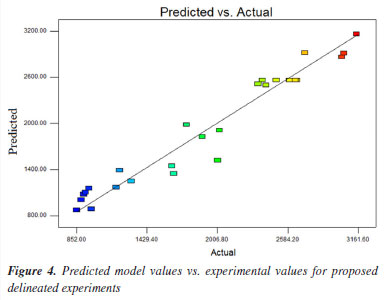
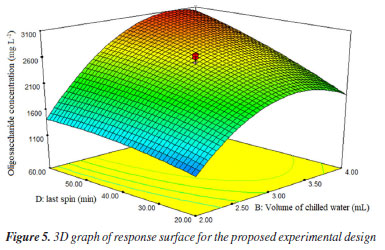
The predicted values are very close to the obtained values. This indicates that the points are aligned with the line, suggesting a good fit for the model. Thus, Figure 5 displays the response surface obtained during the experimental design, showing that reducing the addition of water volume and centrifugation time leads to a decrease in oligosaccharide extraction in human milk. As centrifugation time increases (40 min), there is greater efficiency in defatting the sample and precipitating protein, indicating a higher extraction potential, Figure 5.
The results obtained under the optimal conditions proposed by the experimental design are presented in Table 4.
All values obtained in the optimization of the experimental design are within the proposed range, as shown in Table 4, considering the suggested value for the concentration of 2'-FL in human milk and the coefficient of variation obtained in this design. Additionally, the chromatographic analysis proved adequate, achieving a 99% recovery of the desired 2'-FL content.5 Thus, the model demonstrates efficacy in the extraction for quantification of 2'-FL. Application of 2'-FL extraction To investigate the applicability of 2'-FL extraction under optimized conditions, human milk samples from colostrum and transitional phases of lactation were analyzed. The experimental conditions applied to these samples followed those indicated in experiment 15 (Table 2). This extraction method resulted in 2'-FL concentrations of 4247.33 mg L-1 (colostrum) and 1892.93 mg L-1 (transition). The values found corroborate the range of concentrations reported by Christensen et al.,5 which indicates a variation from 760 to 4780 mg L-1. According to Gan et al.,21 Soyyılmaz et al.,7 and Thurl et al.,22 variations in the oligosaccharide content in human milk are attributed to factors such as the lactation phase and the extraction methods employed. Thus, the results obtained in the present study are consistent with the levels typically found in the literature, thus validating the optimized extraction method used. Therefore, the application of the optimized extraction method proved to be effective, resulting in high levels of 2'-FL, especially in colostrum. Previously, an extraction process of human milk oligosaccharides was described by Erney et al.,23 involving gel filtration and high-efficiency anion exchange chromatography for interference filtration. During the evaluation of different continental regions and postpartum intervals (0 to 452 days), the results indicated a decrease in the content of 2'-FL over the days, from 2780 to 1900 mg L-1. However, oligosaccharide extraction using only water was performed resulting in anterior milk concentrations ranging from 100 to 4000 mg mL-1, and posterior milk concentrations ranging from 10 to 4500 mg mL-1, as described.24 In addition, studies25 involving human milk oligosaccharide extraction by lipid removal through sonication and centrifugation, followed by protein precipitation with ethanol, resulted in concentrations of 679 to 1300 mg L-1 in human milk from 0 to 181 days, as reported. So, factors such as interference removal and lactation stages demonstrate potential variables. Furthermore, according to Ren et al.,26 human milk oligosaccharide concentrations vary between lactation stage, maternal secretor gene status, Lewis blood type, volume of expressed breast milk, and maternal origin. So, previous studies show that methods for quantifying oligosaccharides typically involve chromatographic techniques that require extensive sample preparation procedures and long analysis periods. Regarding sample preparation, they employ matrix stabilization for up to 24 h, in addition to centrifugation times of up to 80 min.5,27 Similarly, the runtime for quantification by chromatographic analysis takes from 19 to 24 min to complete.5,28 However, in the present study, preparation was optimized, reducing the resting time to 90 min, which represents a reduction of 93.75%, and the total centrifugation time to 55 min, resulting in a reduction of 31.25%. Furthermore, the runtime for quantification by chromatography was 3.00 min. Thus, the proposed optimization significantly reduces the total extraction time and highlights a more efficient methodology. The use of gentler and safer methods during extraction ensures the integrity and quality of the oligosaccharide, as well as provides safety for the final product. Especially when it comes to foods intended for infants, such as human milk, it is crucial to ensure that there are no harmful chemical residues.21
CONCLUSIONS Under the optimization of experimental conditions, focusing on variables such as centrifugation time and specific water volumes, significant concentrations of 2'-FL were obtained in human milk from different lactation stages - mature, transitional, and colostrum - with contents of 3046, 1892.93, and 4247.33 mg L-1, respectively. Thus, this study successfully developed an efficient method for extracting 2'-FL from human milk, the results demonstrated that the proposed method is not only effective in extracting and quantifying but also preserves the integrity of the oligosaccharide, offering an economical and safe approach for infant nutrition. For future studies, characterization of the prebiotic integrity extracted by non-toxic solvents is recommended.
DATA AVAILABILITY STATEMENT Data will be made available on request.
ACKNOWLEDGMENTS The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Araucária, Programa Pesquisa Para o SUS (PPSUS), and Fundação Cargill for financial assistance.
AUTHOR CONTRIBUTIONS Elienae da Silva Gomes was responsible for the data curation, formal analysis, investigation, visualization, writing original draft, writing review and editing; Eloize S. Alves for the visualization, writing original draft, writing review and editing; Patrícia D. S. Santos for the data curation, formal analysis, Visualization, writing original draft; Marcela de Souza Zangirolami for the visualization, writing review and editing; Oscar O. Santos for the data curation, project administration, supervision, visualization.
REFERENCES 1. Chen, Y.; Sui, X.; Wang, Y.; Zhao, Z.; Han, T.; Liu, Y.; Zhang, J.; Zhou, P.; Yang, K.; Ye, Z.; Food Chem. 2024, 22, 101289. [Crossref] 2. Liu, Y.; Zhu, Y.; Wang, H.; Wan, L.; Zhang, W.; Mu, W.; J. Agric. Food Chem. 2022, 70, 11481. [Crossref] 3. Pressley, S. R.; McGill, A. S.; Luu, B.; Atsumi, S.; Curr. Opin. Food Sci. 2024, 57, 101154. [Crossref] 4. Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B.; Nutrients 2020, 12, 266. [Crossref] 5. Christensen, A. S.; Skov, S. H.; Lendal, S. E.; Hornshøj, B. H.; J. Food Sci. 2020, 85, 332. [Crossref] 6. Zhou, W.; Jiang, H.; Wang, L.; Liang, X.; Mao, X.; ACS Synth. Biol. 2021, 10, 447. [Crossref] 7. Soyyılmaz, B.; Mikš, M. H.; Röhrig, C. H.; Matwiejuk, M.; Meszaros ‐ Matwiejuk, A.; Vigsnæs, L. K.; Nutrients 2021, 13, 2737. [Crossref] 8. Yan, J.; Ding, J.; Liang, X.; Anal. Methods 2017, 9, 1071. [Crossref] 9. Yu, S.; Liu, J.-J.; Yun, E. J.; Kwak, S.; Kim, K. H.; Jin, Y.-S.; Microb. Cell Fact. 2018, 17, 101. [Crossref] 10. Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M. J.; Linhardt, R. J.; Chem.Rev. 2015, 115, 6811. [Crossref] 11. Huang, X.; Zhu, B.; Jiang, T.; Yang, C.; Qiao, W.; Hou, J.; Han, Y.; Xiao, H.; Chen, L.; J. Agric. Food Chem. 2019, 67, 12237. [Crossref] 12. Minjares-Fuentes, R.; Femenia, A.; Garau, M. C.; Meza-Velázquez, J. A.; Simal, S.; Rosselló, C.; Carbohydr. Polym. 2014, 106, 179. [Crossref] 13. Baş, D.; Boyacı, I. H.; J. Food Eng. 2007, 78, 836. [Crossref] 14. Bezerra, M. A.; Santelli, R. E.; Oliveira, E. P.; Villar, L. S.; Escaleira, L. A.; Talanta 2008, 76, 965. [Crossref] 15. Szpisják-Gulyás, N.; Al-Tayawi, A. N.; Horváth, Z. H.; László, Z.; Kertész, S.; Hodúr, C.; Acta Aliment. 2023, 52, 521. [Crossref] 16. Agência Nacional de Vigilância Sanitária (ANVISA); Resolução da Diretoria Colegiada (RDC) No. 171. Regulamento Técnico para o Funcionamento de Bancos de Leite Humano; Ministério da Saúde, Brasil, 2006. [Link] accessed in June 2025 17. Nijman, R. M.; Liu, Y.; Bunyatratchata, A.; Smilowitz, J. T.; Stahl, B.; Barile, D.; J. Agric. Food Chem. 2018, 66, 6851. [Crossref] 18. Chiavelli, L. U. R.; Galuch, M. B.; Senes, C. E. R.; Maia, L. C.; Lopes, T. A. M.; Rufato, K. B.; Santos, O. O.; Visentainer, J. V.; Food Anal. Methods 2022, 15, 1418. [Crossref] 19. Design-Expert®, version 7.0; Stat-Ease, Inc., Minneapolis, MN, USA, 2007. 20. Remoroza, C. A.; Mak, T. D.; De Leoz, M. L. A.; Mirokhin, Y. A.; Stein, S. E.; Anal. Chem. 2018, 90, 8977. [Crossref] 21. Gan, J.; Cao, C.; Stahl, B.; Zhao, X.; Yan, J.; Trends Food Sci. Technol. 2023, 141, 104203. [Crossref] 22. Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B.; Nutr. Rev. 2017, 75, 920. [Crossref] 23. Erney, R. M.; Malone, W. T.; Skelding, M. B.; Marcon, A. A.; Kleman-Leyer, K. M.; O'Ryan, M. L.; Ruiz-Palacios, G.; Hilty, M. D.; Pickering, L. K.; Prieto, P. A.; J. Pediatr. Gastroenterol. Nutr. 2000, 30, 181. [Crossref] 24. Pu, Z.; Sun, Z.; Liu, J.; Zhang, J.; Cheng, M.; Zhang, L.; Zhou, P.; Food Bioeng. 2023, 2, 105. [Crossref] 25. Li, J.; Bi, Y.; Zheng, Y.; Cao, C.; Yu, L.; Yang, Z.; Chai, W.; Yan, J.; Lai, J.; Liang, X.; Food Chem. 2022, 397, 133750. [Crossref] 26. Ren, X.; Yan, J.; Bi, Y.; Shuttleworth, P. W.; Wang, Y.; Jiang, S.; Wang, J.; Duan, Y.; Lai, J.; Yang, Z.; Nutrients 2023, 15, 1408. [Crossref] 27. Ruhaak, L. R.; Lebrilla, C. B.; Adv. Nutr. 2012, 3, 406S. [Crossref] 28. Yao, Q.; Gao, Y.; Wang, F.; Delcenserie, V.; Wang, J.; Zheng, N.; Food Chem. 2024, 430, 136977. [Crossref]
Associate Editor handled this article: Eduardo M. Richter |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access