Artigo
| Organosolv pretreatment with commercial and crude glycerol together with FeCl3 of the corn straw and corn cob biomasses, both individually and mixed |
|
Joselaine C. Santana* Núcleo de Estudos em Sistemas Coloidais (NUESC), Instituto de Tecnologia e Pesquisa (ITP), Universidade Tiradentes (UNIT), 49032-490 Aracaju - SE, Brasil Received: 02/18/2025 *e-mail: joselainecs@gmail.com Organosolv pretreatment with glycerol was applied to the corn straw and corn cob biomasses individually and mixed. The reactions were carried out to improve the amount of FeCl3 catalyst, the use of crude glycerol, and eliminate the steps of washing and drying the pretreated biomass before it was subjected to enzymatic hydrolysis. The results indicated that the straw and cob behaved very similarly in the different pretreatment situations, with the condition using 0.0125 mol L-1 of FeCl3 being considered the best for both. It was evident that the FeCl3 catalyst reduces the recalcitrance of biomasses, acting in the removal of hemicellulose and lignin to the pretreatment liquor. The 50% cob + 50% straw mixture, pretreated according to the best condition, provided sugar release analogous to that of the biomasses individually. Excellent cellulose recovery was obtained (98.2%), in addition 99.2% of hemicellulose and 89.5% of lignin were removed using commercial glycerol with 0.0125 mol L-1 of FeCl3. From 1 kg of 50% cob + 50% straw in natura, 360.9 g of glucose was obtained in 24 h of hydrolysis. The application of crude glycerol was promising, and the pretreated biomass can be subjected directly to enzymatic hydrolysis, without washing and drying. INTRODUCTION Faced with the current scenario, which requires urgent solutions to environmental and climate issues, the production of bioethanol from lignocellulosic biomass, called cellulosic ethanol or second-generation ethanol, is being widely studied as it is a very promising alternative to fossil fuels.1-4 First-generation ethanol is a biofuel already widely used as a partial or total substitute for gasoline. It is highly relevant worldwide due to its affordable cost, lower polluting power, and neutral CO2 emissions.5-8 However, a problem associated with its production is the competition between food and fuel, since it is obtained from food sources, mainly sugarcane and corn.9,10 Therefore, obtaining bioethanol from lignocellulosic biomass has gained prominence because it is an alternative sugar source that is very abundant, renewable, low-cost, and not intended for food use.2,11 Thus, studies8,10,12-14 are assessing the energy potential of lignocellulosic materials, which are generally considered to be waste, such as sugarcane straw and bagasse, corn straw and cob, wood dust and scraps, among others. The global production of second-generation ethanol is still not very significant, with Brazil standing out as the main producer. To produce second-generation ethanol, an initial pretreatment stage is necessary to break down the complex lignocellulosic structure formed by the lignin-hemicellulose-cellulose polymers. This allows the hydrolysis stage to be carried out, which consists of breaking down cellulose and hemicellulose into their monomers, fermentable sugars. Finally, the monomeric sugars are fermented to produce bioethanol.12,15,16 Pretreatment is essential because it directly influences the yield of the other stages and is one of the challenges in obtaining cellulosic ethanol. Consequently, most research6,8,10,17-19 focuses on the search for more efficient, economical, and environmentally friendly pretreatment methods. One way to make pretreatment more economical and environmentally friendly is to reduce the use of water in the washing step of the pretreated biomass or eliminate this consumption by taking it unwashed and wet directly to the enzymatic hydrolysis step, which also reduces the energy expenditure related to drying the pretreated material.13,20 In addition, pretreatment can be made more sustainable and profitable by introducing the biorefining strategy.12,21-24 One type of pretreatment that has been investigated is organosolv, as it allows the fractionation and utilization of biomass components through the use of organic solvents.25-28 Generally, in organosolv pretreatment, most of the hemicellulose and lignin are removed by the solvent, leaving a solid residue rich in cellulose that is easily digestible in enzymatic hydrolysis.16 In addition, organosolv pretreatment minimizes the degradation of carbohydrates, with low formation of the inhibitors furfural and hydroxymethylfurfural (HMF).13 Glycerol, a non-toxic organic solvent, shows promise in organosolv pretreatment. It has sustainable aspects in the economic and environmental fields, which are reinforced by the possibility of using crude glycerol, the residue from biodiesel production. In addition, it has a high boiling point, which allows it to be operated at atmospheric pressure, making the process safer, without requiring the use of sophisticated equipment.16,20,29-31 Glycerol can also be used with the addition of catalysts, such as the metal salt FeCl3, to intensify fractionation.13,18 Corn straw and corn cob are waste products generated in large quantities from the processing of corn, an important agricultural product in Brazil and many parts of the world. In Brazil, corn production exceeds 100 million tons per year and it is estimated that straw and cobs represent, on average, 30% of the weight of the corn harvested. The use of these wastes for the production of cellulosic ethanol is justified by their high carbohydrate content, as well as providing an appropriate destination that takes advantage of their energy potential, given that they are often underutilized and disposed incorrectly.1,11,32-34 Furthermore, when corn is harvested for processing, a large amount of biomass from the corn stalk is obtained. The potential of this biomass for bioethanol prodution is also being investigated.35-37 The main objective of this work was to improve organosolv pretreatment with glycerol and FeCl3, applied to corn straw and cob. The aim was to understand how these residues, coming from the same agroindustry, are similar or different in the face of the pretreatment reactions, which were carried out with these biomasses individually and mixed. In addition, the application of crude glycerol was investigated, to improve its use, as well as the direct submission of pretreated biomass to hydrolysis, without being subjected to washing and drying steps.
EXPERIMENTAL Collection and preparation of the biomasses Corn ears were collected in September 2022 from a family farming plantation located in the municipality of Itabaiana (Sergipe State, Brazil). The straw was removed and the ears were threshed, leaving the cob. Corn straw and cob were dried by exposure to solar irradiation to a moisture content of less than 10%. After drying, the biomasses were crushed separately to obtain a particle size of 0.5-1.0 mm, which was used in the pretreatment reactions, and a particle size of 1.0-1.7 mm, which was used in the analysis of the lignocellulosic composition of the in natura biomasses. For this purpose, the biomass that passed through a 16 mesh sieve and was retained in a 32 mesh sieve, as well as the biomass that passed through a 10 mesh sieve and was retained in a 16 mesh sieve were used, respectively. Organosolv pretreatment with glycerol The organosolv pretreatment reactions of the corn straw and corn cob biomasses were initially carried out with commercial glycerol (99.5%, Vetec, Brazil) and with each biomass separately. The pretreatment tests were carried out with 1.5 g of in natura, dried, and crushed biomass. The solid/liquid ratio (m/m) was 6% (1.5 g of biomass/20.0 mL of glycerol), and the reaction time was 10 min, at a temperature of 220 ºC. Iron(III) chloride hexahydrate P.A., FeCl3.6H2O (97-102%, Cromoline Química Fina, Brazil), was used as a catalyst, three situations were evaluated: without the use of a catalyst; with 0.0125 mol L-1 of FeCl3; and with 0.025 mol L-1 of FeCl3.13,18 All reactions were carried out in duplicate. To carry out the reactions, the reaction medium was placed in an Erlenmeyer flask which was immersed in an oil bath previously heated to 220 ºC on a hotplate with magnetic stirring. For proper heat transfer and homogenization, both the oil bath and the reaction medium were stirred. The reaction time was measured when the reaction medium reached the required temperature. After the reaction, the reactor was cooled in a water bath at room temperature. The liquid fraction was separated from the solid fraction by fabric filtration, using 50 mL of tap water to ensure complete transfer of the solid residue from the Erlenmeyer flask. The pH of the liquid fraction (liquor) was measured. The solid fraction (pretreated biomass) was washed three times with 100 mL of tap water, dried in an oven at 35 ºC, and subsequently weighed. After determining the best situation regarding the use of the FeCl3 catalyst, reactions were carried out with a mixture of biomasses (50% cob + 50% straw), with both commercial glycerol and crude glycerol. In addition, the direct application of the pretreated biomass in the enzymatic hydrolysis process was evaluated, without washing and drying, after been separated from the liquor by fabric filtration. Crude glycerol The crude glycerol used in the pretreatment reactions was obtained from Indústria e Comércio de Biodiesel Sul Brasil (BSBIOS), based in Passo Fundo, RS. The material received (pH 5.27) was treated with hydrochloric acid at the company itself, to precipitate and separate residual fatty acids from the biodiesel production process, which used a mixture of vegetable and animal fat. Thus, the crude glycerol was provided after undergoing a simple purification, and, due to this fact, no further treatment was required for use in the reactions, and was used under the conditions in which it was ceded. Composition: glycerol 82.74%; moisture 12.20%; methanol 0.71%; ash 4.01%. Lignin recovery The lignin removed from the liquor was recovered by centrifugation for 12 min at 9,500 rpm (Hermle Labortechnik Z36HK centrifuge), followed by washing and drying in an oven at 40 ºC. The washing was performed with the centrifuge using two steps, in order to ensure the removal of glycerol excess. The total volume of water used was equivalent to 40% of the volume of the liquor that was centrifuged. In each washing step, half of the water required was added to the tube containing the solid (lignin), with vigorous stirring for homogenization, followed by centrifugation for 10 min. Enzymatic hydrolysis Enzymatic hydrolysis was carried out using the Cellic CTec3 enzyme complex, from Novozymes Latin America Ltda (Paraná, Brazil), at a charge of 15 FPU g-1 (filter paper unit g-1biomass) which is related to the enzyme activity (63,43 FPU mL-1) determined according to Ghose.38 Erlenmeyer flasks were filled with 0.250 g of pretreated biomass, 30 mL of 50 mmol L-1 citrate buffer solution (pH 4.85), and the enzyme in a volume corresponding to 15 FPU g-1. The flasks were incubated for 48 h at 50 ºC, shaking at 100 rpm. Aliquots were taken from the reaction medium at predetermined time intervals and filtered through 0.45 μm nylon filters. In the case of enzymatic hydrolysis tests carried out with wet biomass (without washing and drying), the entire pretreated mass, from a reaction with 1.5 g of in natura biomass, was used together with 50 mL of buffer. The volume of the enzyme was proportional to the dry mass of pretreated biomass obtained in an equivalent reaction. The calculations of the concentration of glucose and xylose present in these hydrolysates took into account the proportionality of mass and volume to obtain comparable results. Methods of analysis Moisture, ash and extractives content determinations The methodologies employed for the determination of the moisture, ash and extractives contents of the biomasses were adapted from the procedures used by the National Renewable Energy Laboratory (NREL), respectively: NREL/TP-510-42621,39 NREL/TP-510-42622,40 and NREL/TP-510-42619.41 Determination of reducing sugars and biomass composition The pentoses and hexoses were determined by high-performance liquid chromatography (HPLC), using a Shimadzu Prominence chromatographic system (Kyoto, Japan), equipped with a refractive index detector (RID). The column used was an Aminex HPX-87P (300 × 7.8 mm), operating in isocratic elution mode, with ultrapure water as mobile phase at a flow rate of 0.6 mL min-1. The column oven temperature was maintained at 83 ºC and the detector cell temperature at 60 ºC. The running time was 18 min and the injection volume was 10 μL. Carbohydrate standards (purity ≥ 99.0%) were purchased from Sigma-Aldrich (Brazil). The lignin, hemicellulose, and cellulose contents of the in natura and pretreated biomasses were determined according to NREL procedures (NREL/TP-510-42618).42 The solid yield, the percentage recovery of the components (cellulose, hemicellulose and lignin), the degree of removal, the cellulose digestibility and the glucose yield were calculated using Equations 1-5: 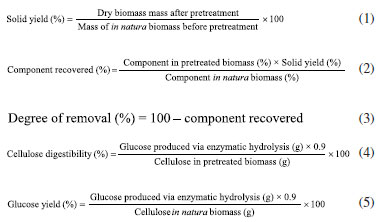 Determinations of HMF and furfural HMF and furfural were determined by HPLC, using a Prominence instrument (Shimadzu, Kyoto, Japan) equipped with a UV-Vis detector and a Lichrospher RP18-5 column (25 cm × 4.6 mm, 5 μm) maintained at 35 ºC. An isocratic elution mode was employed, using a mobile phase of acetonitrile:water (20:80 v/v), at a flow rate of 0.8 mL min-1. The run time was 10 min and the injection volume was 10 μL. The two compounds were quantified at 280 nm. HMF and furfural standards (purity ≥ 99.0%) were acquired from Sigma-Aldrich (Brazil). Determination of acetic acid and formic acid Acetic acid and formic acid were determined by HPLC, using a Prominence instrument (Shimadzu, Kyoto, Japan) equipped with a UV-Vis detector and a Supelcogel C-610H column (30 cm × 7.8 mm) maintained at 60 ºC. An isocratic elution mode was employed, using a mobile phase of H2SO4 0.005 mol L-1, at a flow rate of 0.6 mL min-1. The run time was 18 min and the injection volume was 10 μL. The two compounds were quantified at 210 nm. The standards were acquired from Sigma-Aldrich (Brazil). Fourier transform infrared spectroscopy (FTIR) FTIR spectra were obtained using an Agilent Cary 630 instrument, in the range 4000-500 cm-1, with a resolution of 4 cm-1.
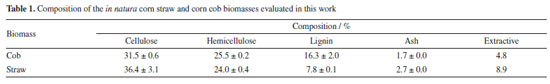
RESULTS AND DISCUSSION Composition of the in natura biomasses The composition of the in natura biomasses (Table 1) indicates that they are suitable for second-generation ethanol production since they have a high content of structural carbohydrates (cellulose and hemicellulose). It can be seen that the composition of corn straw and cob is similar, with the greatest differences being in the lignin and extractive content. The cob has about twice as much lignin as the straw, which explains its greater hardness.
The composition can change depending on the region and time of collection. The corn straw and cob used in this study were comparable in composition to those found in the literature.43,44 Organosolv pretreatment of the biomasses individually The corn straw and corn cob biomasses, pretreated separately with the commercial glycerol, were subjected to enzymatic hydrolysis to identify how the different pretreatment conditions influenced enzymatic digestibility. Figures 1a and 1b show, respectively, the concentration of glucose and xylose obtained in the hydrolysates.
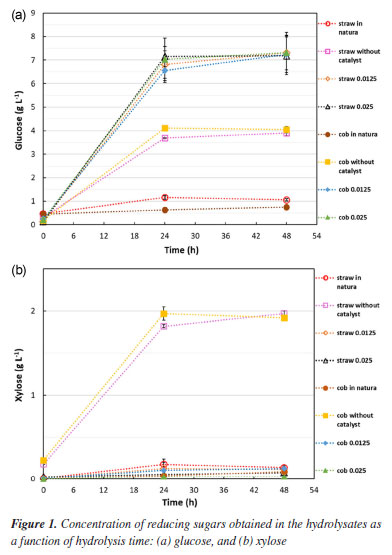
The quantification of glucose and xylose in the hydrolysates allowed the identification of the best pretreatment condition. The hydrolysates from the reactions with 0.0125 and 0.025 mol L-1 FeCl3 showed practically identical glucose levels for both straw and cob, around 7.0 g L-1 after 48 h of enzymatic hydrolysis. In addition, these hydrolysates contained very little xylose and no other monosaccharides. The reactions without catalyst were less efficient for glucose production. The concentration of glucose in both straw and cob hydrolysates was approximately 4.0 g L-1 after 48 h of hydrolysis. These reactions were also not very selective, the hydrolysates had considerable levels of xylose (around 2.0 g L-1), which is less preferable to the fermentation process. Given this, it is clear that the FeCl3 catalyst intensifies the reduction in the recalcitrance of the biomasses, allowing the enzymes better access to the cellulose. It also has a strong effect on the hemicellulosic fraction, facilitating its removal, since the hydrolysates from the pretreatment reactions using FeCl3 showed a negligible amount of xylose, the monosaccharide that makes up hemicellulose. In the literature, several works9,34,44-46 obtained high hemicellulose removal in biomass pretreatment processes using FeCl3. The pretreatment condition using 0.0125 mol L-1 of FeCl3 was considered the best for both biomasses. It uses less catalyst, bringing economic benefits to the process, and produces an amount of glucose equivalent to that of the pretreatment condition using 0.025 mol L-1. A test carried out in a previous study13 to evaluate the reduction in the amount of FeCl3 catalyst, indicated this same situation for corn straw. In the present study, it was interesting to note that corn straw and corn cob showed the same behavior about the amount of FeCl3. An analysis of Figure 1 also shows that, in 24 h, practically the same amount of reducing sugars is produced as in 48 h, making it possible to interrupt hydrolysis after just 24 h without damaging the result, this contributes to the economic and environmental sustainability of the process. Figure 1 also illustrates the amount of reducing sugars released in the hydrolysates of the in natura biomasses, proving the importance of carrying out the pretreatment. Table 2 shows the amount of acid-insoluble lignin recovered from the liquors. The reactions carried out with the catalyst released more lignin into the pretreatment liquor, proving that FeCl3 facilitates the fractionation of biomass. Table 2 also shows that the cob released more lignin during pretreatment, which is related to the fact that this biomass has a higher amount of lignin (Table 1).
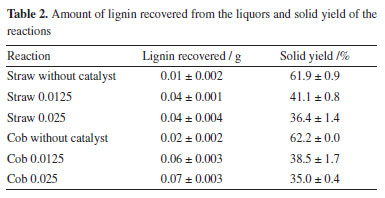
Figure 2 shows the FTIR spectra of the lignins from corn straw and cob. The profile of these is similar to those of lignin spectra obtained in literature studies.28,30,47,48 They presented a broad band in the 3600-3200 cm-1 region attributed to hydroxyl groups present in alcohols and phenolic compounds, and peaks related to the vibration of the aromatic structure in the 1600-1450 cm-1 region.
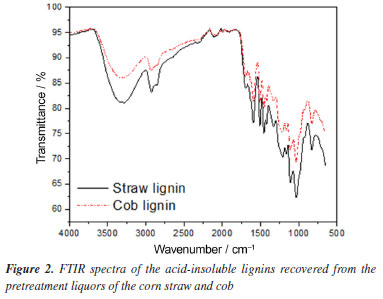
It is important to note that in this study, as in a previous study13 it was not necessary to add acid to the liquor to precipitate the lignin, a common practice in the literature23,30,49 since it was already dispersed in the acidic pH liquor (pH 5-6). Thus, only the separation was carried out using the centrifugation technique. The lignin was also washed in the centrifuge, and the water consumption was managed and corresponded to the minimum necessary to remove excess glycerol and enable drying. Thus, the recovery of lignin in this research took place through a simple and sustainable process. The hydrolysis and lignin recovery results corroborate with the solid yield of the reactions (Table 2), which were very similar for the two biomasses. Thus, despite the relevant difference in the composition of straw and cob concerning lignin, organosolv pretreatment with glycerol resulted in very similar materials, as evidenced by the equivalent release of glucose and xylose in the two pretreated biomasses. Organosolv pretreatment with mixture of the biomasses Given the very similar behavior of corn straw and cob in pretreatment reactions, additional experiments were conducted using a mixture of these biomasses (50% cob + 50% straw), an approach not previously reported in the literature. The best pretreatment condition was adopted, i.e., using 0.0125 mol L-1 of FeCl3. The lignocellulosic composition of the biomass pretreated in this situation, as well as the percentage of components recovery and removal, the concentration of glucose obtained in the hydrolysate, the glucose yield, and the cellulose digestibility are shown in Table 3.
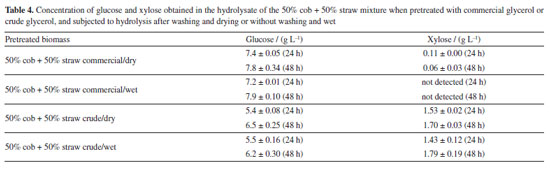
The analysis of Table 3 reveals that this pretreated biomass showed excellent recovery of cellulose, as well as high removal of hemicellulose and lignin. These are aspects sought in the pretreatment of biomass for the production of cellulosic ethanol, since they indicate fractionation, reduction of recalcitrance by removing lignin and hemicellulose for the liquid fraction (liquor), and preservation of cellulose in the solid fraction. Cellulose is the component of greatest interest in the production of bioethanol, as it is the main source of glucose that will later be fermented. This result was reflected in the amount of glucose produced in the hydrolysate and the excellent cellulose enzymatic digestibility, which reached 94.5% in 24 h and 99.9% in 48 h, i.e., practically all the cellulose was converted into glucose during enzymatic hydrolysis. It is therefore clear that mixing these two lignocellulosic residues, coming from the same agroindustry, is a good strategy. The amount of glucose produced in the hydrolysate of the mixture was very similar to that obtained with the cob and straw individually (Figure 1). The molecular mechanism that occurs in the hydrolysis of lignocellulose was investigated by Zhang et al.50 This contributes to the understanding of the effect of the optimal condition of pretreatment with glycerol/FeCl3 on increasing the saccharification of the biomasses evaluated here. Based on the work cited, the high production of glucose in the hydrolysis process, in addition to being a result of the large removal of hemicellulose and lignin in the pretreatment, is attributed to the preservation of cellulose with many amorphous regions that act as interruption points to initiate and complete the enzymatic hydrolysis of cellulose nanofibers. Organosolv pretreatment with a mixture of the biomasses: commercial glycerol versus crude glycerol and dry biomass versus wet biomass Pretreatment reactions with a mixture of 50% cob and 50% straw were also carried out with the crude glycerol from biodiesel production. Additional reactions using this biomass mixture were conducted with both commercial glycerol and crude glycerol, and the pretreated biomass without washing and wet was subjected directly to the enzymatic hydrolysis stage, to verify the possibility of eliminating the washing and drying stages of the pretreated biomass (Table 4).
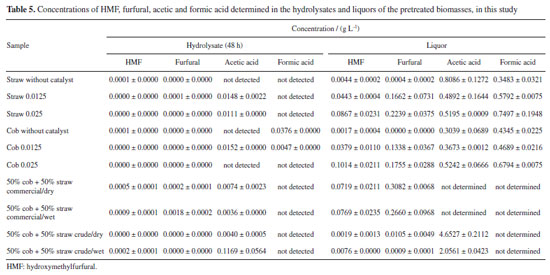
The results of Table 4 are promising. It can be seen that crude glycerol has the potential to be used in corn cob and straw pretreatment reactions. Although the production of glucose in the hydrolysate have been smaller than that obtained with commercial glycerol, was offset by the production of xylose, which indicates a lower removal of hemicellulose with the use of crude glycerol. The lower selectivity for glucose production should not have much impact on the fermentation process, given that glucose remains the majority sugar. In addition, xylose can contribute to the ethanol yield, provided that a more careful choice of fermenting microorganism is made, as research indicates that some are capable of fermenting glucose and xylose simultaneously.51 The analysis in Table 4 also shows that carrying out hydrolysis immediately after the pretreatment reaction with the wet pretreated biomass, without being subjected to the washing and drying steps, does not cause any harm to sugar production, whether using commercial or raw glycerol. This result is a differentiator and is of great importance, significantly improving the sustainability of the process, since it leads to significant savings in water and energy resources. In previous work13 an investigation was carried out regarding the use of crude glycerol in the pretreatment of water hyacinth biomass. Among the various tests carried out, the situation using crude glycerol subjected to simple treatment and keeping the reactor open resulted in the release of total reducing sugars equivalent to that using commercial glycerol, but the released sugars were not discriminated. An evaluation of Table 4 shows that, in this study, the release of total reducing sugars (glucose + xylose) using crude glycerol was also equivalent to that obtained using commercial glycerol. The same previous work13 tested the direct submission of wet and without washing pretreated biomass to the enzymatic hydrolysis process, and the results corresponded to those of this study, there was no loss in the production of reducing sugars. Ji et al.20 studied the pretreatment of Moso bamboo biomass with various sources of glycerol and, also, evaluated the non-washing of the pretreated biomass. They used a pressure filtration process, without the use of water, which keeps glycerol residues under the pretreated solid. Enzymatic hydrolysis was carried out, and with one of the glycerol sources, resulting from the production of biodiesel with KOH, the glucose yield was not impaired. The works of Zhang et al.52 and Zhu et al.28 investigated the effect of the presence of certain concentrations of glycerol in the enzymatic hydrolysis reaction medium. In both cases, glycerol had no negative impact at concentrations of up to 2% by weight. Thus, in this study, filtration in fabric, in which the biomass was squeezed through the fabric to remove excess liquid, meant that few glycerol residues remained adhered to it, since there was no unfavorable effect on the release of sugars in the enzymatic hydrolysis carried out with the unwashed biomass. Being that, the addition of a little water to the pretreatment reactor before filtration, to enable the content to be transferred, helped to reduce the adherence of the glycerol to the pretreated biomass. Determination of HMF, furfural, acetic acid, and formic acid Table 5 shows the concentrations of HMF, furfural, acetic and formic acid that were determined in the hydrolysates and liqueurs. These compounds, at certain concentration levels, can inhibit the action of the microorganisms responsible for fermentation. Thus, the quantification of these by-products makes it possible to assess the capacity for producing ethanol from the hydrolysate and the pretreatment liquor.
Studies53-55 indicate that the presence of 0.5 g L-1 of acetic acid is enough to negatively affect the fermentation process, with ethanol yields reduced by 20-40%. Complete inhibition of ethanol production was observed at acetic acid concentrations in the range of 3.5-4.0 g L-1. For formic acid, it was observed55 that a concentration of 2.0 g L-1 almost completely inhibited ethanol production, with inhibition being less pronounced at lower concentrations. Furfural exerts some relevant inhibitory effect from 2.0 g L-1, and HMF from 0.5 g L-1.53 The results in Table 5 show that the hydrolysates can be subjected to the fermentation process without undergoing detoxification treatment, since acetic acid is present at a concentration well below 0.5 g L-1, and formic acid, when detected, is present at a very low concentration. In addition, HMF and furfural are found at negligible levels in the hydrolysates, well below what is necessary to cause inhibitory action. In the liqueurs, HMF and furfural have slightly higher concentrations than in the hydrolysates, but these are well below those needed to cause inhibition. In the case of formic acid, the concentration in the liqueurs may be sufficient to cause slight inhibition. The concentration of acetic acid in the liqueurs is higher than in the hydrolysates, enough to cause moderate inhibition and strong inhibition in the case of the liqueur obtained with crude glycerol. As a result, it does not seem feasible to subject the liqueurs to a fermentation process without prior treatment to extract the acetic acid. This is formed as a result of the high removal of hemicellulose by pretreatment, and is, therefore, obtaining it is a natural consequence.33,44 One possibility is to recover this by-product through distillation and add value to it by setting up a biorefinery. Mass balance and comparison with other studies Figure 3 shows the mass balance for the 50% cob + 50% straw mixture, pretreated according to the chosen condition and using commercial glycerol. A comparison with studies from the literature11,16,23,56 revealed that the results of this research are promising. From one kilogram of 50% cob + 50% straw in natura, 360.9 g of glucose were obtained in the hydrolysate in just 24 h of enzymatic hydrolysis, and 381.5 g after 48 h. Considering the yield of 0.51 g of ethanol per gram of glucose and the density of ethanol as 0.789 g mL-1, the theoretical ethanol production after 24 h corresponds to 233 L per ton of biomass, which falls within the expected range for second-generation ethanol production.
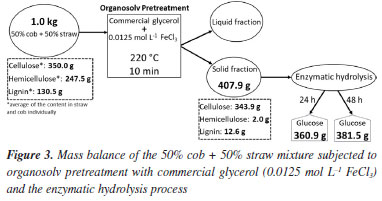
The work of Cai et al.56 performed pretreatment on corn cob with sulfuric acid. The pretreatment was carried out in an autoclave at 120 ºC, and the condition that provided the highest production of glucose in the hydrolysate after 72 h (296.4 g kg-1) used 0.25 wt.% H2SO4 for 120 min. Thus, the present study obtained a higher glucose yield in a short hydrolysis time, using a more sustainable and safer pretreatment, carried out under atmospheric pressure in just 10 min. In the work of David et al.,11 an optimization of corn cob pretreatment was carried out using green liquor dregs, a residue from the chemical kraft pulping industry. The process was carried out in an autoclave at 121 ºC. The percentages of cob and dregs green liquor, the solid load, and the time were optimized. The yield was presented in g of glucose per g of dry pretreated cob, and the optimized condition resulted in 0.42 g g-1 after 72 h of enzymatic hydrolysis. In this study, 0.88 g g-1 was obtained after just 24 h of hydrolysis and 0.93 g g-1 after 48 h. This result is due to the high recovery of cellulose in the pretreated biomass and its high digestibility during enzymatic hydrolysis. In the work of Wu et al.,23 corn straw was pretreated in two stages. The first was a hydrothermal pretreatment with seawater carried out at 190 ºC for 40 min. The second was a pretreatment with electro-generated alkaline hydrogen peroxide, carried out at 70 ºC for 4 h. After the two stages, enzymatic hydrolysis was carried out and after 72 h, 33.18 kg of glucose was obtained from 100 kg of straw. In this study, a single pretreatment strategy, lasting only 10 min, obtained 36.09 kg of glucose in 24 h and 38.15 kg in 48 h, considering 100 kg of the raw material 50% cob + 50% straw in natura. It is clear that in this work, the production of glucose was more expressive and obtained through a much simpler and faster method. In the work of Luo et al.,16 corn straw underwent an organosolv pretreatment with glycerol (80%) assisted by L-cysteine, at 220 ºC for 0.5 h. The enzymatic hydrolysis was carried out and, after 72 h, 20.80 g of glucose per 100 g of straw were obtained in the hydrolysate. The present study also used an organosolv pretreatment with glycerol, but with FeCl3 at 220 ºC for 10 min. In this case, for every 100 g of the mixture of 50% cob + 50% straw in natura, 36.09 g of glucose was obtained in the hydrolysate in 24 h and 38.15 g in 48 h of enzymatic hydrolysis. Thus, in addition to the much higher production of glucose, both the pre-treatment and hydrolysis were carried out in less time compared to Luo et al.,16 resulting in an energy gain. As already discussed, corn straw and corn cob behaved in a similar way to pretreatment, and increasing the concentration of FeCl3 from 0.0125 to 0.025 mol L-1 did not alter the result of sugar release. This fact related to the concentration of FeCl3 was also observed in the previous study13 for corn straw. However, in the present study the biomasses were processed to a more limited particle size range (0.5-1.0 mm) than that of Santana et al.13 in the optimized condition (smaller than 0.85 mm). It was noted that in this current study, cellulose recovery in the 50% cob + 50% straw pretreated biomass (98.2%) was much higher than that obtained in the previous study13 for corn straw pretreated under the optimized condition (54.6%). This great improvement in cellulose recovery was certainly due to the more limited particle size range, which excluded very fine particles that are more susceptible to excessive degradation. As a result, practically all the cellulose was preserved in the pretreated biomass, and there was an excellent reduction in recalcitrance due to the high removal of hemicellulose and lignin. Consequently, the production of glucose in the hydrolysate in this present study was much better than that obtained by Santana et al.13 Thus, in this study, as in the previous one, the importance of particle size was evident. In the study by Santana et al.13 the particle size, in the ranges studied, showed relevance for water hyacinth biomass, which had a much-improved result when pretreated with smallest particle size evaluated (smaller than 0.85 mm), for the other biomasses studied (corn straw, sugarcane bagasse, coconut husks) particle size was not significant for the response, and the smallest particle size tested was adopted as the optimized condition, due to standardization criteria with water hyacinth biomass. In the present study, the adoption of a particle size range very similar to the smallest size evaluated by Santana et al.,13 but excluding very fine particles, enabled greater preservation of cellulose without harming the reduction in recalcitrance. In this way, the present study represents an advance over the previous one,13 since it made more explicit the importance of properly reducing the size of the biomass before it is subjected to pretreatment. Based on both studies, it can be concluded that the most suitable particle size for biomass pretreatment reactions aimed at obtaining second-generation ethanol is the 0.5-1.0 mm range.
CONCLUSIONS The organosolv pretreatment with glycerol of the corn straw and corn cob biomasses individually revealed that they behave similarly, giving rise to pretreated biomasses with equivalent characteristics. The pretreatment condition using 0.0125 mol L-1 of FeCl3 was considered the best, since it produces an amount of glucose equivalent to that of the condition using 0.025 mol L-1, and the reactions without catalyst were less efficient for glucose production. Pretreatment with the 50% cob + 50% straw mixture showed that it is feasible to use this waste simultaneously, which can facilitate processing. The application of crude glycerol proved promising, given that glucose remains the majority sugar. It became evident that the pretreated biomass can be subjected directly to enzymatic hydrolysis without going through the washing and drying stages.
DATA AVAILABILITY STATEMENT All data are available in the text.
ACKNOWLEDGMENTS The authors thank the Brazilian funding agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant 165052/2020-1) and Universidade de Tiradentes (UNIT) for the structure provided for the development of the research. The samples of crude glycerol were kindly provided by Indústria e Comércio de Biodiesel Sul Brasil (BSBIOS).
REFERENCES 1. Madhuvanthi, S.; Jayanthi, S.; Suresh, S.; Pugazhendhi, A.; Chemosphere 2022, 304, 135242. [Crossref] 2. Shankar, K.; Kulkarni, N. S.; Jayalakshmi, S. K.; Sreeramulu, K.; Biomass Bioenergy 2019, 127, 105298. [Crossref] 3. Wang, L. Q.; Cai, L. Y.; Ma, Y. L.; RSC Adv. 2020, 10, 38409. [Crossref] 4. Yuan, X.; Shen, G.; Chen, S.; Chen, X.; Zhang, C.; Liu, S.; Jin, M.; Energy 2022, 247, 123488. [Crossref] 5. Grisales Díaz, V. H.; Willis, M. J.; Energy Convers. Manage. 2019, 202, 112200. [Crossref] 6. Joy, S. P.; Krishnan, C.; Ind. Crops Prod. 2022, 177, 114409. [Crossref] 7. Mata, T. M.; Rodrigues, S.; Caetano, N. S.; Martins, A. A.; Energy Reports 2022, 8, 468. [Crossref] 8. Santos, F. A.; de Queiróz, J. H.; Colodette, J. L.; Fernandes, S. A.; Guimarães, V. M.; Rezende, S. T.; Quim. Nova 2012, 35, 1004. [Crossref] 9. Chen, L.; Chen, R.; Fu, S.; ACS Sustainable Chem. Eng. 2015, 3, 1794. [Crossref] 10. Guragain, Y. N.; de Coninck, J.; Husson, F.; Durand, A.; Rakshit, S. K.; Bioresour. Technol. 2011, 102, 4416. [Crossref] 11. David, A. N.; Sewsynker-Sukai, Y.; Sithole, B.; Gueguim Kana, E. B.; Fuel 2020, 274, 117797. [Crossref] 12. Qiao, H.; Han, M.; Ouyang, S.; Zheng, Z.; Ouyang, J.; Renewable Energy 2022, 191, 775. [Crossref] 13. Santana, J. C.; da Silva, A. C. M.; Abud, A. K. S.; Wisniewski, A.; Romão, L. P. C.; J. Braz. Chem. Soc. 2022, 33, 1117. [Crossref] 14. Trinh, L. T. P.; Lee, Y. J.; Lee, J. W.; Lee, H. J.; Biomass Bioenergy 2015, 81, 1. [Crossref] 15. Bensah, E. C.; Mensah, M.; Int. J. Chem. Eng. 2013, 2013, 719607. [Crossref] 16. Luo, H.; Gao, L.; Xie, F.; Shi, Y.; Zhou, T.; Guo, Y.; Yang, R.; Bilal, M.; Bioresour. Technol. 2022, 363, 127975. [Crossref] 17. Pascal, K.; Ren, H.; Sun, F. F.; Guo, S.; Hu, J.; He, J.; ACS Omega 2019, 4, 20015. [Crossref] 18. Santana, J. C.; Abud, A. K. S.; Wisniewki Junior, A.; Navickiene, S.; Romão, L. P. C.; Biomass Bioenergy 2020, 133, 105454. [Crossref] 19. Zhang, R.; Gao, H.; Wang, Y.; He, B.; Lu, J.; Zhu, W.; Peng, L.; Wang, Y.; Bioresour. Technol. 2023, 369, 128315. [Crossref] 20. Ji, L.; Lei, F.; Zhang, W.; Song, X.; Jiang, J.; Wang, K.; Bioresour. Technol. 2019, 276, 300. [Crossref] 21. Das, S.; Bhattacharya, A.; Haldar, S.; Ganguly, A.; Gu, S.; Ting, Y. P.; Chatterjee, P. K.; Sustainable Mater. Technol. 2015, 3, 17. [Crossref] 22. Lopes, A. M. C.; João, K. G.; Rubik, D. F.; Bogel-Łukasik, E.; Duarte, L. C.; Andreaus, J.; Bogel-Łukasik, R.; Bioresour. Technol. 2013, 142, 198. [Crossref] 23. Wu, Y.; Li, X.; Li, F.; Ling, Z.; Meng, Y.; Chen, F.; Ji, Z.; Bioresour. Technol. 2022, 351, 127066. [Crossref] 24. Wang, M.; Wang, Y.; Liu, J.; Yu, H.; Liu, P.; Yang, Y.; Sun, D.; Kang, H.; Wang, Y.; Tang, J.; Fu, C.; Peng, L.; Green Carbon 2024, 2, 164. [Crossref] 25. Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S.; Fuel Process. Technol. 2017, 160, 196. [Crossref] 26. Sun, F. F.; Wang, L.; Hong, J.; Ren, J.; Du, F.; Hu, J.; Zhang, Z.; Zhou, B.; Bioresour. Technol. 2015, 187, 354. [Crossref] 27. Zhang, Z.; Harrison, M. D.; Rackemann, D. W.; Doherty, W. O. S.; O'Hara, I. M.; Green Chem. 2016, 18, 360. [Crossref] 28. Zhu, Y.; Qi, B.; Liang, X.; Luo, J.; Wan, Y.; Renewable Energy 2021, 178, 1456. [Crossref] 29. Ebrahimi, M.; Villaflores, O. B.; Ordono, E. E.; Caparanga, A. R.; Bioresour. Technol. 2017, 228, 264. [Crossref] 30. Romaní, A.; Ruiz, H. A.; Teixeira, J. A.; Domingues, L.; Renewable Energy 2016, 95, 1. [Crossref] 31. Sun, F.; Chen, H.; Bioresour. Technol. 2008, 99, 5474. [Crossref] 32. Hodaifa, G.; Martínez Nieto, L.; Kowalska, M.; J. Taiwan Inst. Chem. Eng. 2022, 131, 104202. [Crossref] 33. Van Eylen, D.; van Dongen, F.; Kabel, M.; de Bont, J.; Bioresour. Technol. 2011, 102, 5995. [Crossref] 34. Wei, W.; Jin, Y.; Wu, S.; Yuan, Z.; Ind. Crops Prod. 2019, 140, 111663. [Crossref] 35. Wu, L.; Feng, S.; Deng, J.; Yu, B.; Wang, Y.; He, B.; Peng, H.; Li, Q.; Hu, R.; Peng, L.; Green Chem. 2019, 21, 4388. [Crossref] 36. Li, T.; Peng, H.; He, B.; Hu, C.; Zhang, H.; Li, Y.; Yang, Y.; Wang, Y.; Bakr, M. M. A.; Zhou, M.; Peng, L.; Kang, H.; Int. J. Biol. Macromol. 2024, 264, 130448. [Crossref] 37. Liu, J.; Zhang, X.; Peng, H.; Li, T.; Liu, P.; Gao, H.; Wang, Y.; Tang, J.; Li, Q.; Qi, Z.; Peng, L.; Xia, T.; Molecules 2023, 28, 2060. [Crossref] 38. Ghose, T. K.; Pure Appl. Chem. 1987, 59, 257. [Crossref] 39. Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J.; NREL/TP-510-42621: Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; National Renewable Energy Laboratory: Colorado. [Link] accessed in August 2025 40. Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; NREL/TP-510-42622:Determination of Ash in Biomass; National Renewable Energy Laboratory: Colorado, 2008. [Link] accessed in August 2025 41. Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; NREL/TP-510-42619:Determination of Extractives in Biomass; National Renewable Energy Laboratory: Colorado, 2008. [Link] accessed in August 2025 42. Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.; NREL/TP-510-42618: Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Colorado, 2012. [Link] accessed in August 2025 43. Du, C.; Li, Y.; Zong, H.; Yuan, T.; Yuan, W.; Jiang, Y.; Bioresour. Technol. 2020, 310, 123427. [Crossref] 44. Kamireddy, S. R.; Li, J.; Tucker, M.; Degenstein, J.; Ji, Y.; Ind. Eng. Chem. Res. 2013, 52, 1775. [Crossref] 45. Liu, L.; Sun, J.; Cai, C.; Wang, S.; Pei, H.; Zhang, J.; Bioresour. Technol. 2009, 100, 5865. [Crossref] 46. Zhang, H.; Zhang, S.; Yuan, H.; Lyu, G.; Xie, J.; Bioresour. Technol. 2018, 249, 395. [Crossref] 47. Kim, D.; Cheon, J.; Kim, J.; Hwang, D.; Hong, I.; Kwon, O. H.; Park, W. H.; Cho, D.; Carbon Lett. 2017, 22, 81. [Crossref] 48. Minu, K.; Jiby, K. K.; Kishore, V. V. N.; Biomass Bioenergy 2012, 39, 210. [Crossref] 49. Zhong, L.; Zhang, X.; Tang, C.; Chen, Y.; Shen, T.; Zhu, C.; Ying, H.; Bioresour. Technol. 2018, 268, 677. [Crossref] 50. Zhang, R.; Hu, Z.; Wang, Y.; Hu, H.; Li, F.; Li, M.; Ragauskas, A.; Xia, T.; Han, H.; Tang, J.; Yu, H.; Xu, B.; Peng, L.; Nat. Commun. 2023, 14, 1100. [Crossref] 51. Li, J.; Liu, D.; Zhang, M.; Huang, H.; Wang, D.; Ind. Crops Prod. 2019, 140, 111728. [Crossref] 52. Zhang, Z.; Wong, H. H.; Albertson, P. L.; Harrison, M. D.; Doherty, W. O. S.; O'Hara, I. M.; Bioresour. Technol. 2015, 192, 367. [Crossref] 53. Bellido, C.; Bolado, S.; Coca, M.; Lucas, S.; González-Benito, G.; García-Cubero, M. T.; Bioresour. Technol. 2011, 102, 10868. [Crossref] 54. Colombi, B. L.; Ortiz, M. A.; Zanoni, P. R. S.; Magalhães, W. L. E.; Tavares, L. B. B.; Engevista 2017, 19, 339. [Link] accessed in August 2025 55. Toquero, C.; Bolado, S.; Bioresour. Technol. 2014, 157, 68. [Crossref] 56. Cai, D.; Dong, Z.; Wang, Y.; Chen, C.; Li, P.; Qin, P.; Wang, Z.; Tan, T.; Bioresour. Technol. 2016, 211, 677. [Crossref]
Associate Editor handled this article: Eduardo M. Richter |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Química Nova
Publicações da Sociedade Brasileira de Química
Caixa Postal: 26037
05513-970 São Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access